Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetite Nanoparticles
2.3. Synthesis of Fe (III)-Oleate
2.4. Ligand Modification
2.5. Characterization
2.6. Cell Culture
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results and Discussion
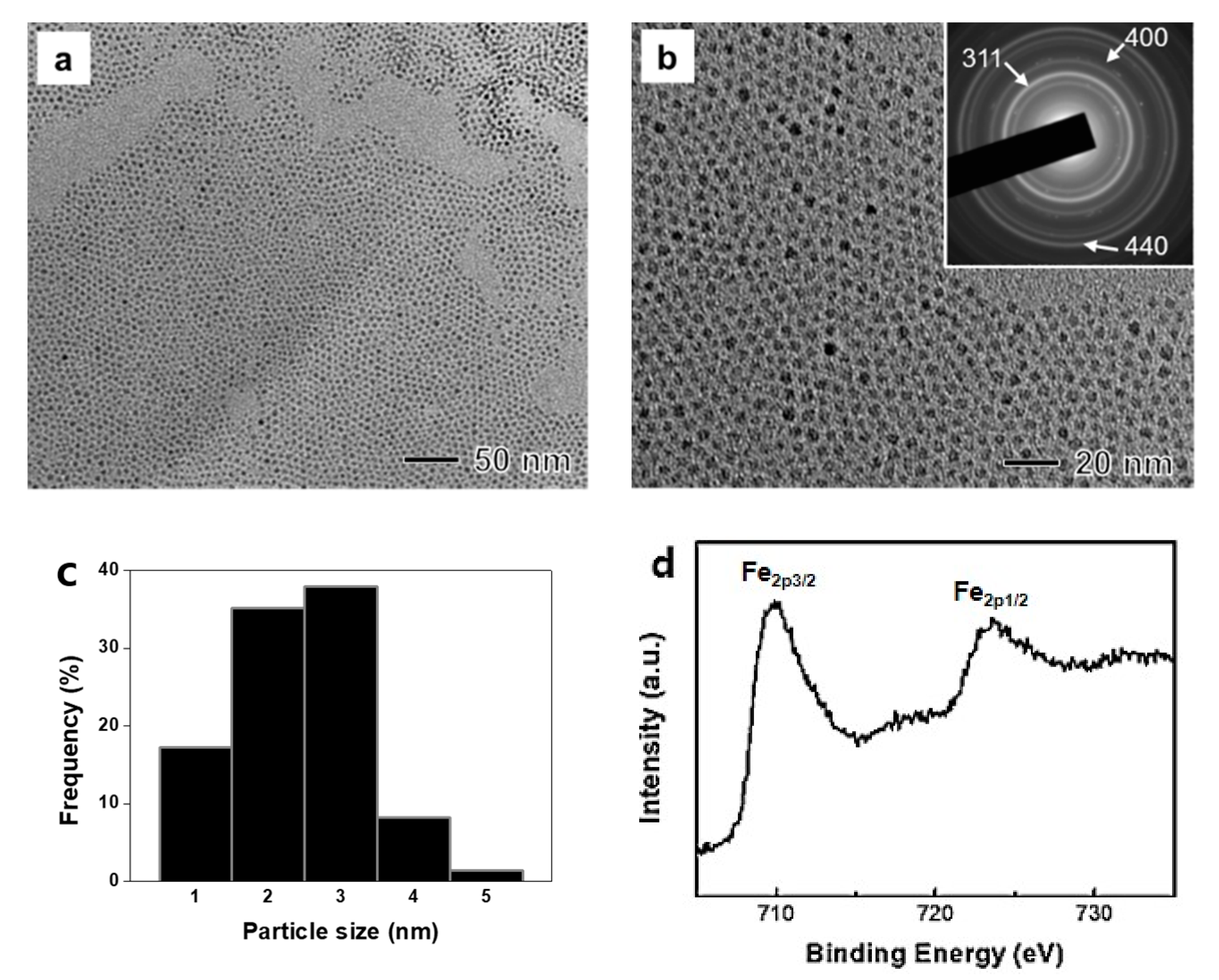
3.1. Characterizaion of the Magnetite Nanoparticles
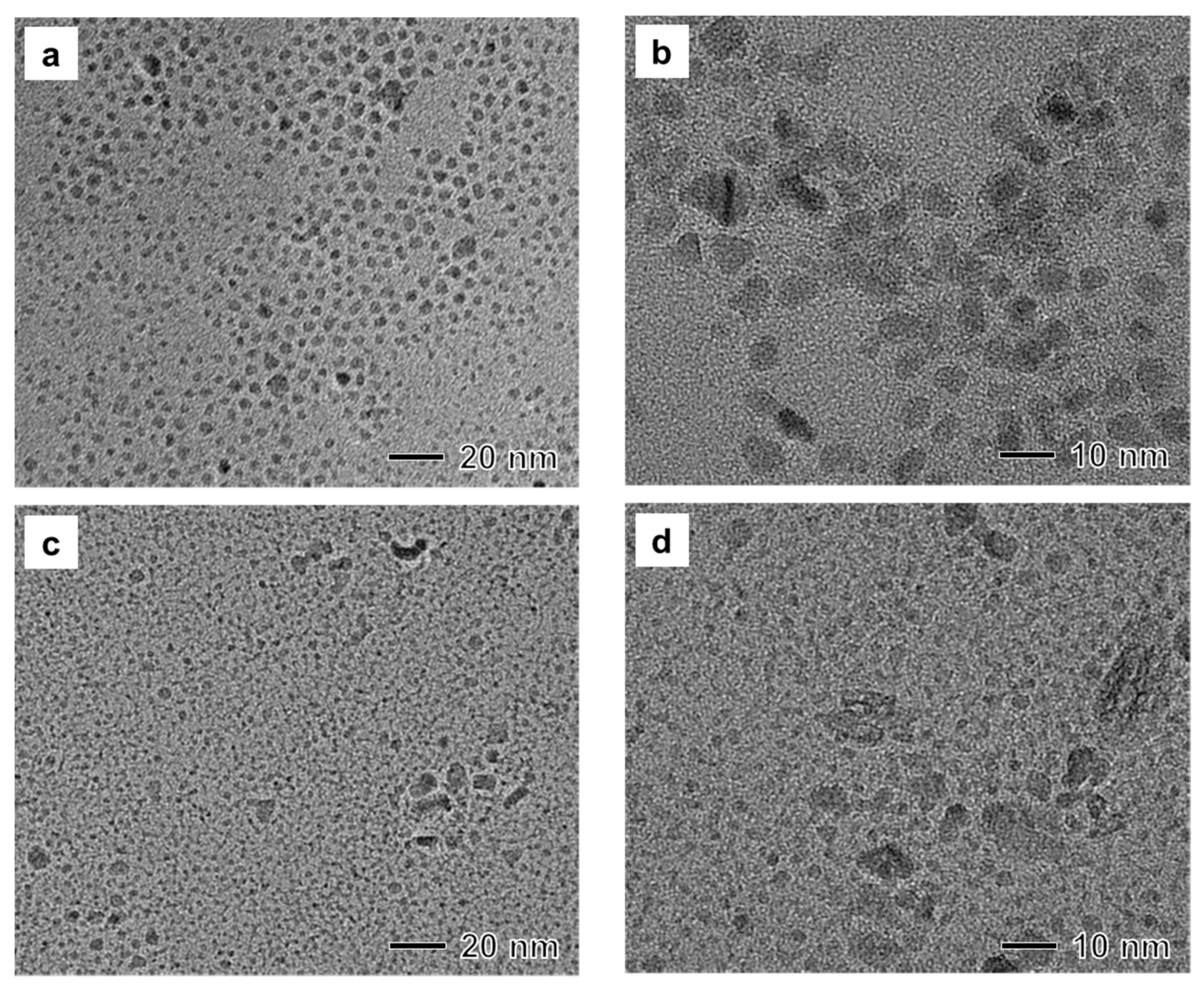
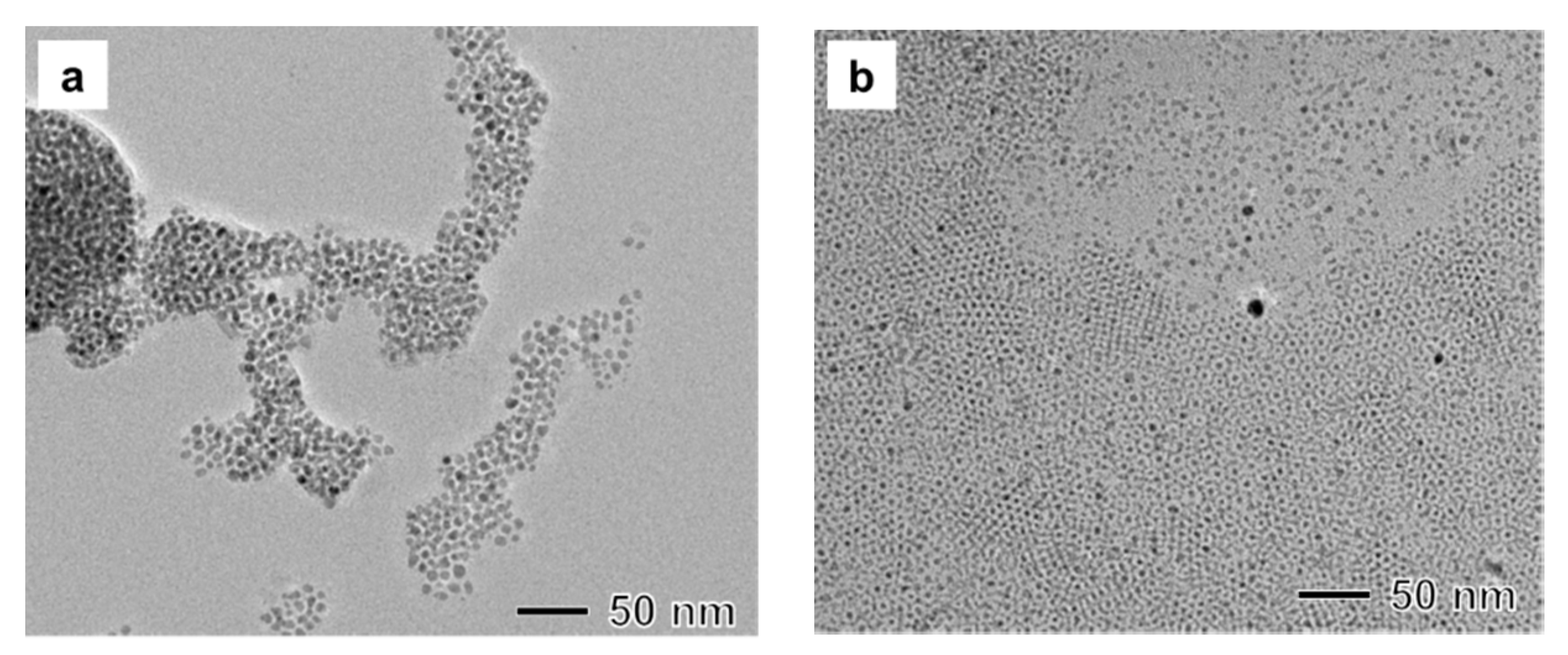
3.2. Study on the Role of Stabilizer
3.3. Influence of Precursor
3.4. Toxicity Study of the Synthesized Magnetite Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Riwotzki, K.; Meyssamy, H.; Schnablegger, H.; Kornowski, A.; Haase, M. Liquid-phase synthesis of colloids and redispersible powders of strongly luminescing LaPO4: Ce, Tb nanocrystals. Angew. Chem. Int. Ed. 2001, 40, 573–576. [Google Scholar] [CrossRef]
- Fominykh, K.; Feckl, J.M.; Sicklinger, J.; Döblinger, M.; Böcklein, S.; Ziegler, J.; Peter, L.; Rathousky, J.; Scheidt, E.-W.; Bein, T.; et al. Ultrasmall dispersible crystalline nickel oxide nanoparticles as high-performance catalysts for electrochemical water splitting. Adv. Funct. Mater. 2014, 24, 3123–3129. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Huang, W.; Tsung, C.K.; Zhang, Y.; Somorjai, G.A. Structure sensitivity of carbon-nitrogen ring opening: Impact of platinum particle size from below 1 to 5 nm upon pyrrole hydrogenation product selectivity over monodisperse platinum nanoparticles loaded onto mesoporous silica. J. Am. Chem. Soc. 2008, 130, 14026–14027. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: Account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Usov, N.A.; Serebryakova, O.N.; Tarasov, V.P. Interaction Effects in Assembly of Magnetic Nanoparticles. Nanoscale Res. Lett. 2017, 12, 489. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Hwang, N.M.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 2872–2877. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S. Nanoscale size effect of magnetic manocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yu, B.; Li, X.; Li, K. Facile solvothermal synthesis of monodisperse Fe3O4 nanocrystals with precise size control of one nanometre as potential MRI contrast agents. J. Mater. Chem. 2011, 21, 2476–2481. [Google Scholar] [CrossRef]
- Hu, F.; MacRenaris, K.W.; Waters, E.A.; Liang, T.; Schultz-Sikma, E.A.; Eckermann, A.L.; Meade, T.J. Ultrasmall, water-soluble magnetite nanoparticles with high relaxivity for magnetic resonance imaging. J. Phys. Chem. C 2009, 113, 20855–20860. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Brougham, D.F.; Fox, E.K.; Feliu, N.; Bushmelev, A.; Schmidt, A.; Mertens, N.; Kiessling, F.; Valldor, M.; et al. Water-soluble superparamagnetic magnetite nanoparticles with biocompatible coating for enhanced magnetic resonance imaging. ACS Nano 2011, 5, 6315–6324. [Google Scholar] [CrossRef]
- Pileni, M.P. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Mater. 2003, 2, 145–150. [Google Scholar] [CrossRef]
- Lin, J.; Lin, Y.; Liu, P.; Meziani, M.J.; Allard, L.F.; Sun, Y.P. Hot-fluid annealing for crystalline titanium dioxide nanoparticles in stable suspension. Am. Chem. Soc. 2002, 124, 11514–11518. [Google Scholar] [CrossRef]
- Tartaj, P.; Serna, C.J. Microemulsion-assisted synthesis of tunable superparamagnetic composites. Chem. Mater. 2002, 14, 4396–4402. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, J.; Liu, X.; Chan, H.S.O. Synthesis of Fe3O4 nanoparticles from emulsions. J. Mater. Chem. 2001, 11, 1704–1709. [Google Scholar] [CrossRef]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Ngo, A.T.; Pileni, M.P. Nanoparticles of cobalt ferrite: Influence of the applied field on the organization of the nanocrystals on a substrate and on their magnetic properties. Adv. Mater. 2000, 12, 276–279. [Google Scholar] [CrossRef]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Func. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wisniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Smolensky, E.D.; Park, H.-Y.E.; Zhou, Y.; Rolla, G.A.; Marjanska, M.; Botta, M.; Pierre, V.C. Scaling laws at the nanosize: The effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J. Mater. Chem. B 2013, 1, 2818–2828. [Google Scholar] [CrossRef]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic iron oxide nanoparticles with variable size and an iron oxidation state as prospective imaging agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Dong, B.; Cao, M.; Wei, B.; Hu, C. Hierarchical dendrite-like magnetic materials of Fe3O4, γ-Fe2O3, and Fe with high performance of microwave absorption. Chem. Mater. 2011, 23, 1587–1593. [Google Scholar] [CrossRef]
- Yu, T.; Moon, J.; Park, J.; Park, Y.I.; Na, H.B.; Kim, B.H.; Song, I.C.; Moon, W.K.; Hyeon, T. Various-shaped uniform Mn3O4 nanocrystals synthesized at low temperature in air atmosphere. Chem. Mater. 2009, 21, 2272–2279. [Google Scholar] [CrossRef]
- Shahzad, A.; Kim, W.S.; Yu, T. Synthesis, stabilization, growth behavior, and catalytic activity of highly concentrated silver nanoparticles using a multifunctional polymer in an aqueous-phase. RSC Adv. 2015, 5, 28652–28661. [Google Scholar] [CrossRef]
- Yu, N.; Cai, T.; Sun, Y.; Jiang, C.; Xiong, H.; Li, Y.; Peng, H. A novel antibacterial agent based on AgNPs and Fe3O4 loaded chitin microspheres with peroxidase-like activity for synergistic antibacterial activity and wound-healing. Int. J. Pharm. 2018, 552, 277–287. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, Y.; Li, W.S.; Su, Z.; Fadhil, J.I.; Zhang, C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials 2019, 225, 119515. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Roberts, E.R.; Stafford, A.R.; Banyard, K.L.; Matteucci, P.; Mace, K.A.; Hardman, M. Tissue Iron Promotes Wound Repair via M2 Macrophage Polarization and the Chemokine (C-C Motif) Ligands 17 and 22. Am. J. Pathol. 2019, 19, 30671–30676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Mao, F.; Wang, W.; Yang, Y.; Bai, Z. Sulfhydryl-modified Fe3O4@SiO2 Core/Shell nanocomposite: Synthesis and toxicity assessment in vitro. ACS Appl. Mater. Interfaces. 2015, 7, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Hanot, C.C.; Choi, Y.S.; Anani, T.B.; Soundarrajan, D.; David, A.E. Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells. Int. J. Mol. Sci. 2015, 17, 54. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Z.; Li, X.; Zhang, Y.; Yin, M.; Li, J.; Song, H.; Shi, J.; Ling, D.; Wang, L.; et al. Deciphering active biocompatibility of iron oxide nanoparticles from their intrinsic antagonism. Nano Res. 2018, 11, 2746–2755. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]


| Primer Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| RT-PCR | - | - |
| Human β-actin | GCA CTC TTC CAG CCT TCC TTC C | TCA CCT TCA CCG TTC CAG TTT TT |
| Human BAX | GTG CAC CAA GGT GCC GGA AC | TCA GCC CAT CTT CTT CCA GA |
| Human BCL2 | TGT GGC TGC ACT TGC TCT AA | CGA TGG CCA TAG ACC CTG TC |
| qRT-PCR | - | - |
| Human β-actin | CAC CCT GAA GTA CCC CAT CG | TGC CAG ATT TTC TCC ATG TCG |
| Human BAX | GCA ACT TCA ACT GGG GCC GGG | GAT CCA GCC CAA CAG CCG CTC |
| Human BCL2 | CTT GAC AGA GGA TCA TGC TGT AC | GGA TGC TTT ATT TCA TGA GGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.; Kim, S.-W.; Cho, A.; Kim, Y.-J.; Jeong, G.-J.; Kim, J.; Bhang, S.H.; Yu, T. Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application. Materials 2019, 12, 3850. https://doi.org/10.3390/ma12233850
Jung E, Kim S-W, Cho A, Kim Y-J, Jeong G-J, Kim J, Bhang SH, Yu T. Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application. Materials. 2019; 12(23):3850. https://doi.org/10.3390/ma12233850
Chicago/Turabian StyleJung, Euiyoung, Sung-Won Kim, Ahyoung Cho, Yu-Jin Kim, Gun-Jae Jeong, Jinheung Kim, Suk Ho Bhang, and Taekyung Yu. 2019. "Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application" Materials 12, no. 23: 3850. https://doi.org/10.3390/ma12233850
APA StyleJung, E., Kim, S.-W., Cho, A., Kim, Y.-J., Jeong, G.-J., Kim, J., Bhang, S. H., & Yu, T. (2019). Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application. Materials, 12(23), 3850. https://doi.org/10.3390/ma12233850








