Effect of Heat Treatment on the Phase Composition and Corrosion Resistance of 321 SS Welded Joints Produced by a Defocused Laser Beam
Abstract
1. Introduction
2. Experimental
2.1. Laser Welding Process and Optical Microscopy
2.2. Heat Treatment
2.3. Intergranular Corrosion
2.4. X-Ray Diffraction
2.5. SEM Microstructure and Chemical Composition
3. Results and Discussion
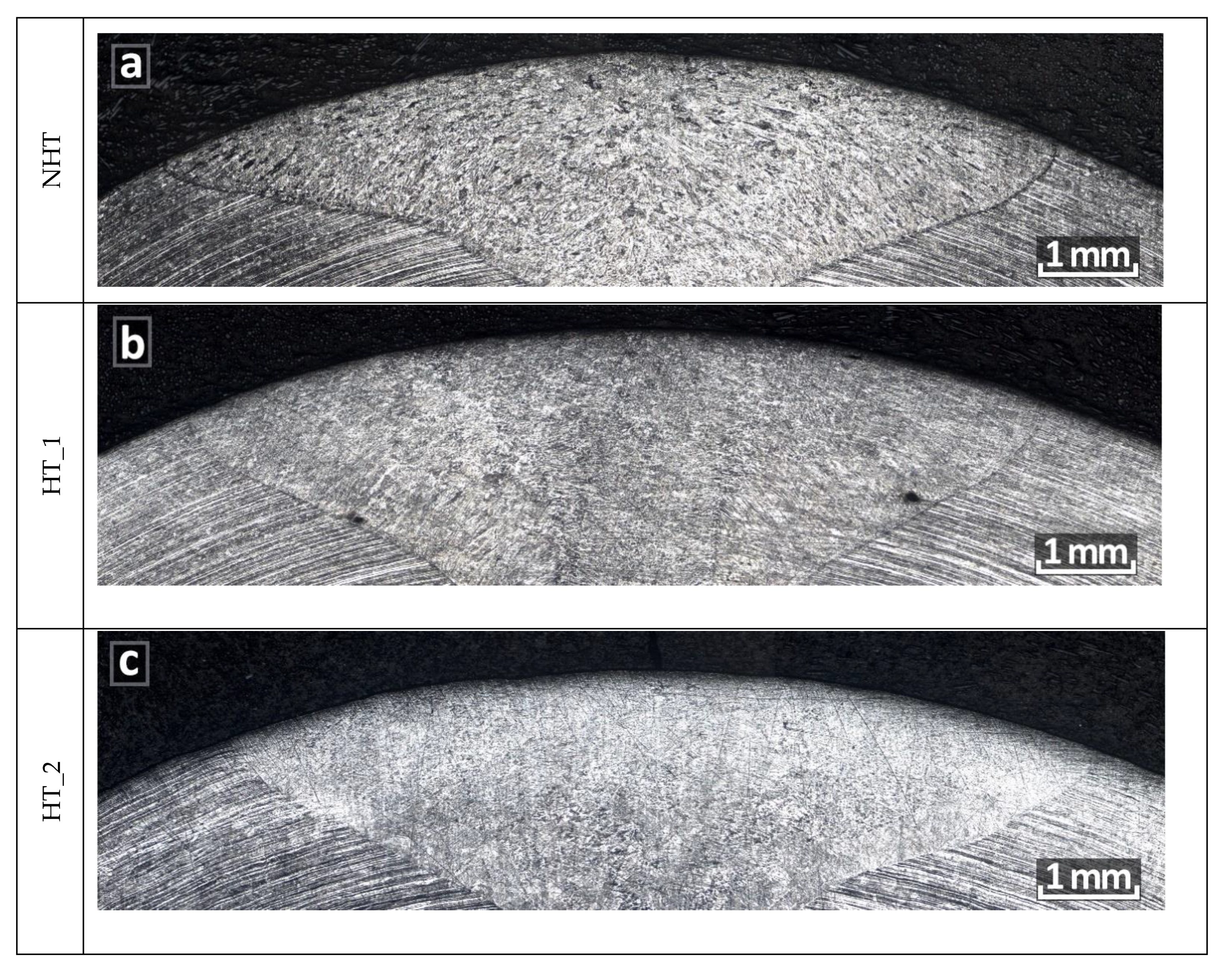
3.1. Microstructure after Welding
3.2. Microstructure after Heat Treatment
3.3. SEM
3.3.1. Chemical Composition
3.3.2. SEM Microstructure
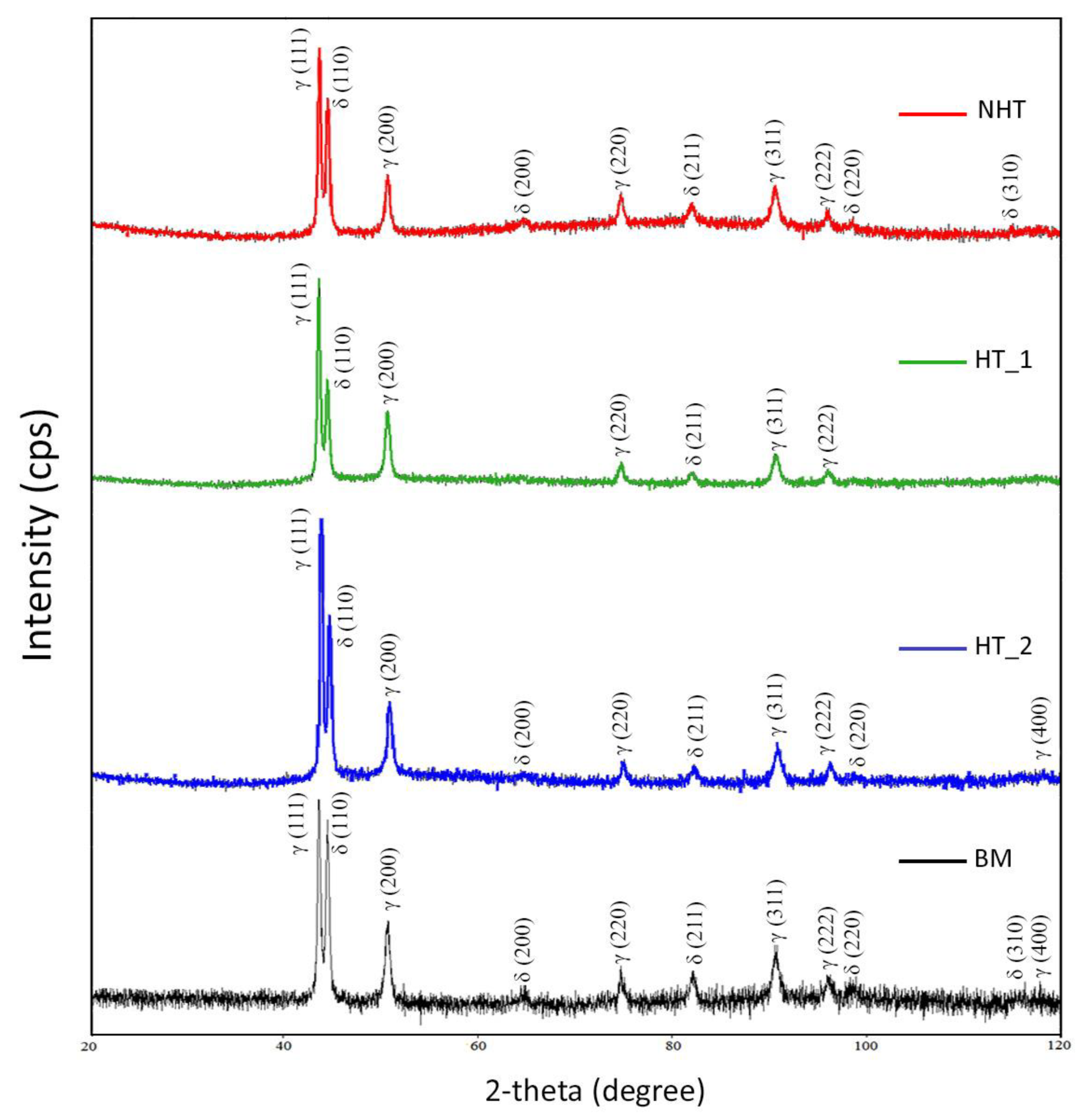
3.4. X-Ray Diffraction
3.4.1. Phase Composition
3.4.2. Residual Stress Measurement
3.5. Intergranular Corrosion Resistance
4. Conclusions
Funding
Conflicts of Interest
References
- Hand book corrosion Metals Handbook. In Metals Handbook, 9th ed.; American Society for Metals: Cleveland, OH, USA, 1987; Volume 13.
- Seitovirta, M. Hand Book of Stainless Steel; Outokumpu Oyj: Avesta, Sweden, 2013. [Google Scholar]
- Verma, J.; Taiwade, R.V. Effect of welding processes and conditions on the microstructure, mechanical properties and corrosion resistance of duplex stainless steel weldments—A review. J. Manuf. Process. 2017, 25, 134–152. [Google Scholar] [CrossRef]
- Handbook Welding, Brazing and Soldering; ASM International: Cleveland, OH, USA, 1993; Volume 6.
- Lippold, J.; Kotecki, D. Welding Metallurgy and Weldability of Stainless Steel; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Westin, E.M.; Stelling, K.; Gumenyuk, A. Single-pass laser-GMAW hybrid welding of 13.5 mm thick duplex stainless steel. Weld. World 2011, 55, 39–49. [Google Scholar] [CrossRef]
- Kuryntsev, S.V.; Morushkin, A.E.; Shigapov, A.I. Investigation of the structure and properties of steel 30khgsa after volume laser-beam treatment. Metal Sci. Heat Treat. 2019, 60, 802–807. [Google Scholar] [CrossRef]
- Molian, P.A. Solidification behavior of laser welded stainless steel. J. Mater. Sci. Lett. 1985, 4, 281–283. [Google Scholar] [CrossRef]
- Yazdian, N.; Mohammadpour, M.; Razavi, R.; Kovacevic, R. Hybrid laser/arc welding of 304L stainless steel tubes, part 2–Effect of filler wires on microstructure and corrosion behavior. Int. J. Press. Vessel. Pip. 2018, 163, 45–54. [Google Scholar] [CrossRef]
- Sabooni, S.; Karimzadeh, F.; Enayati, M.H.; Ngan, A.H.W.; Jabbari, H. Gas tungsten arc welding and friction stir welding of ultrafine grained AISI 304L stainless steel: Microstructural and mechanical behavior characterization. Mater. Charact. 2015, 109, 138–151. [Google Scholar] [CrossRef]
- Shorshorov, M.K.; Belov, V.V. Atlas. In Phase Transformations and Changes of Steel Properties During Welding; Nauka Publishing house: Moscow, Russia, 1972. [Google Scholar]
- Karhu, M.; Kujanpaa, V.; Lippold, J.C. Hot Cracking Phenomena in Welds III; Springer-Verlag: Berlin, Germany, 2011. [Google Scholar]
- Lippold, J.C. Welding Metallurgy and Weldability; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kadoi, K.; Fujinaga, A.; Yamamoto, M.; Shinozaki, K. The effect of welding conditions on solidification cracking susceptibility of type 310S stainless steel during laser welding using an in-situ observation technique. Weld. World 2013, 57, 383–390. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Gou, G.; Tian, H.; Chen, Y. Modeling of corrosion fatigue life of stainless steel joints welded by laser-metal active gas hybrid welding. Corrosion 2018, 74, 1132–1140. [Google Scholar] [CrossRef]
- Lee, C.; Lee, Y.; Lee, C.; Hong, S. Possibility of Mn substitution of Ni through evaluation of mechanical properties and corrosion resistance in superaustenitic stainless steel weld metal. Mater. Sci. Eng. A 2018, 733, 16–23. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Sun, Y.; Jiang, Y.; Li, J. A Study on the pitting initiation of duplex stainless steel (DSS 2205) welded joints using SEM–EDS, SKPFM and electrochemistry methods. Int. J. Electrochem. Sci. 2018, 13, 11607–11619. [Google Scholar] [CrossRef]
- Qi, K.; Li, R.; Wang, G.; Li, G.; Liu, B.; Wu, M. Microstructure and Corrosion Properties of Laser-Welded SAF 2507 Super Duplex Stainless Steel Joints. J. Mater. Eng. Perform. 2019, 28, 287–295. [Google Scholar] [CrossRef]
- Lee, C.; Roh, S.; Lee, C.; Hong, S. Influence of Si on sigma phase precipitation and pitting corrosion in superaustenitic stainless steel weld metal. Mater. Chem. Phys. 2018, 207, 91–97. [Google Scholar] [CrossRef]
- Kuryntsev, S.; Gilmutdinov, A.K. Welding of stainless steel using defocused laser beam. J. Constr. Steel Res. 2015, 114, 305–313. [Google Scholar] [CrossRef]
- De Pablos-Martin, A.; Benndorf, G.; Tismer, S.; Mittang, M. Laser-welded fused silica substrates using a luminescent fresnoite-based sealant. Opt. Laser Technol. 2016, 80, 176–185. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Shah, L.H.; Gerlich, A.P. Dissimilar laser welding of an AA6022-AZ31 lap-joint by using Ni-interlayer: Novel beam-wobbling technique, processing parameters, and metallurgical characterization. Opt. Laser Technol. 2019, 112, 349–362. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Chen, Y.; Zhu, X.; Ji, S. Numerical Simulation Analysis of Dual-Beam Laser Welding of Tailored Blanks with Different Thicknesses. Metals 2019, 9, 135. [Google Scholar] [CrossRef]
- Mann, V.; Hofmann, K.; Schaumberger, K.; Weigert, T.; Schuster, S.; Hafenecker, J.; Hübner, S.; Lipinski, L.; Roth, S.; Schmidt, M. Influence of oscillation frequency and focal diameter on weld pool geometry and temperature field in laser beam welding of high strength steels. Procedia CIRP 2018, 74, 470–474. [Google Scholar] [CrossRef]
- Schaumberger, K.; Mann, V.; Mödl, M.; Hofmann, K.; Stein, S.; Roth, S.; Schmidt, M. Influence of focal spot diameter and beam oscillation on the process efficiency of laser beam welding utilizing a direct diode laser. Procedia CIRP 2018, 74, 466–469. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, J.; Zhao, J.; Zhang, Z. Effect of Defocusing Amount on Hot Cracking in Laser Welding of 316 Stainless Steel. At. Energy Sci. Technol. 2018, 52, 210–214. [Google Scholar]
- Kumar, N.; Sahoo, P.; Bandyopadhyay, A. Effect of process parameters on bead geometry, microstructure and hardness of a pulsed Nd: YAG laser welded AISI 304 stainless steel sheet. Lasers Eng. 2018, 40, 65–93. [Google Scholar]
- Sun, J.; Feng, K.; Zhang, K.; Guo, B.; Li, Z. Fiber laser welding of thick AISI 304 plate in a horizontal (2G) butt joint configuration. Mater. Des. 2017, 118, 53–65. [Google Scholar] [CrossRef]
- Grünenwald, S.; Unt, A.; Poutiainen, I.; Sokolov, M. High power fiber laser welding of single sided T-joint on shipbuilding steel with different processing setups. Appl. Sci. 2017, 7, 1276–1288. [Google Scholar]
- Garcia, C.; De Tiedra, M.P.; Blanco, Y.; Martin, O.; Martin, F. Intergranular corrosion of welded joints of austenitic stainless steels studied by using an electrochemical minicell. Corros. Sci. 2008, 50, 2390–2397. [Google Scholar] [CrossRef]
- Mori, G.; Prohaska, M.; Lackner, R.; Rokosz, K.; Albu, M.; Kothleitner, G. Depletion zones and intergranular corrosion of superaustenitic stainless steel Alloy 926. Mater. Corros. 2017, 68, 284–294. [Google Scholar] [CrossRef]
- Otero Huerta, E. Materials Corrosion and Degradation; Editorial Síntesis: Madrid, Spain, 1997. [Google Scholar]
- Kwok, C.T.; Fong, S.L.; Cheng, F.T.; Man, H.C. Pitting and galvanic corrosion behavior of laser welded stainless steels. J. Mater. Process. Technol. 2006, 176, 168–178. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.H.; Chan, W.K. Stress corrosion cracking of laser-welded stainless steels. In Proceedings of the 1st International Joint Symposium on Joining and Welding, Osaka, Japan, 6–8 November 2013; pp. 547–552. [Google Scholar]
- Gennari, C.; Lago, M.; Bögre, B.; Meszaros, I.; Calliari, I.; Pezzato, L. Microstructural and Corrosion Properties of Cold Rolled Laser Welded UNS S32750 Duplex Stainless Steel. Metals 2018, 8, 1074. [Google Scholar] [CrossRef]
- Sedriks, A.J. Corrosion of Stainless Steel, 2nd ed.; Corrosion Monograph Series; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Lu, B.T.; Chen, Z.K.; Luo, J.L.; Patchett, B.M.; Xu, Z.H. Pitting and stress corrosion cracking behavior in welded austenitic stainless steel. Electrochim. Acta 2005, 50, 1391–1403. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Cordovilla, F.; Tur, A.; García-Beltrán, Á.; Diaz, M.; Angulo, I.; Ocaña, J.L. Minimization of the Thermal Impact in the Laser Welding of Dissimilar Stainless Steels. Metals 2018, 8, 650. [Google Scholar] [CrossRef]
- Saadi, S.; Yi, Y.; Cho, P.; Jang, C.; Beeley, P. Passivity breakdown of 316L stainless steel during potentiodynamic polarization in NaCl solution. Corros. Sci. 2016, 111, 720–727. [Google Scholar] [CrossRef]
- Malik, A.U.; Siddioi, N.A.; Ahmad, S.; Andijani, I.N. The effect of dominant alloy additions on the corrosion behavior of some conventional and high alloy stainless steel in seawater. Corros. Sci. 1995, 37, 1521–1535. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Jiang, Z.; Zhang, B.; Li, Z.; Wu, J.; Fan, S.; Feng, H.; Zhu, H. Effects of Cr and Mo on precipitation behavior and associated intergranular corrosion susceptibility of superaustenitic stainless steel S32654. Mater. Charact. 2019, 152, 141–150. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Tan, H.; Hong, J.; Jiang, Y.; Jiang, L.; Li, J. Effect of a brief post-weld heat treatment on the microstructure evolution and pitting corrosion of laser beam welded UNS S31803 duplex stainless steel. Corros. Sci. 2012, 65, 472–480. [Google Scholar] [CrossRef]
- Cervo, R.; Ferro, P.; Tiziani, A.; Zucchi, F. Annealing temperature effects on super duplex stainless steel UNS S32750 welded joints, II: Pitting corrosion resistance evaluation. J. Mater. Sci. 2010, 45, 4378–4389. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Zhou, C. Effects of nitrogen in shielding gas on microstructure evolution and localized corrosion behavior of duplex stainless steel welding joint. Appl. Surf. Sci. 2017, 404, 110–128. [Google Scholar] [CrossRef]
- Lai, R.; Cai, Y.; Wu, Y.; Li, F.; Hua, X. Influence of absorbed nitrogen on microstructure and corrosion resistance of 2205 duplex stainless steel joint processed by fiber laser welding. J. Mater. Process. Technol. 2016, 231, 397–405. [Google Scholar] [CrossRef]
- Filho, D.F.; Reis, R.P.; Ferraresi, V.A. Effect of shielding gas composition on intergranular corrosion of stabilized ferritic stainless steel GMA welds. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 73. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Bobić, B.M.; Radojković, B.M.; Alić, B. Influence of the welding current intensity and nitrogen content on the corrosion resistance of austenitic stainless steels. Mater. Corros. 2018, 69, 1–12. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Bobić, B.M.; Radojković, B.M.; Alić, B. Influence of welding parameters on pit initiation and pit growth in welded joints of X5CrNi18-10 stainless steel. Weld. World 2019, 63, 167–179. [Google Scholar] [CrossRef]
- Ali, A.; Farzam, M.; Golozar, M.A.; Danaee, I. The effect of repeated repair welding on mechanical and corrosion properties of stainless steel 316L. Mater. Des. 2014, 54, 331–341. [Google Scholar]
- Sadeghi, B.; Sharifi, H.; Rafiei, M.; Tayebi, M. Effects of post weld heat treatment on residual stress and mechanical properties of GTAW: The case of joining A537CL1 pressure vessel steel and A321 austenitic stainless steel. Eng. Fail. Anal. 2018, 94, 396–406. [Google Scholar] [CrossRef]
- Kiyoku, R.; Kato, T.; Hirose, T.; Tanikawa, H.; Mori, H. Effect of heat-treatment conditions on toughness in laser beam welds of reduced activation ferritic/martensitic steel F82H. Q. J. Jpn. Weld. Soc. 2018, 36, 104–110. [Google Scholar] [CrossRef][Green Version]
- Kuryntsev, S.V.; Gilmutdinov, A.K. Heat treatment of welded joints of steel 0.3С–1Cr–1Si produced by high-power fiber lasers. Opt. Laser Technol. 2015, 74, 125–131. [Google Scholar] [CrossRef]
- Saravanan, S.; Raghukandan, K.; Sivagurumanikandan, N. Pulsed Nd: YAG laser welding and subsequent post-weld heat treatment on super duplex stainless steel. J. Manuf. Process. 2017, 25, 284–289. [Google Scholar] [CrossRef]
- Yi, G.; Xu, J.; Zheng, C. Semiconductor properties of passive film and corrosion resistance of 2205 duplex stainless steel joint welded by laser hybrid welding. Anti-Corros. Methods Mater. 2018, 66, 327–335. [Google Scholar] [CrossRef]
- Mankari, K.; Acharyya, S.G. Development of stress corrosion cracking resistant welds of 321 stainless steel by simple surface engineering. Appl. Surf. Sci. 2017, 426, 944–950. [Google Scholar] [CrossRef]
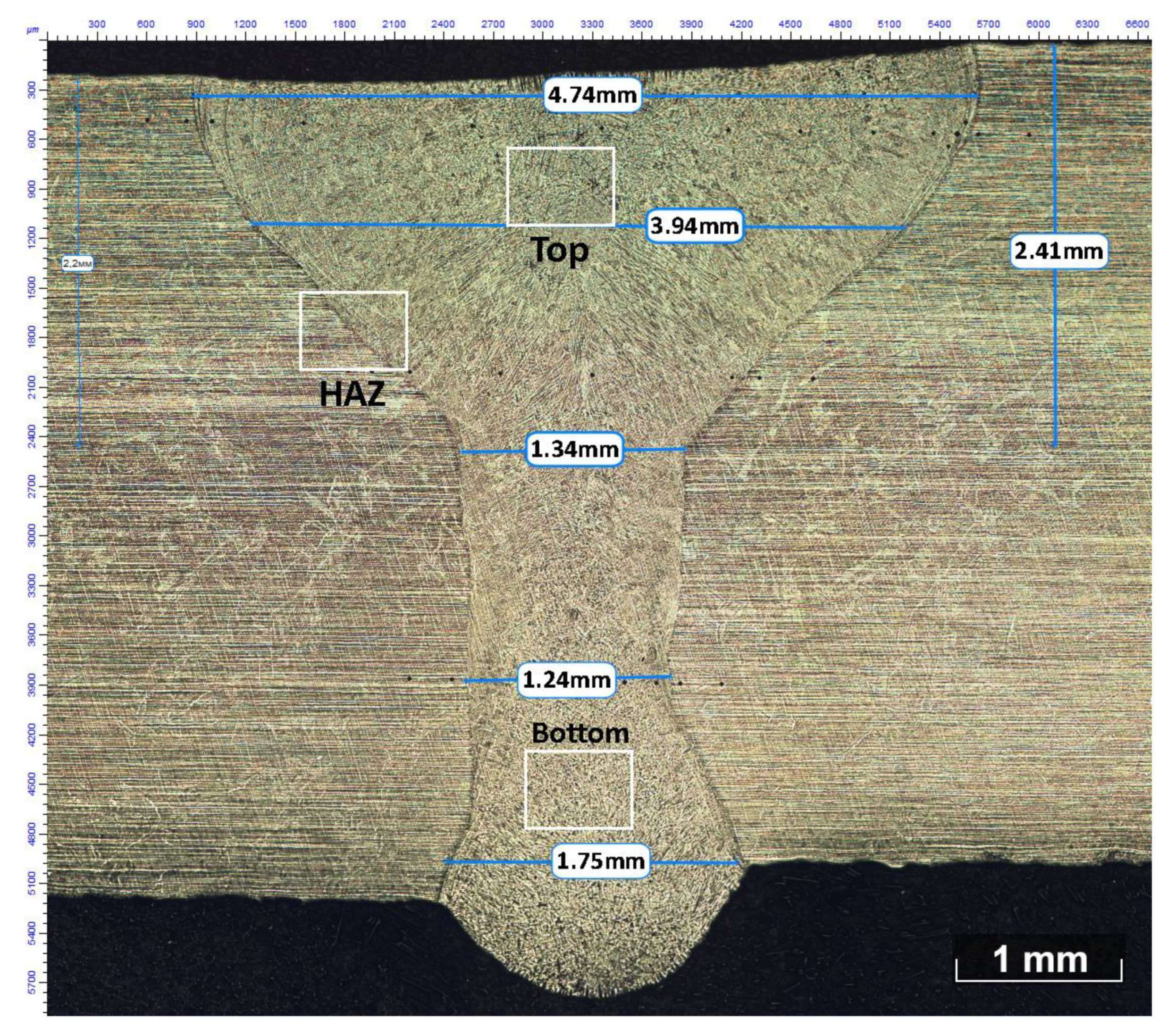
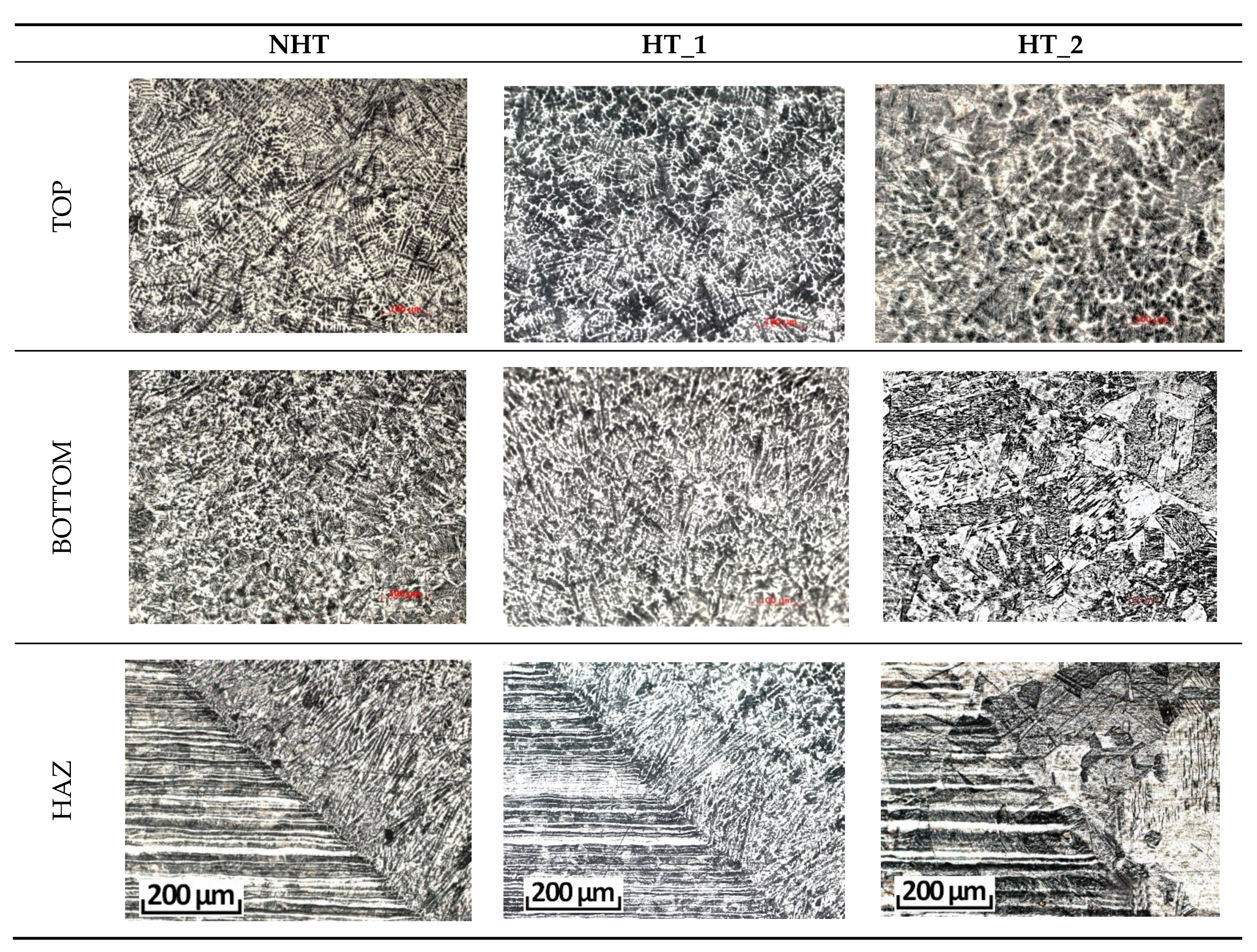
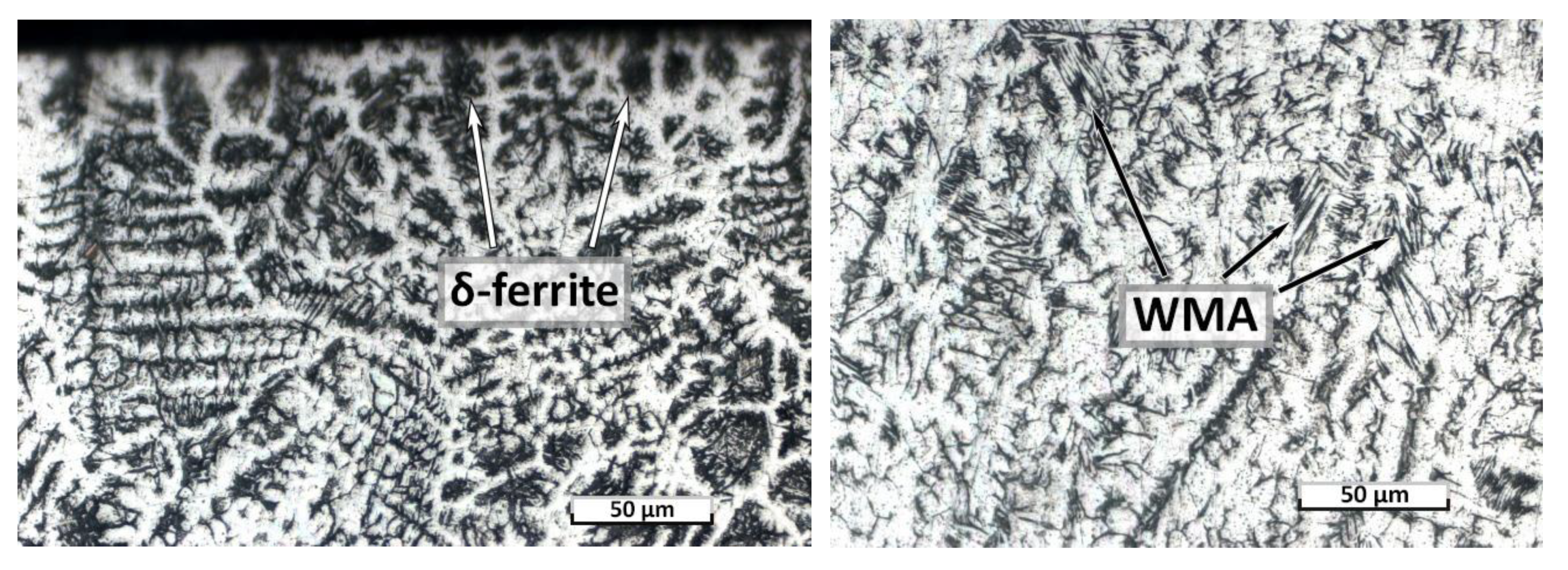
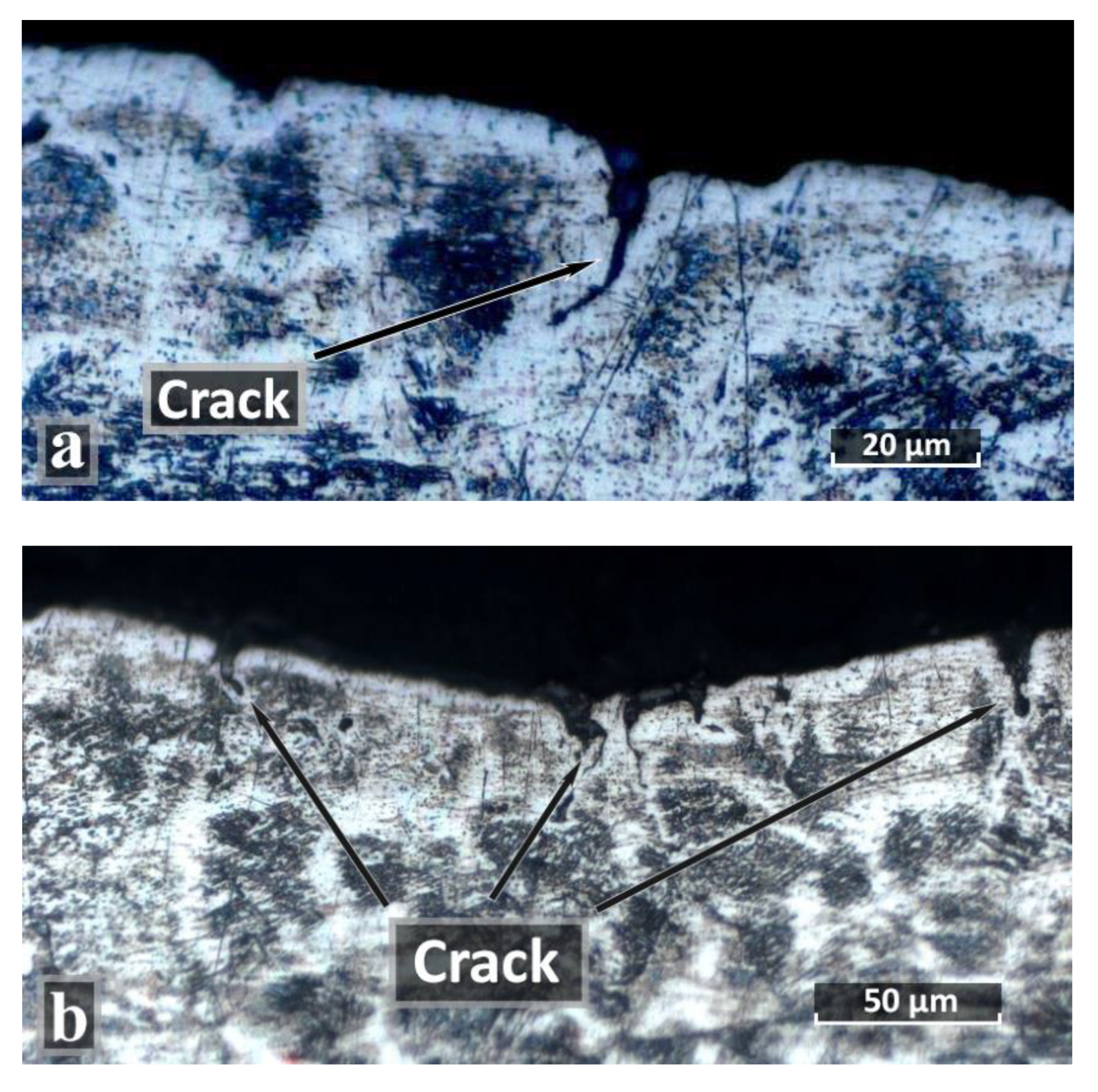
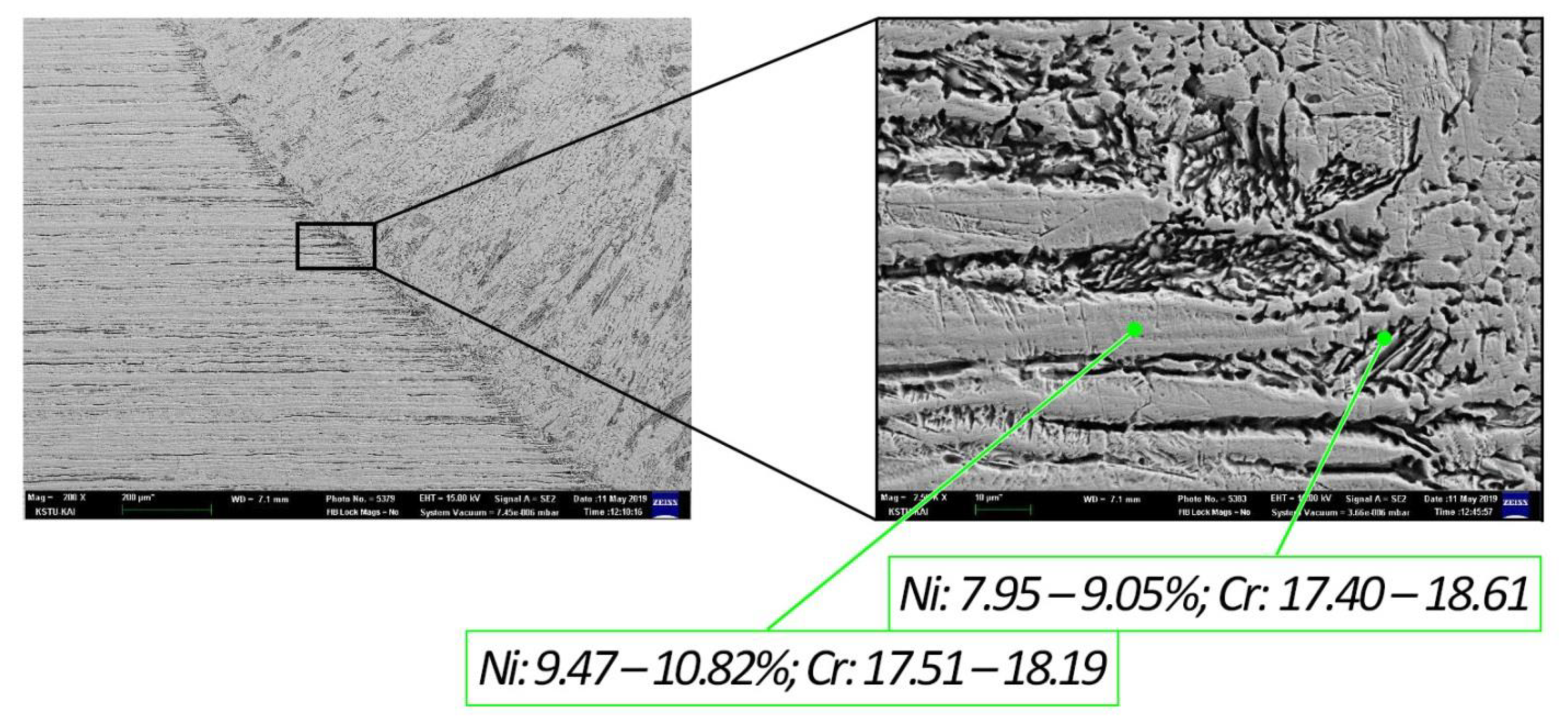
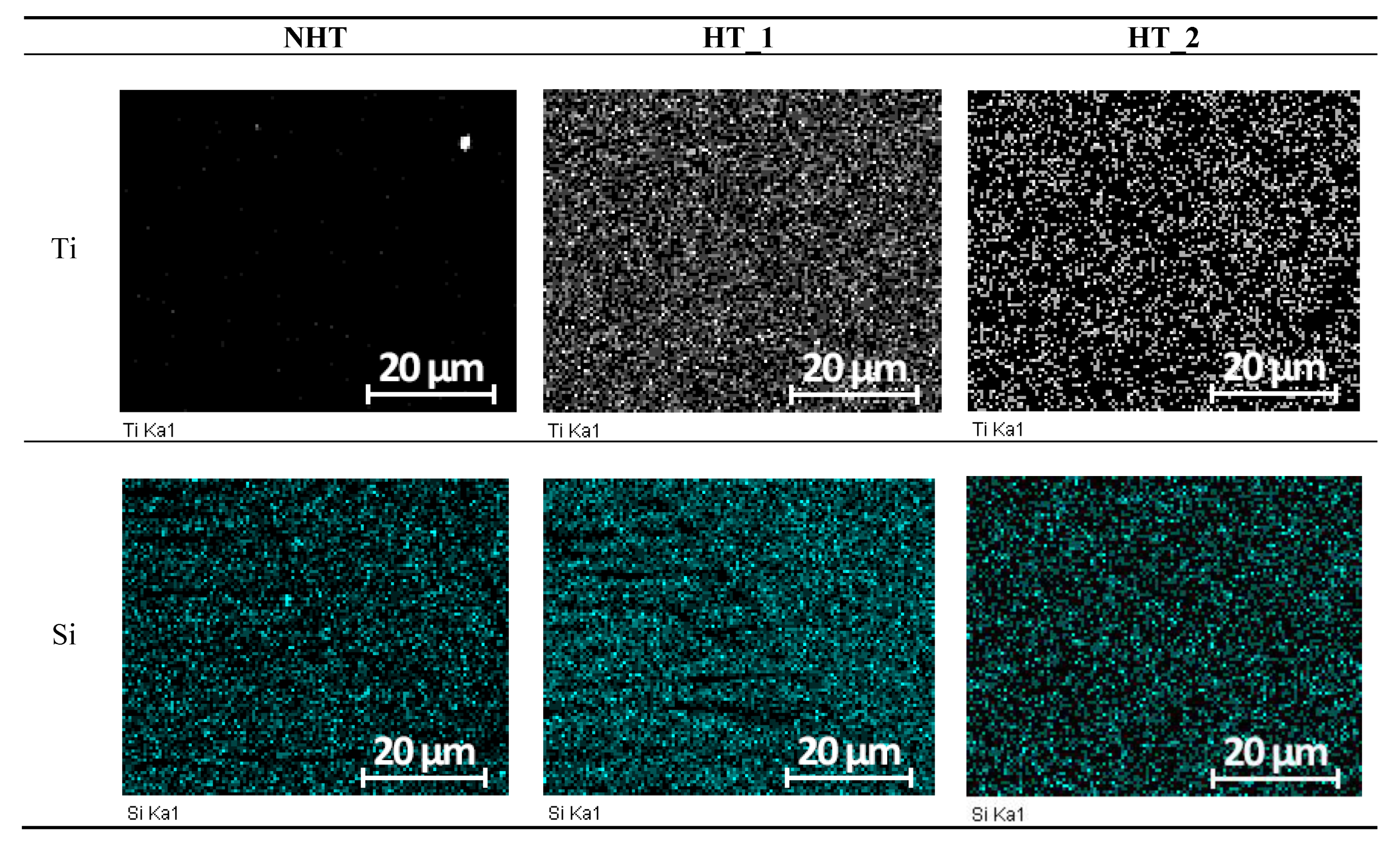
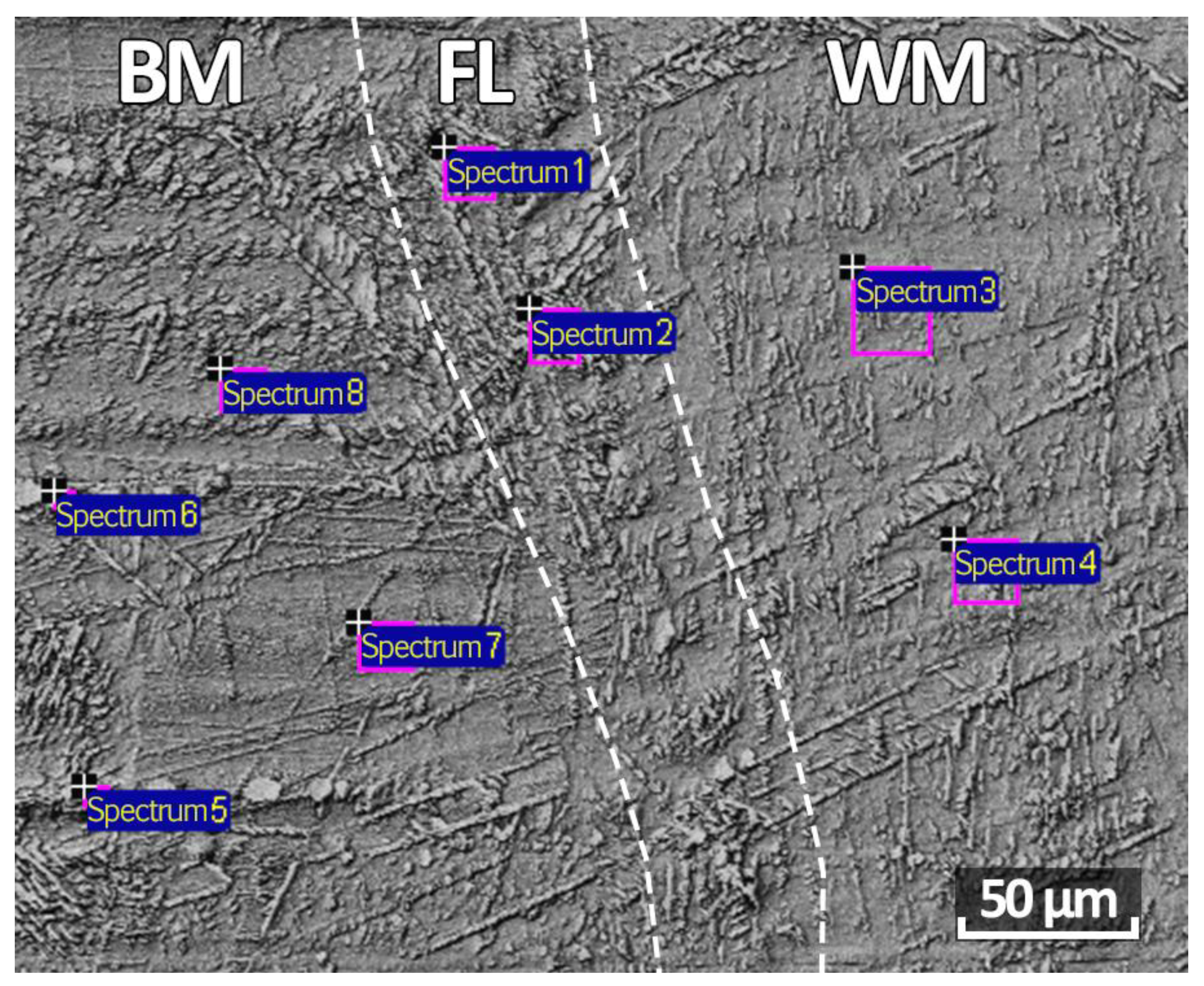

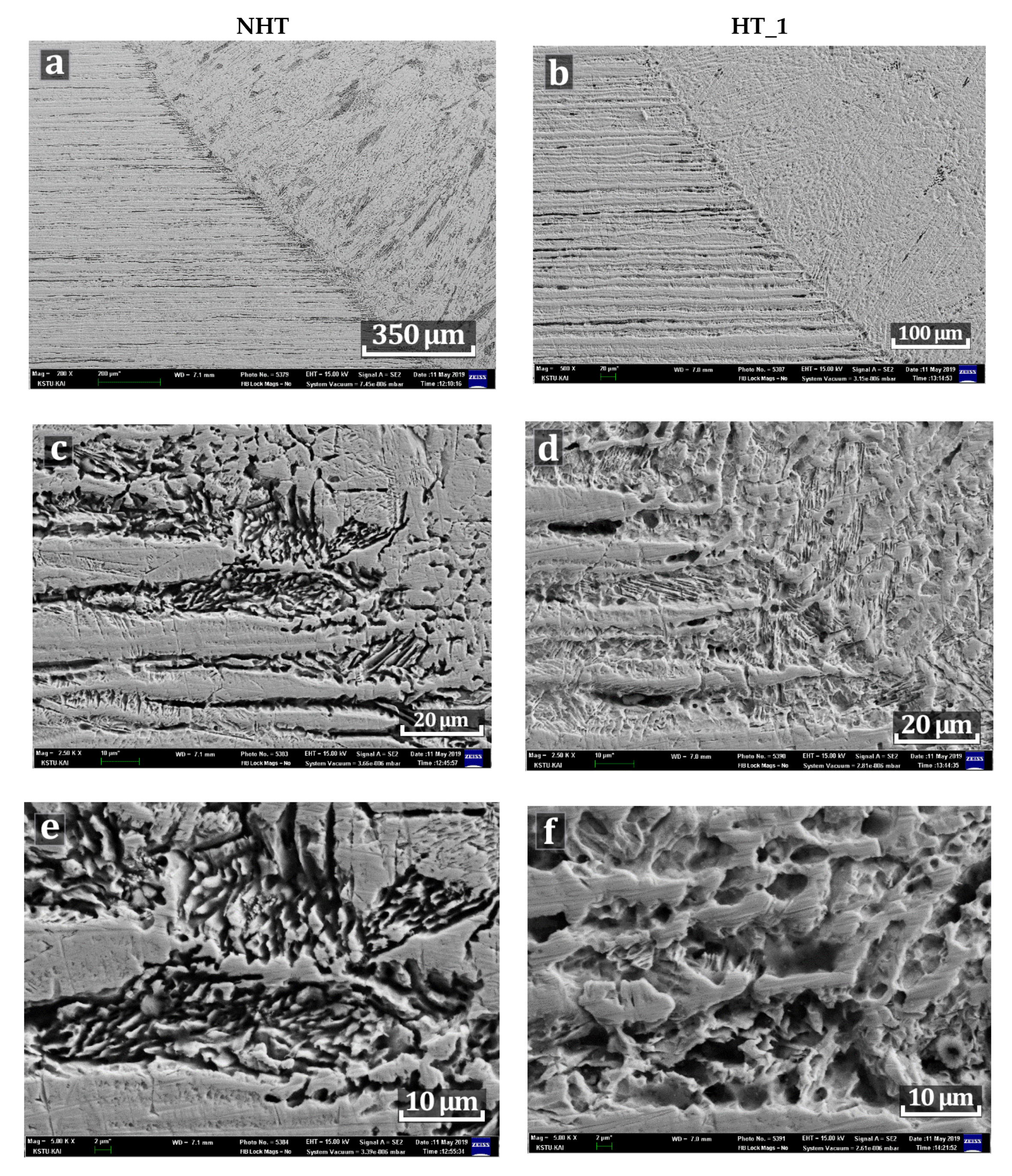
| C | Si | Mn | S | P | Cr | Ni | Ti | As | Cu |
|---|---|---|---|---|---|---|---|---|---|
| <0.12 | <0.80 | <2.00 | <0.020 | <0.035 | 17.0–19.0 | 9.0–11.0 | <1.00 | – | – |
| Sample | NHT | HT_1 | HT_2 |
|---|---|---|---|
| Temperature, °C | – | 880 | 1080 |
| Treatment time, min | – | 120 | 120 |
| Cooling area | – | Furnace, until 500 °C, than water | Water |
| Cr, wt% | Ni, wt% | Ti, wt% | Si, wt% | |||||
|---|---|---|---|---|---|---|---|---|
| NHT | HT_1 | NHT | HT_1 | NHT | HT_1 | NHT | HT_1 | |
| Spectrum 1 | 17.86 | 17.93 | 10.30 | 10.69 | – | 0.22 | 0.58 | 0.47 |
| Spectrum 2 | 18.19 | 17.51 | 9.47 | 10.82 | – | 0.25 | 0.52 | 0.53 |
| Spectrum 3 | 17.40 | 18.36 | 8.74 | 9.03 | 0.37 | 0.35 | 0.66 | 0.56 |
| Spectrum 4 | 18.47 | 18.61 | 7.95 | 9.05 | – | 0.36 | 0.53 | 0.56 |
| Cr, wt% | Ni, wt% | Ti, wt% | Si, wt% | |
|---|---|---|---|---|
| Spectrum 1 | 18.50 | 8.40 | 2.74 | 0.48 |
| Spectrum 2 | 18.97 | 8.13 | 0.43 | 0.54 |
| Spectrum 3 | 18.22 | 9.40 | 0.32 | 0.48 |
| Spectrum 4 | 18.77 | 9.31 | 0.40 | 0.62 |
| Spectrum 1 | 20.39 | 6.97 | 2.03 | 0.41 |
| Spectrum 2 | 21.21 | 6.63 | 0.50 | 0.37 |
| Spectrum 3 | 18.80 | 9.22 | 0.26 | 0.57 |
| Spectrum 4 | 18.69 | 8.98 | 0.23 | 0.46 |
| Sample | Residual Stress σ, MPa | ||
|---|---|---|---|
| 0° | 45° | 90° | |
| NHT | −265 ± 133 | −363 ± 53 | −240 ± 71 |
| HT_1 | −228 ± 87 | −368 ± 81 | −359 ± 81 |
| HT_2 | −298 ± 24 | −281 ± 26 | −368 ± 87 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryntsev, S.V. Effect of Heat Treatment on the Phase Composition and Corrosion Resistance of 321 SS Welded Joints Produced by a Defocused Laser Beam. Materials 2019, 12, 3720. https://doi.org/10.3390/ma12223720
Kuryntsev SV. Effect of Heat Treatment on the Phase Composition and Corrosion Resistance of 321 SS Welded Joints Produced by a Defocused Laser Beam. Materials. 2019; 12(22):3720. https://doi.org/10.3390/ma12223720
Chicago/Turabian StyleKuryntsev, Sergey Vyacheslavovich. 2019. "Effect of Heat Treatment on the Phase Composition and Corrosion Resistance of 321 SS Welded Joints Produced by a Defocused Laser Beam" Materials 12, no. 22: 3720. https://doi.org/10.3390/ma12223720
APA StyleKuryntsev, S. V. (2019). Effect of Heat Treatment on the Phase Composition and Corrosion Resistance of 321 SS Welded Joints Produced by a Defocused Laser Beam. Materials, 12(22), 3720. https://doi.org/10.3390/ma12223720




