Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cocktail Preparation
2.2. Ham Slices
2.3. High Pressure Processing (HPP) Treatment
2.4. Preparation and Application of Na-Alginate Edible Films
2.5. Microbiological Analysis
2.6. Isolation of Listeria Cells and Strain Differentiation
2.7. pH and Color Determination
2.8. Sensory Evaluation
2.9. FTIR Analysis
2.9.1. Data Analysis and Partial Least Squares (PLS) Modeling
2.9.2. Evaluation of Model Performance
2.10. Statistical Analysis
3. Results
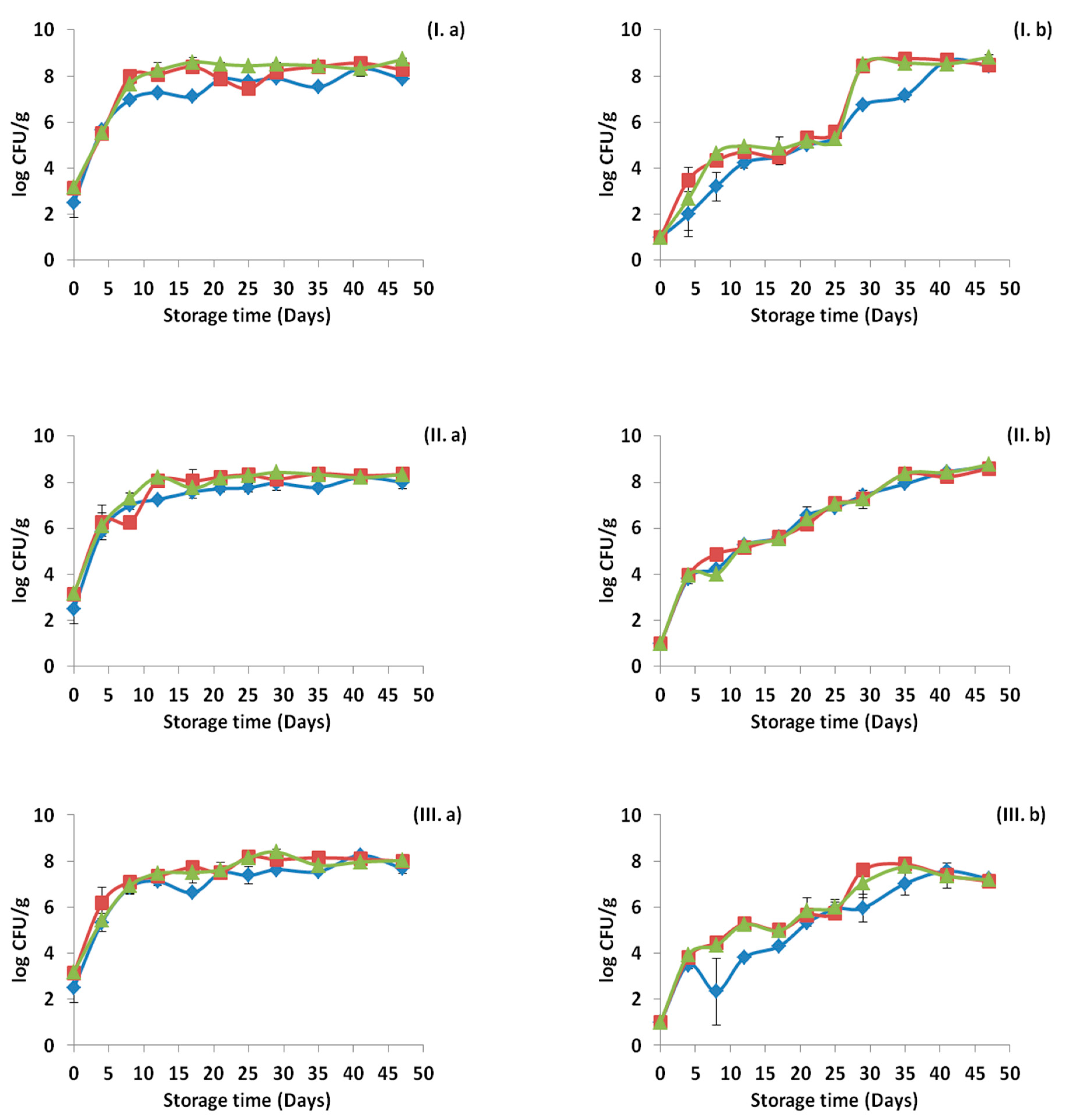
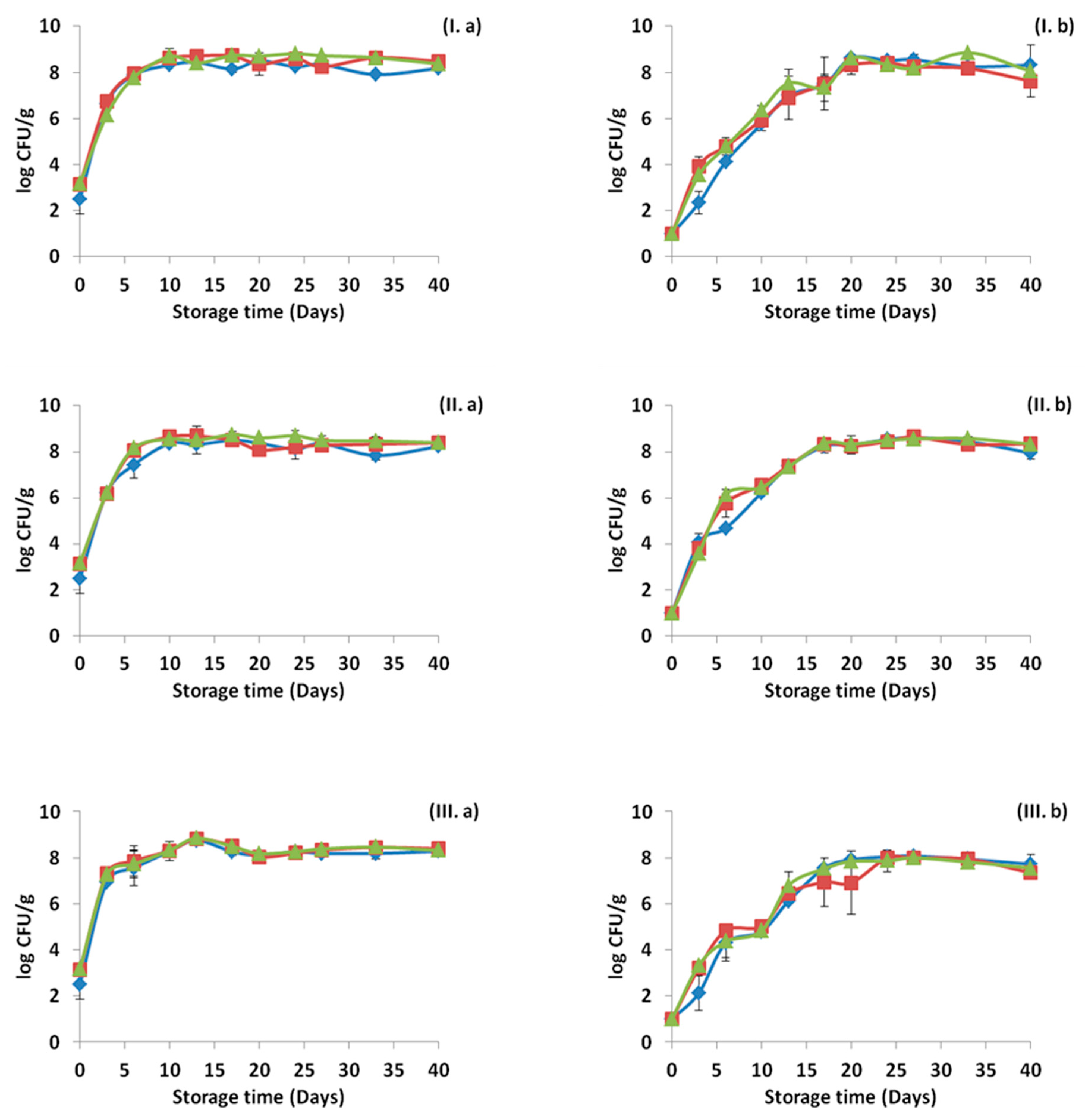
3.1. Microbiological Results
3.2. pH and Color Measurements
3.3. Sensory Assessment
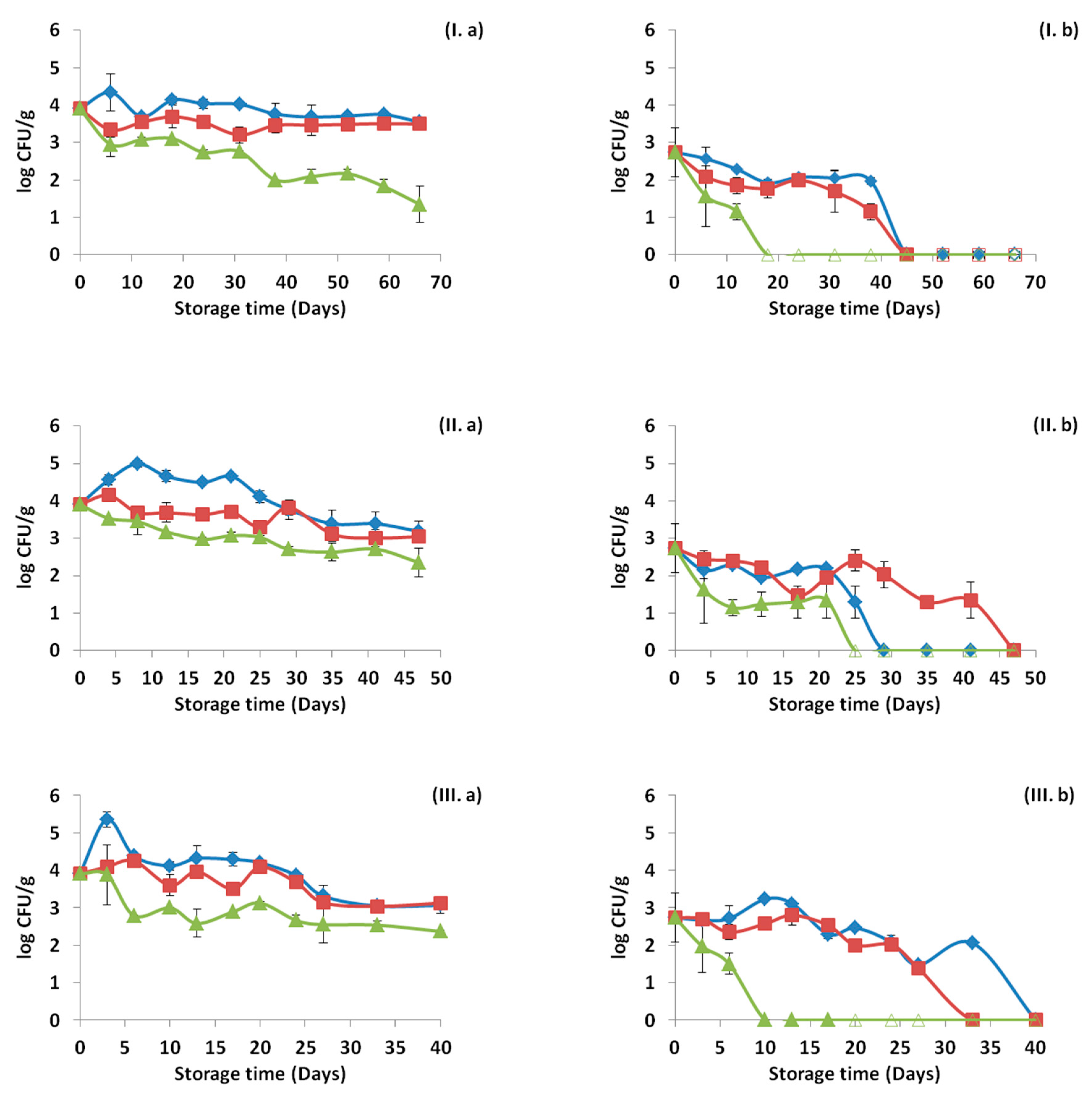
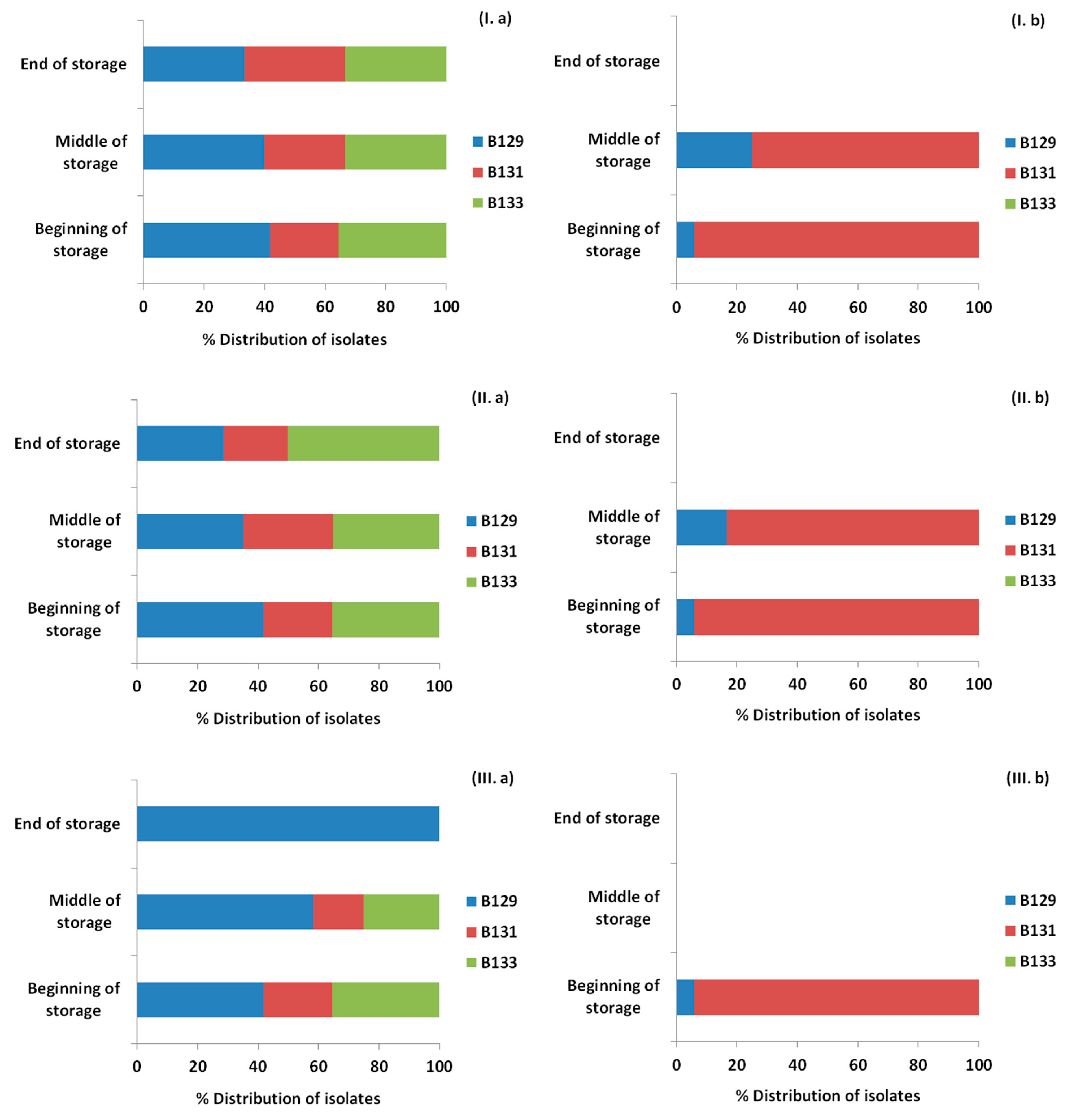
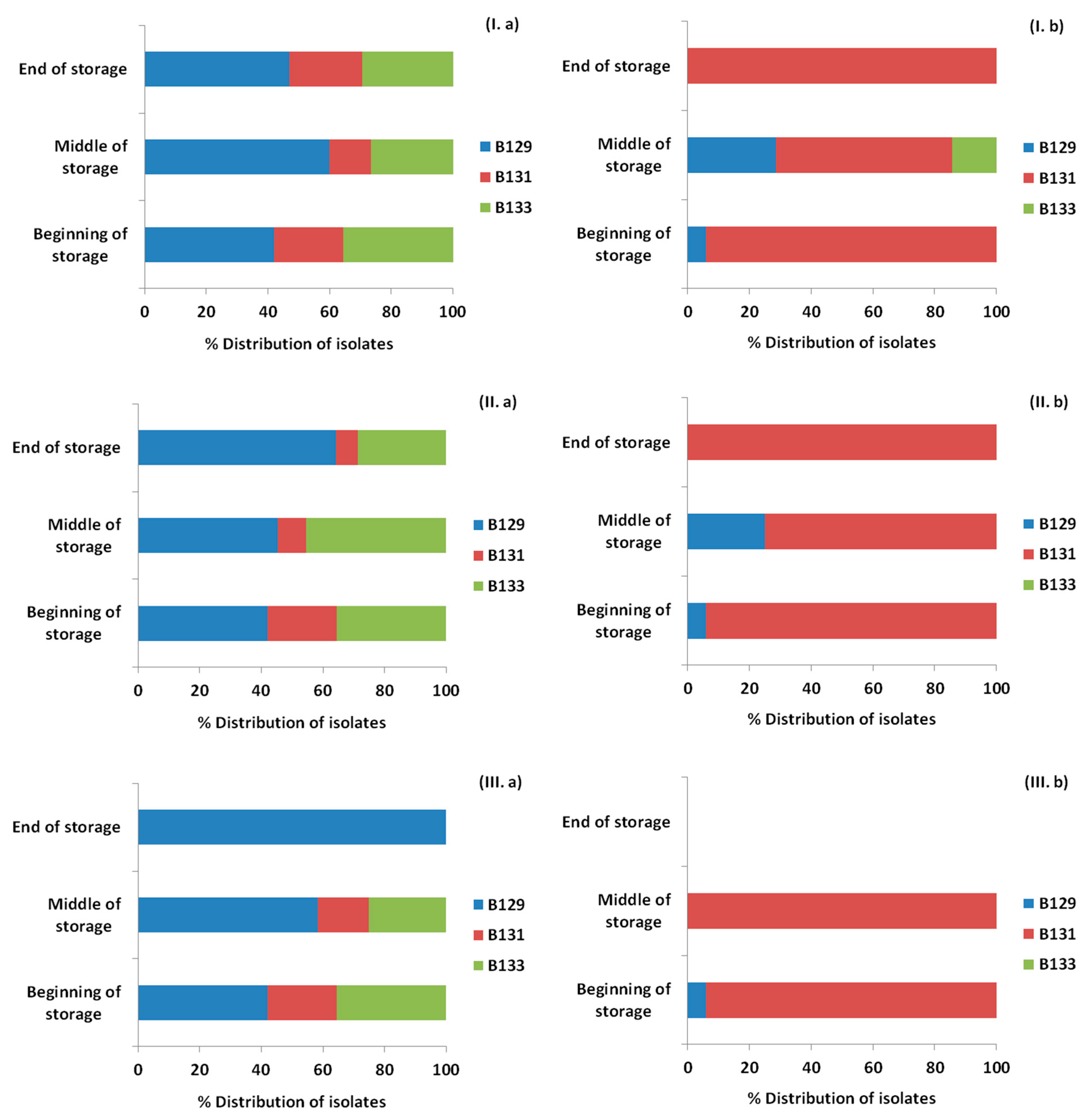
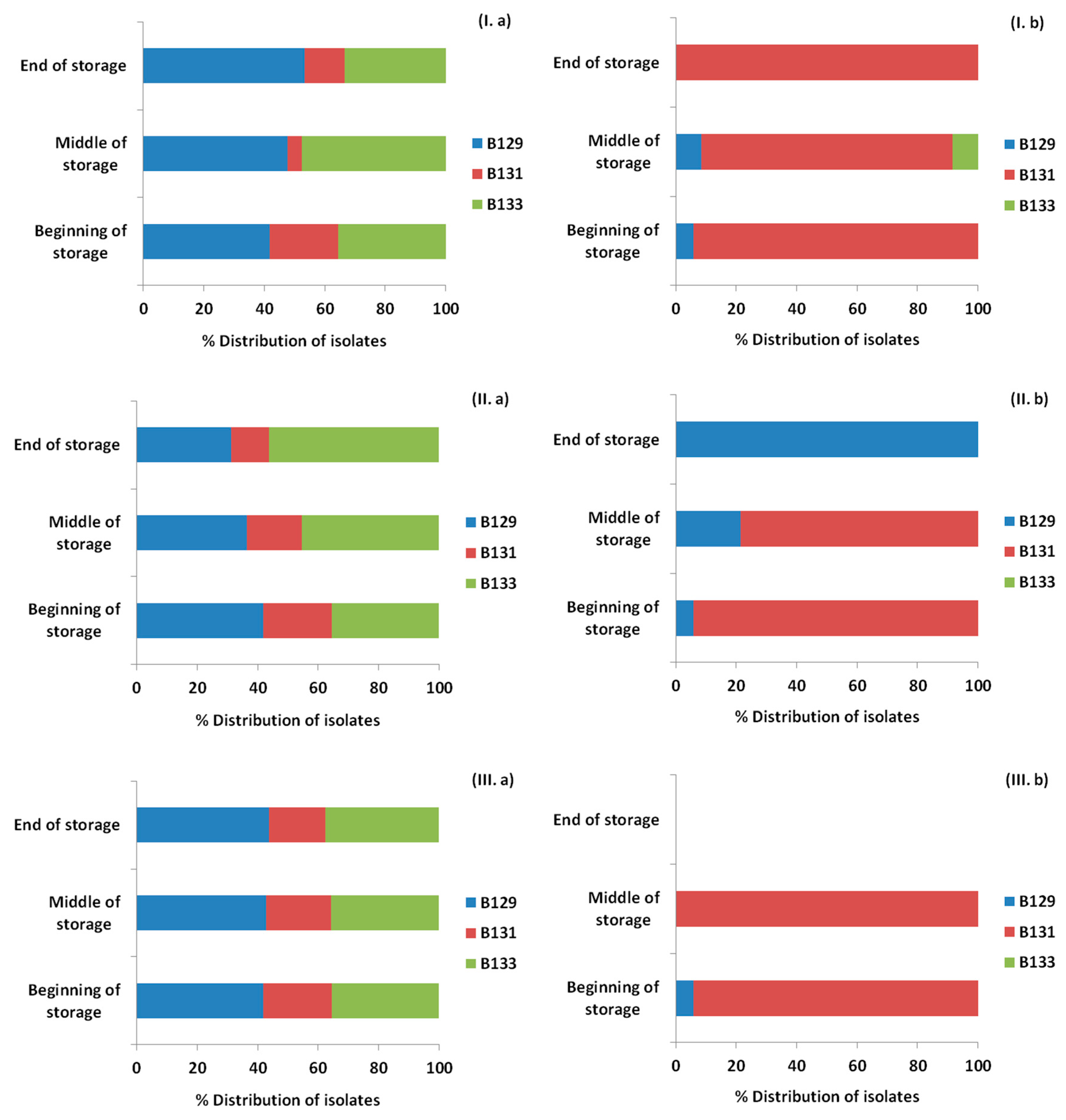
3.4. Monitoring Survival and Strain Differentiation of Listeria Monocytogenes
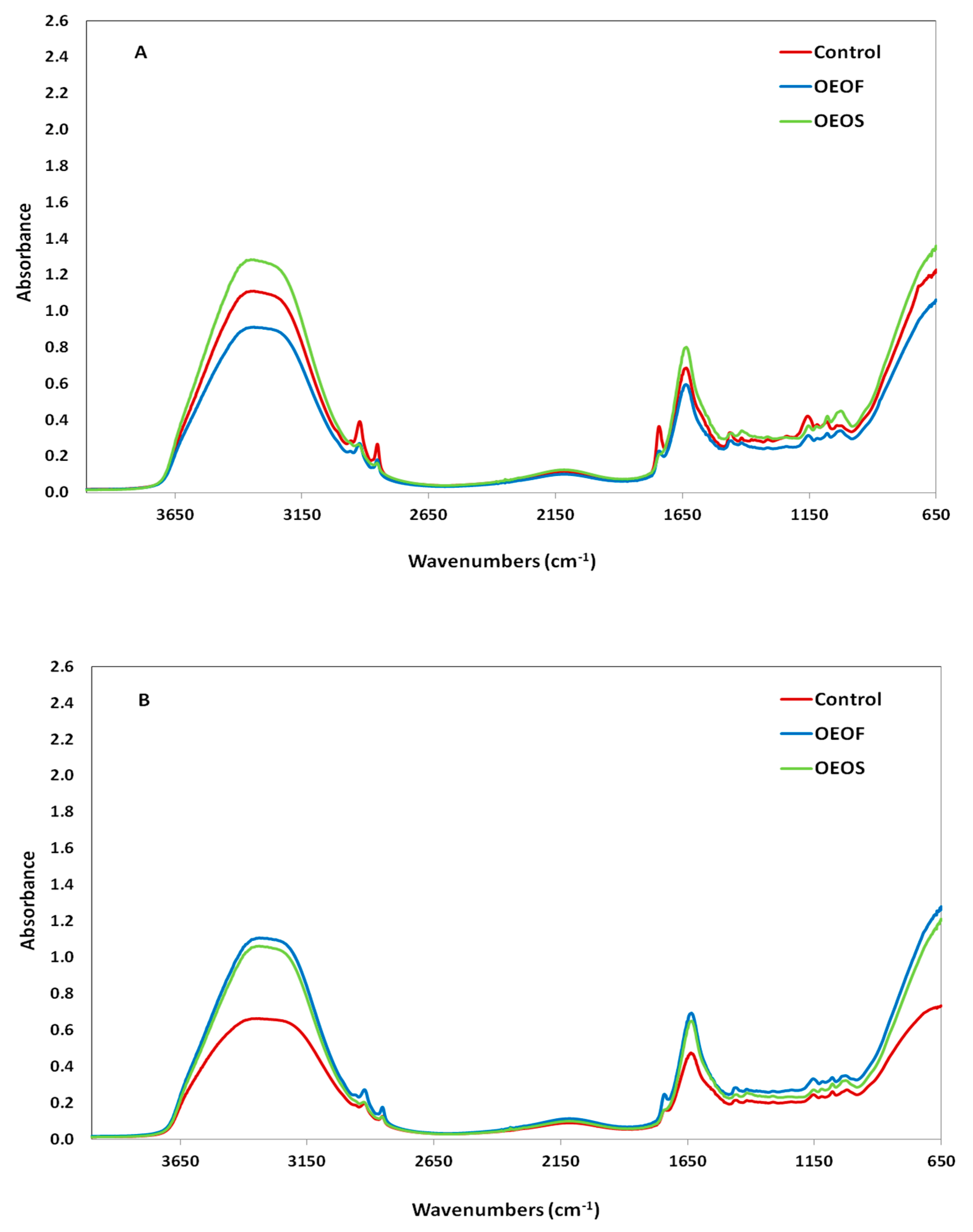
3.5. FTIR Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Dussault, D.; Dang Vu, K.; Lacroix, M. Developing of a model describing the inhibitory effect of selected preservatives on the growth of Listeria monocytogenes in a meat model system. Food Microbiol. 2016, 53, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zilelidou, E.; Manthou, E.; Skandamis, P. Growth differences and competition between Listeria monocytogenes strains determine their predominance on ham slices and lead to bias during selective enrichment with the ISO protocol. Int. J. Food Microbiol. 2016, 235, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Y.; Gui, M.; Zheng, H.; Dai, R.; Li, P. Combined effect of high hydrostatic pressure and enterocin LM-2 on the refrigerated shelf-life of ready-to-eat sliced vacuum-packed cooked ham. Food Control 2012, 24, 64–71. [Google Scholar] [CrossRef]
- Ramaroson, M.; Guillou, S.; Rossero, A.; Reze, S.; Anthoine, V.; Moriceau, N.; Martin, J.-L.; Duranton, F.; Zagorec, M. Selection procedure of bioprotective cultures for their combined use with high pressure processing to control spore-forming bacteria in cooked ham. Int. J. Food Microbiol. 2018, 276, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Blanco, G.; Delgado-Adámez, J.; Martín, M.J.; Ramírez, R. Active packaging using an olive leaf extract and high pressure processing for the preservation of sliced-dry cured shoulders from Iberian pigs. Innov. Food Sci. Emerg. Technol. 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Monteiro, M.J.P.; Gomes, A.; Pintado, M. Impact of whey protein coating incorporated with Bifidobacterium and Lactobacillus on sliced ham properties. Meat Sci. 2018, 139, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22. [Google Scholar] [CrossRef]
- Bak, K.H.; Lindahl, G.; Karlsson, A.H.; Lloret, E.; Ferrini, G.; Arnau, J.; Orlien, V. High pressure effect on the color of minced cured restructured ham at different levels of drying, pH, and NaCl. Meat Sci. 2012, 90, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Garriga, M.; Monfort, J.M. New mild technologies in meat processing: High pressure as a model technology. Meat Sci. 2002, 62, 359–371. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Culioli, J. Effects of high pressure on meat: A review. Meat Sci. 1997, 46, 211–236. [Google Scholar] [CrossRef]
- Torres, J.A.; Velazquez, G. Commercial opportunities and research challenges in the high pressure processing of foods. J. Food Eng. 2005, 67, 95–112. [Google Scholar] [CrossRef]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New insights into the high pressure processing of meat and meat products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- McMillin, K.W. Advancements in meat packaging. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, L 135/3, 9.
- Kapetanakou, A.E.; Skandamis, P.N. Applications of active packaging for increasing microbial stability in foods: Natural volatile antimicrobial compounds. Curr. Opin. Food Sci. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Ramos de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Karyotis, D.; Skandamis, P.N. Control of Listeria monocytogenes by applying ethanol-based antimicrobial edible films on ham slices and microwave-reheated frankfurters. Food Microbiol. 2016, 54, 80–90. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Coma, V. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar] [CrossRef] [PubMed]
- De Azeredo, H.M.C. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Atares, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Skandamis, P.; Tsigarida, E.; Nychas, G.-J.E. The effect of oregano essential oil on survival/death of Salmonella typhimurium in meat stored at 5 °C under aerobic, VP/MAP conditions. Food Microbiol. 2002, 19, 97–103. [Google Scholar] [CrossRef]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems-A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oil against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Dimitrijević, S.I.; Mihajlovski, K.R.; Antonović, D.G.; Milanović-Stevanović, M.R.; Mijin, D.Ž. A study of the synergistic antilisterial effects of a sub-lethal dose of lactic acid and essential oils from Thymus vulgaris L., Rosmarinus officinalis, L. and Origanum vulgare, L. Food Chem. 2007, 104, 774–782. [Google Scholar] [CrossRef]
- .Menezes, N.M.C.; Martins, W.F.; Longhi, D.A.; de Aragão, G.M.F. Modelling the effect of oregano essential oil on shelf-life extension of vacuum-packed cooked sliced ham. Meat Sci. 2018, 139, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tsigarida, E.; Skandamis, P.; Nychas, G.-J.E. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation and antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paparella, A.; Mazzarrino, G.; Chavez-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of alginate-based films containing essential oils on Listeria monocytogenes and Salmonella typhimurium present in bologna and ham. J. Food Protect. 2007, 70, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Pattanayaiying, R.; H-Kittikun, A.; Cutter, C.N. Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, P.; Fiszman, S.M. Hydrocolloids in fried foods. A review. Food Hydrocoll. 2011, 25, 1801–1812. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C.; Manitsa, C.; Mallidis, C. Modelling the effect of high pressure on the inactivation kinetics of a pressure-resistant strain of Pediococcus damnosus in phosphate buffer and gilt-head seabream (Sparus aurata). J. Appl. Microbiol. 2007, 102, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Panagou, E.Z.; Samaras, F.J.; Galiatsatou, P.; Mallidis, C.G. Temperature-assisted high hydrostatic pressure inactivation of Staphylococcus aureus in a ham model system: Evaluation in selective and nonselective medium. J. Appl. Microbiol. 2008, 104, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes. Part 1: Detection Method; ISO Standard 11290-1:1996 and Amd.1:2004; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Kagkli, D.M.; Iliopoulos, V.; Stergiou, V.; Lazaridou, A.; Nychas, G.-J.E. Differential Listeria monocytogenes strain survival and growth in Katiki, a traditional Greek soft cheese, at different storage temperatures. Appl. Environ. Microbiol. 2009, 75, 3621–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavli, F.; Kovaiou, I.; Apostolakopoulou, G.; Kapetanakou, A.; Skandamis, P.; Nychas, G.-J.E.; Tassou, C.; Chorianopoulos, N. Alginate-Based edible films delivering probiotic bacteria to sliced ham pretreated with high pressure processing. Int. J. Mol. Sci. 2017, 18, 1867. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Jeremiah, L.E. The storage life of non-muscle offals packaged under vacuum or carbon dioxide. Food Microbiol. 1991, 8, 339–353. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Nychas, G.-J.E.; Tassou, C.; Chorianopoulos, N. Use of Fourier transform infrared spectroscopy for monitoring the shelf life of ham slices packed with probiotic supplemented edible films after treatment with high pressure processing. Food Res. Int. 2018, 106, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar] [PubMed]
- Oscar, T.P. Predictive model for survival and growth of Salmonella typhimurium DT104 on chicken skin during temperature abuse. J. Food Protect. 2009, 72, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Validation of lag time and growth rate models for Salmonella Typhimurium: Acceptable prediction zone method. J. Food Sci. 2005, 70, 129–137. [Google Scholar] [CrossRef]
- Moreirinha, C.; Nunes, A.; Barros, A.; Almeida, A.; Delgadillo, I. Evaluation of the potential of mid-infrared spectroscopy to assess the microbiological quality of ham. J. Food Saf. 2015, 35, 270–275. [Google Scholar] [CrossRef]
- Böcker, U.; Ofstad, R.; Wu, Z.; Bertram, H.C.; Sockalingum, G.D.; Manfait, M.; Egelandsdal, B.; Kohler, A. Revealing covariance structures in Fourier transform infrared and Raman microspectroscopy spectra: A study on pork muscle fiber tissue subjected to different processing parameters. Appl. Spectrosc. 2007, 61, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Ammor, S.A.; Argyri, A.; Nychas, G.J.E. Rapid monitoring of the spoilage of minced beef stored under conventionally and active packaging conditions using Fourier transform infrared spectroscopy in tandem with chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.T.; Zumalacarregui, J.M.; Alaiz-Rodriguez, R.; Gusman-Martinez, R.; Englesen, S.B.; Mateo, J. Differentiation of perirenal and omental quality of suckling lamps according to the rearing system from Fourier transforms mid-infrared spectra using partial least squares and artificial neural networks analysis. Meat Sci. 2009, 83, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jofré, A.; Aymerich, T.; Grèbol, N.; Garriga, M. Efficiency of high hydrostatic pressure at 600 MPa against food-borne microorganisms by challenge tests on convenience meat products. LWT Food Sci. Technol. 2009, 42, 924–928. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Xu, X.; Sun, X.; Xu, B.; Zhou, G. Effect of high pressure treatment on microbial populations of sliced vacuum-packed cooked ham. Meat Sci. 2011, 88, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, A.; Vanoirbeek, K.G.A.; Lurquin, I.; Steen, L.; Goemaere, O.; Szczepaniak, S.; Paelinck, H.; Hendrickx, M.E.G.; Michiels, C.W. Shelf-Life extension of cooked ham model product by high hydrostatic pressure and natural preservatives. Innov. Food Sci. Emerg. Technol. 2011, 12, 407–415. [Google Scholar] [CrossRef]
- Myers, K.; Montoya, D.; Cannon, J.; Dickson, J.; Sebranek, J. The effect of high hydrostatic pressure, sodium nitrite, and salt concentration of the growth of Listeria monocytogenes on RTE ham and turkey. Meat Sci. 2013, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Belletti, N.; Garriga, M.; Aymerich, T.; Bover-Cid, S. Inactivation of Serratia liquefaciens on dry-cured ham by high pressure processing. Food Microbiol. 2013, 35, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Hereu, A.; Dalgaard, P.; Garriga, M.; Aymerich, T.; Bover-Cid, S. Analysing and modelling the growth behavior of Listeria monocytogenes on RTE cooked meat products after a high pressure treatment at 400 MPa. Int. J. Food Microbiol. 2014, 186, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, Z.; Gaudette, N.J.; Johnston, S.P. The use of high pressure processing to enhance the quality and shelf life of reduced sodium naturally cured restructured cooked hams. Meat Sci. 2016, 116, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Pingen, S.; Sudhaus, N.; Becker, A.; Krischek, C.; Klein, G. High pressure as an alternative processing step for ham production. Meat Sci. 2016, 118, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Possas, A.; Rincón, F.; García-Gimeno, R.M.; Martínez, B. Model for Listeria monocytogenes inactivation by high hydrostatic pressure processing in Spanish chorizo sausage. Food Microbiol. 2018, 69, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Stollewerk, K.; Jofré, A.; Comaposada, J.; Arnau, J.; Garriga, M. The effect of NaCl-free processing and high pressure on the fate of Listeria monocytogenes and Salmonella on sliced smoked dry-cured ham. Meat Sci. 2012, 90, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Mizuno, Y.; Yamamoto, K. Predictive modelling of the recovery of Listeria monocytogenes on sliced cooked ham after high pressure processing. Int. J. Food Microbiol. 2007, 119, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Belletti, N.; Garriga, M.; Aymerich, T. Model for Listeria monocytogenes inactivation on dry-cured ham by high hydrostatic pressure processing. Food Microbiol. 2011, 28, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Jofrè, A.; Aymerich, T.; Monfort, J.M.; Garriga, M. Combined effect of natural antimicrobials and high pressure processing to prevent Listeria monocytogenes growth after a cold chain break during storage of cooked ham. Food Control 2008, 19, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jofré, A.; Garriga, M.; Aymerich, T. Inhibition of Salmonella sp., Listeria monocytogenes and Staphylococcus aureus in cooked ham by combining antimicrobials, high hydrostatic pressure and refrigeration. Meat Sci. 2008, 78, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Delgado-Pando, G.; Linton, M.; Patterson, M.F.; Koidis, A. Synergism between high-pressure processing and active packaging against Listeria monocytogenes in ready-to-eat chicken breast. Innov. Food Sci. Emerg. Technol. 2014, 27, 41–47. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Repková, L.; Gänzle, M.G.; McMullen, L.N. Effect of pressure, reconstituted RTE meat microbiota, and antimicrobials on survival and post-pressure growth of Listeria monocytogenes on ham. Front. Microbiol. 2018, 9, 1979. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.L.; Yang, S.Y.; Song, K.B. Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar]
- Ravishankar, S.; Jaroni, D.; Zhu, L.; Olsen, C.; McHugh, T.; Friedman, M. Inactivation of Listeria monocytogenes on ham and bologna using pectin-based apple, carrot, and hibiscus edible films containing carvacrol and cinnamaldehyde. J. Food Sci. 2012, 77, M377–M382. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-Y.; Jang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Chai, M.; Li, L.; Cao, J.-X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, O.; Panagou, E.Z.; Tassou, C.C.; Nychas, G.J.E. Contribution of Fourier transform infrared (FTIR) spectroscopy data on the quantitative determination of minced pork meat spoilage. Food Res. Int. 2011, 44, 3264–3271. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Mohareb, F.R.; Argyri, A.A.; Bessa, C.M.; Nychas, G.J.E. A comparison of artificial neural networks and partial least squares modeling for the rapid detection of the microbial spoilage of beef fillets based on Fourier transform infrared spectral fingerprints. Food Microbiol. 2011, 28, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyri, A.A.; Jarvis, R.M.; Wedge, D.; Xu, Y.; Panagou, E.Z.; Goodacre, R.; Nychas, G.-J.E. A comparison of Raman and FT-IR spectroscopy for the prediction of meat spoilage. Food Control. 2013, 29, 461–470. [Google Scholar] [CrossRef]
- Estelles-Lopez, L.; Ropodi, A.; Pavlidis, D.; Fotopoulou, J.; Gkousari, C.; Peyrodie, A.; Panagou, E.; Nychas, G.-J.E.; Mohareb, F. An automated ranking platform for machine learning regression models for meat spoilage prediction using multi-spectral imaging and metabolic profiling. Food Res. Int. 2017, 99, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.-J.E. Rapid detection of frozen-then-thawed minced beef using multispectral imaging and Fourier transform infrared spectroscopy. Meat Sci. 2018, 135, 142–147. [Google Scholar] [CrossRef] [PubMed]




| Performance Indice | Without HPP Treatment | With HPP Treatment | ||||
|---|---|---|---|---|---|---|
| LAB | Cocci | TVC | LAB | Cocci | TVC | |
| Prediction error (PE, %) | 66.38 | 74.29 | 77.40 | 30.72 | 37.57 | 47.54 |
| Root mean square error (RMSE) | 0.730 | 0.624 | 0.560 | 1.548 | 1.735 | 1.452 |
| Bf | 0.973 | 0.987 | 0.988 | 0.942 | 0.961 | 1.001 |
| Af | 1.082 | 1.066 | 1.059 | 1.305 | 1.311 | 1.235 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials 2019, 12, 3726. https://doi.org/10.3390/ma12223726
Pavli F, Argyri AA, Skandamis P, Nychas G-J, Tassou C, Chorianopoulos N. Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials. 2019; 12(22):3726. https://doi.org/10.3390/ma12223726
Chicago/Turabian StylePavli, Foteini, Anthoula A. Argyri, Panagiotis Skandamis, George-John Nychas, Chrysoula Tassou, and Nikos Chorianopoulos. 2019. "Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing" Materials 12, no. 22: 3726. https://doi.org/10.3390/ma12223726
APA StylePavli, F., Argyri, A. A., Skandamis, P., Nychas, G.-J., Tassou, C., & Chorianopoulos, N. (2019). Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials, 12(22), 3726. https://doi.org/10.3390/ma12223726







