Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator
Abstract
1. Introduction
2. Experimental
2.1. Materials and Procedures
2.2. Compressive Strength
2.3. Thermogravimetric/Differential Thermal Gravimetry
2.4. X-Ray Diffraction
2.5. Solid-State Nuclear Magnetic Resonance
3. Results and Analysis
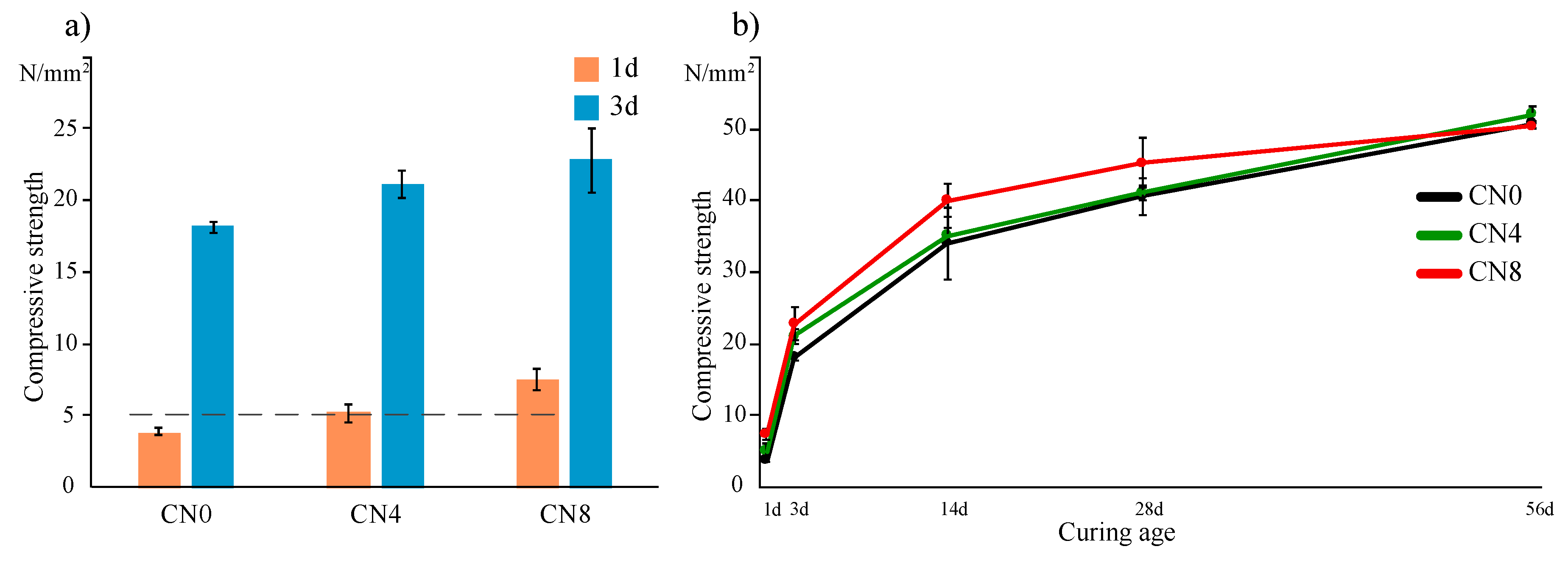
3.1. Compressive Strength
3.2. Hydrate Formation Behavior
3.2.1. TG/DTG
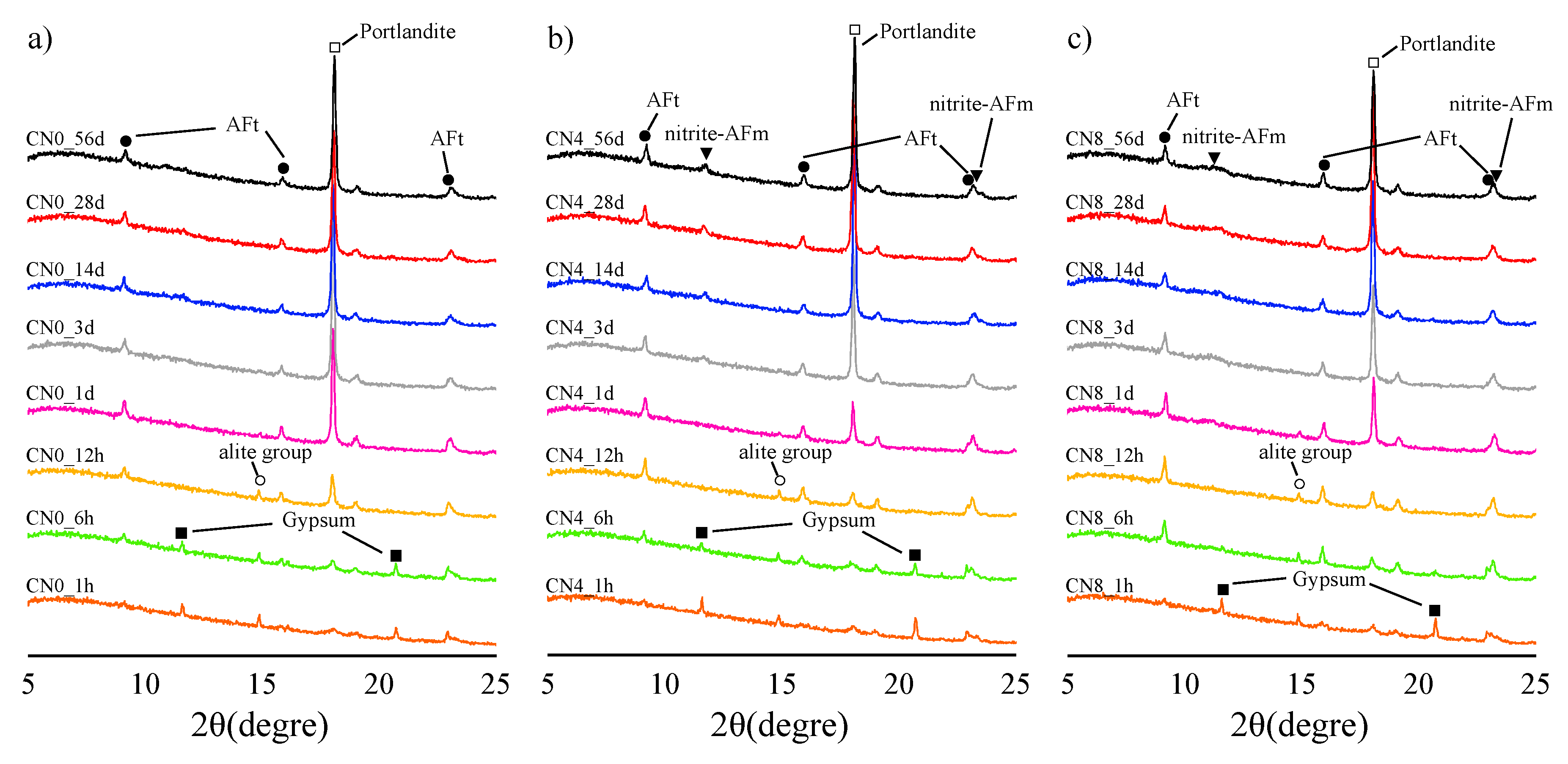
3.2.2. XRD
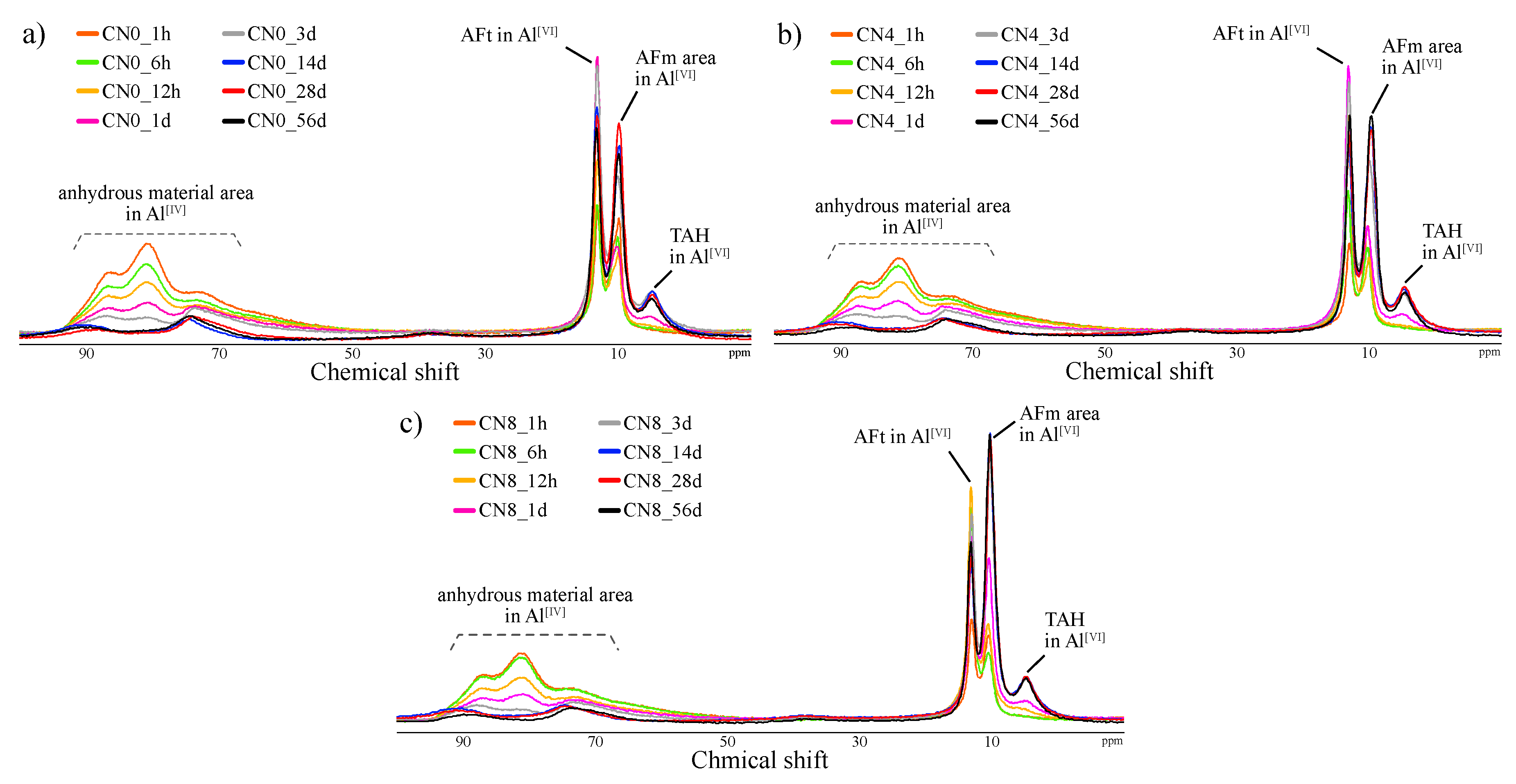
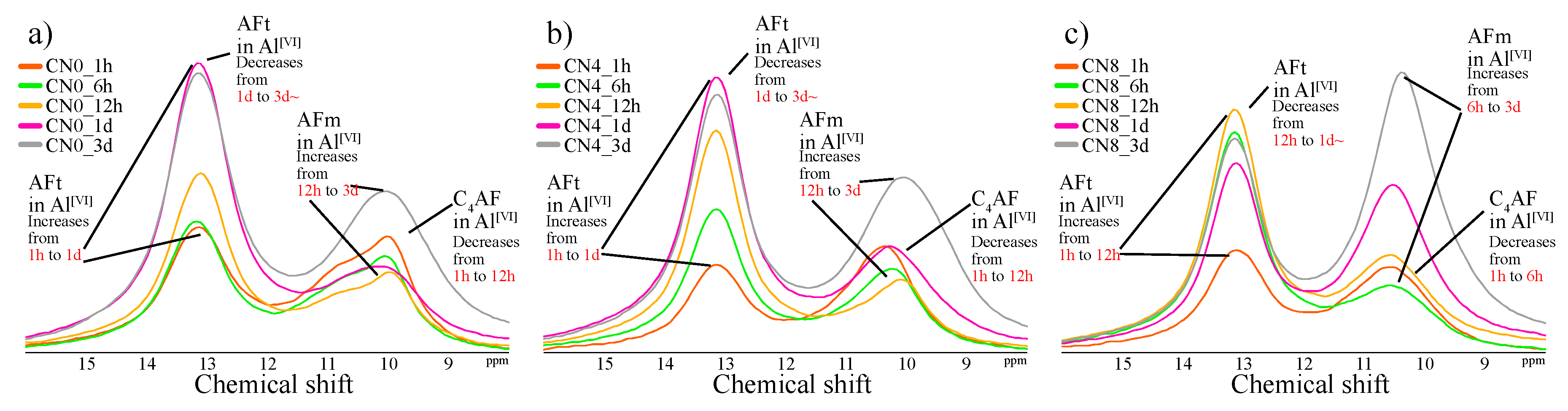
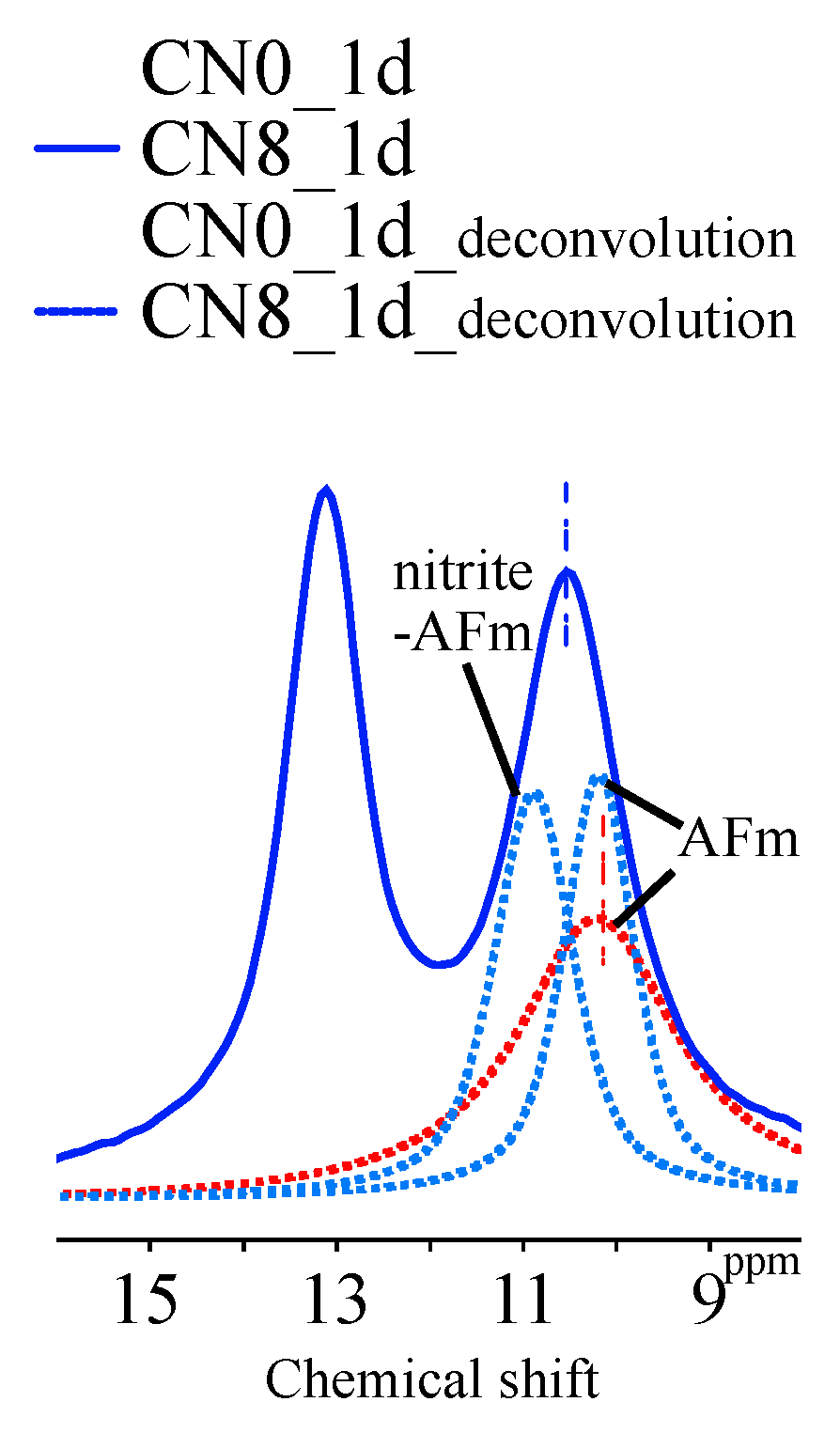
3.2.3. Al MAS NMR
3.2.4. Si MAS NMR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akama, T.; Inoue, M.; Sudoh, U.; Mikami, S. Fresh properties and early strength development of concrete using calcium nitrite and water-reducing agents. Proc. Jpn. Concr. Inst. 2012, 34, 155–159. [Google Scholar]
- Iwasawa, M.; Inoue, M.; Choi, H.; Sudoh, Y.; Ayuta, K. Characteristics of concrete using nitrite-based accelerator and chemical admixtures in low-temperature environments. In Proceedings of the 7th International Conference of Asian Concrete Federation, Hanoi, Vietnam, 30 October–2 November 2016; pp. 1–8. [Google Scholar]
- Practical Guideline for Investigation. Recommendation for Practice of Cold Weather Concreting; Architectural Institute of Japan: Tokyo, Japan, 2010; p. 57. [Google Scholar]
- Grochoski, C. Cold Weather Concreting with Hydronic Heaters. J. Am. Concr. Inst. ACI 2000, 22, 51–55. [Google Scholar]
- ACI Committee 306. Cold-Weather Concreting, ACI 306R-88; American Concrete Institute (ACI): Farmington Hills, MI, USA, 1997. [Google Scholar]
- Choi, H. Control of curing temperature of cold-weather concrete through effective utilization of energy-saving heat-curing systems. In Proceedings of the 2017 World Congress on Advances in Structural Engineering and Mechanics, Seoul, Korea, 28 August–1 September 2017; pp. 1–8. [Google Scholar]
- Construction Promotion Council. Operation Manual of Anti-Freezing Agent; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2005.
- Hama, Y.; Kamada, E. Effects on Protection Fresh Concrete against Frost Damage by Non-chloride and Non-alkali Type Antifreezing Admixtures. Concr. Res. Technol. 1996, 7, 113–122. [Google Scholar] [CrossRef][Green Version]
- Ramachanran, V.S. Concrete Admixture Handbook; Noyes Publications: Park Ridge, NJ, USA, 1995; pp. 741–799. [Google Scholar]
- Kojima, T. Concrete Admixture Handbook; The Society of Materials Science: Tokyo, Japan, 2004; pp. 172–175. [Google Scholar]
- Balonis, M.; Glasser, F.P.; Medala, M. Influence of calcium nitrate and nitrite on the constitution of AFm and AFt cement hydrates. Adv. Cem. Res. 2011, 23, 129–143. [Google Scholar] [CrossRef]
- Japanese Industrial Standards. Method of Test for Compressive Strength of Concrete, JIS A 1108; Japanese Standards Association: Tokyo, Japan, 2018. [Google Scholar]
- Paulo, J.M. CONCRETE, Microstructure, Properties, and Materials, 2nd ed.; Mc Graw Hill: New York, NY, USA, 1995; pp. 181–227. [Google Scholar]
- Falzone, G.; Balonis, M.; Sant, G. X-AFm stabilization as a mechanism of bypassing conversion phenomena in calcium aluminate cements. Cem. Concr. Res. 2015, 72, 54–68. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997; p. 459. [Google Scholar]
- Choi, H.; Inoue, M.; Choi, H.; Kim, J.; Sudoh, Y.; Kwon, S.; Lee, B.; Yoneyama, A. Physicochemical Study on the Strength Development Characteristics of Cold Weather Concrete Using a Nitrite–Nitrate Based Accelerator. Materials 2019, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Dumm, J.Q.; Brown, P.W. Phase assemblages in the system Ca (OH)2-Al2O3-Ca (NO3)2-H2O. Adv. Cem. Res. 1996, 8, 143–153. [Google Scholar] [CrossRef]
- Sakai, E.; Ueda, Y.; Aikawa, Y.; Nito, N. Influence of calcium nitrite on the hydration of high volume slag cement. J. Cem. Sci. Concr. Technol. 2017, 71, 62–67. [Google Scholar] [CrossRef]
- Chudek, J.A.; Hunter, G.; Jones, M.R.; Scrimgeour, S.N.; Hewlett, P.C.; Kudryavtsev, A.B. Aluminium-27 solid state NMR spectroscopic studies of chloride binding in Portland cement and blends. J. Mater. Sci. 2000, 35, 4275–4288. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- L’Hopital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Richardson, I.G.; Brough, A.R.; Brydson, R.; Groves, G.W.; Dobson, C.M. Location of Aluminum in Substituted Calcium Silicate Hydrate (C-S-H) Gels as Determined by Si-29 and Al-27 Nmr and Eels. J. Am. Ceram. Soc. 1993, 76, 2285–2288. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. A new Aluminium-hydrate species in hydrated Portland cements characterized by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2006, 36, 3–17. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R.J. 29Si MAS NMR study of the structure of calcium silicate hydrate. Adv. Cem. Based Mater. 1996, 3, 144–156. [Google Scholar] [CrossRef]
- Thomas, S.; Meise Gresch, K.; Müller-Warmuth, W.; Odler, I. MAS NMR Studies of Partially Carbonated Portland Cement and Tricalcium Silicate Pastes. J. Am. Ceram. Soc. 1993, 76, 1998–2004. [Google Scholar] [CrossRef]




| Type | Index | CN Content [Cement × wt%] | W/C wt% | Curing Condition | Curing Age h: hour, d: day | Analysis Method | |
|---|---|---|---|---|---|---|---|
| Cement paste | CN0 CN4 CN8 | 0 4 8 | 50 | +10 °C Sealed | 1 h 6 h 12 h | 1 d 3 d 14 d 28 d 56 d | TG/DTG XRD 27Al MAS NMR 29Si MAS NMR |
| Mortar | - | 1 d 3 d 14 d 28 d 56 d | Compressive Strength | ||||
| Component | Component Ratio | Specific Gravity of Aqueous Solution | pH of Aqueous Solution |
|---|---|---|---|
| Ca(NO2)2 | 31.84 wt% | 1.308 | 10.5 |
| Ca(NO3)2 | 3.17 wt% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Honda, D.; Choi, H.; Hama, Y. Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. Materials 2019, 12, 3936. https://doi.org/10.3390/ma12233936
Kim J, Honda D, Choi H, Hama Y. Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. Materials. 2019; 12(23):3936. https://doi.org/10.3390/ma12233936
Chicago/Turabian StyleKim, Jihoon, Daiki Honda, Heesup Choi, and Yukio Hama. 2019. "Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator" Materials 12, no. 23: 3936. https://doi.org/10.3390/ma12233936
APA StyleKim, J., Honda, D., Choi, H., & Hama, Y. (2019). Investigation of the Relationship between Compressive Strength and Hydrate Formation Behavior of Low-Temperature Cured Cement upon Addition of a Nitrite-Based Accelerator. Materials, 12(23), 3936. https://doi.org/10.3390/ma12233936





