Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel
Abstract
1. Introduction
2. Experiment
2.1. Material and Specimen Preparation
2.2. Tempering
2.3. Analysis
3. Results
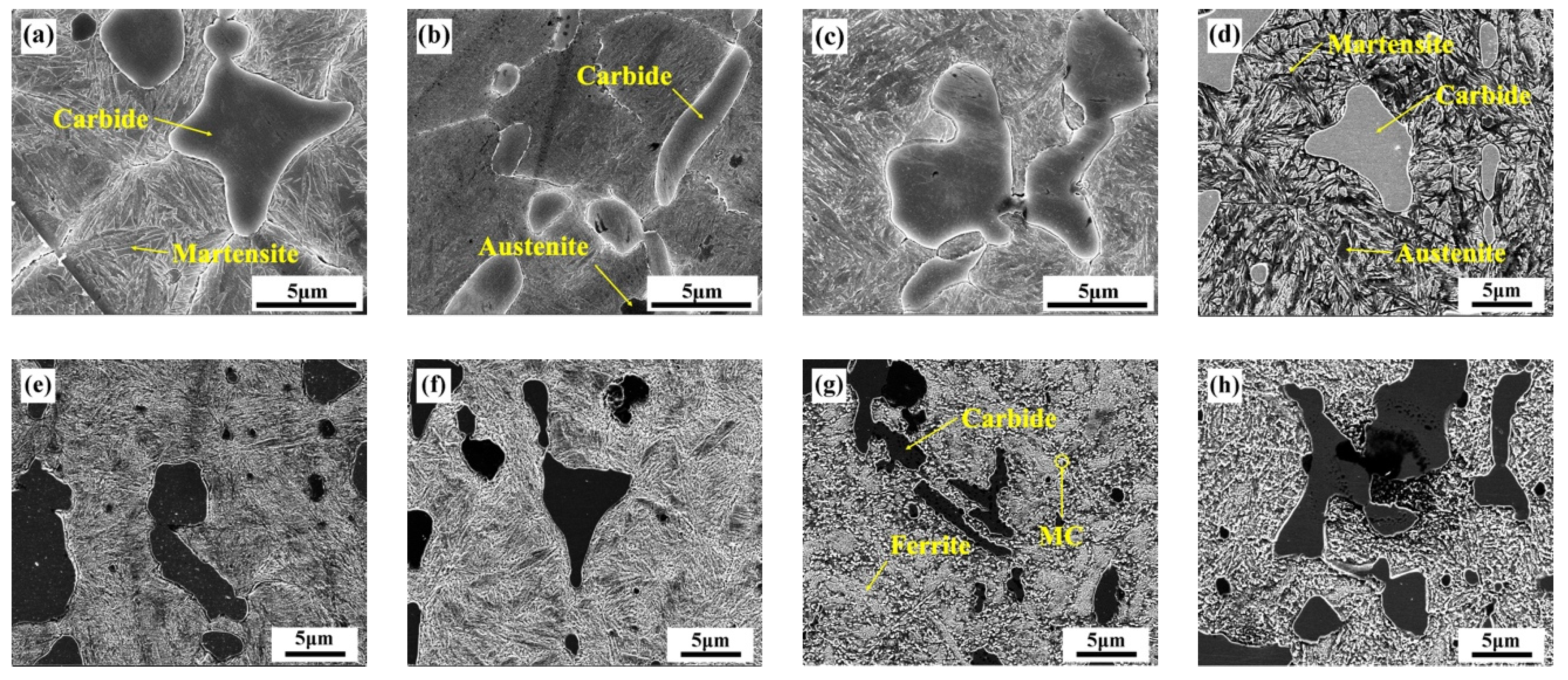
3.1. Microstructure of M42 Evolution
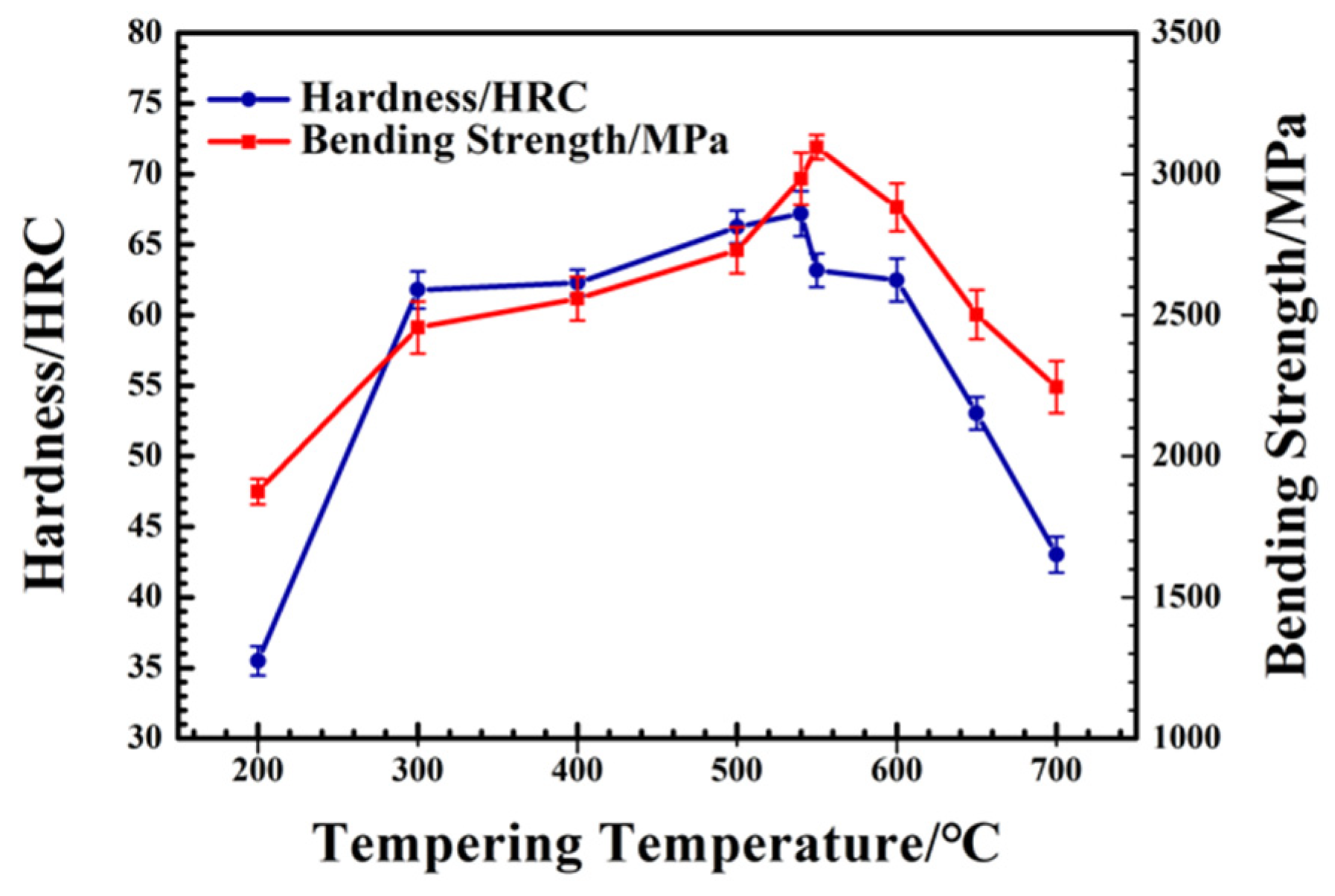
3.2. Evolution of Mechanical Properties
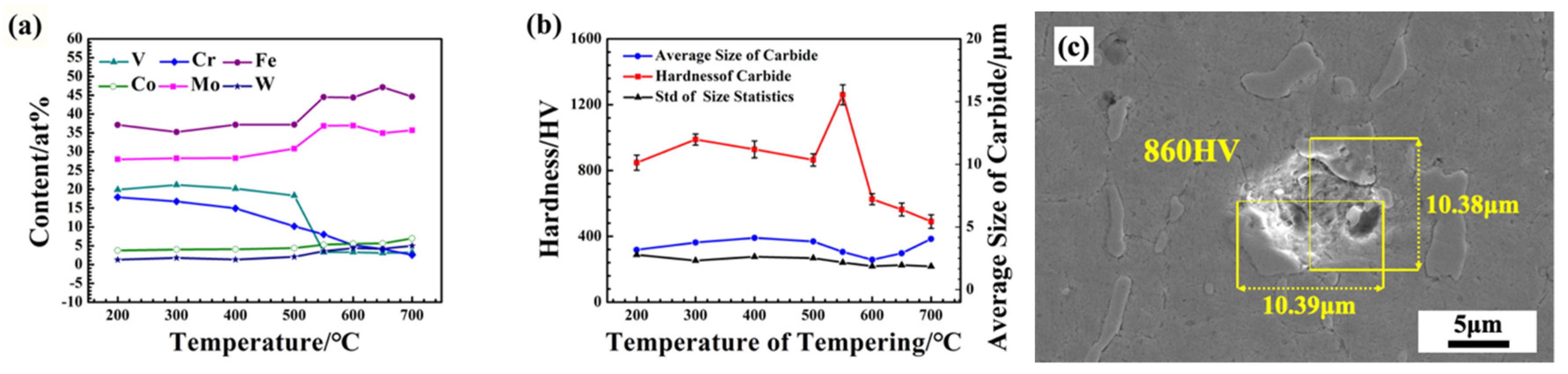
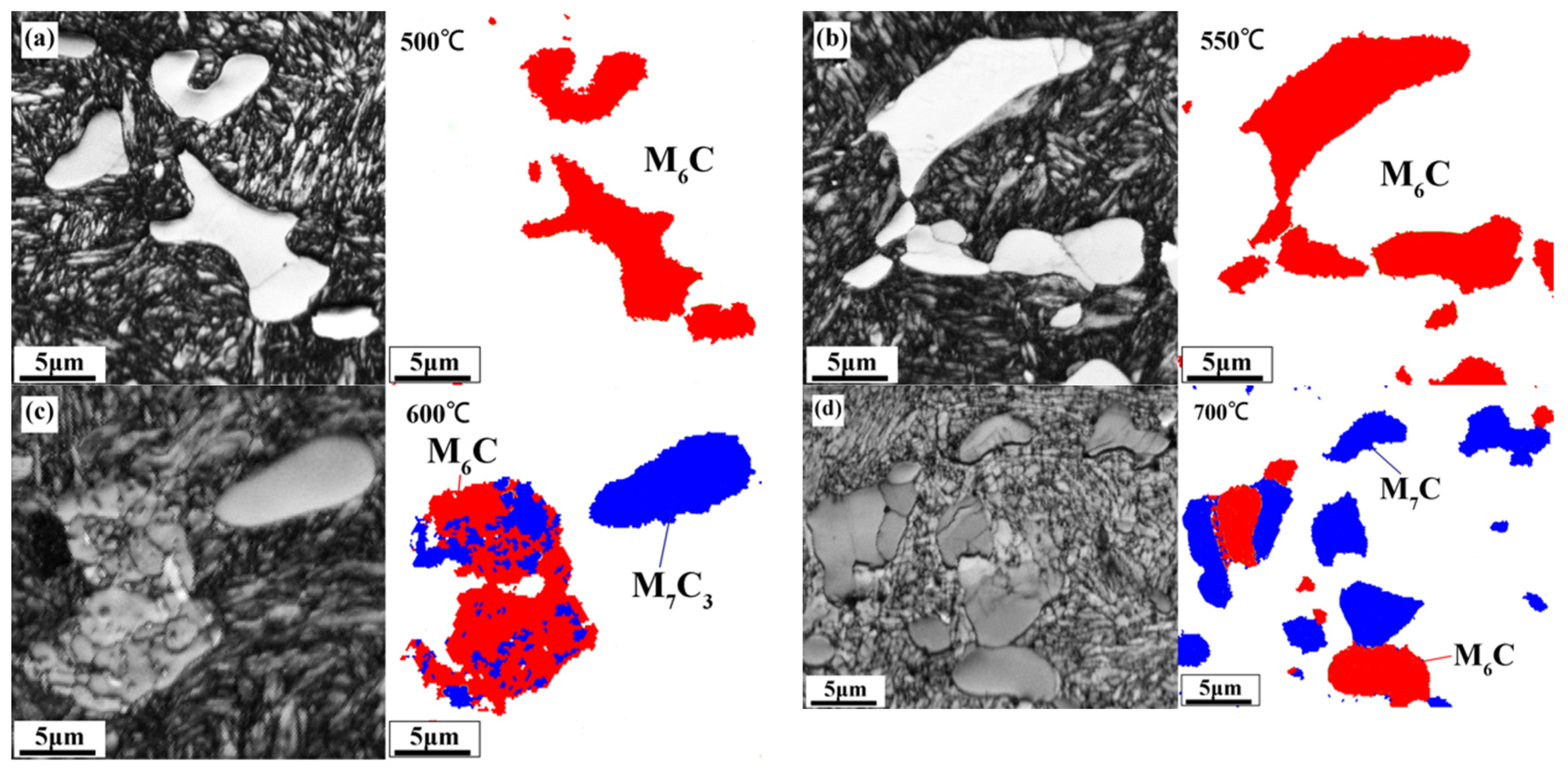
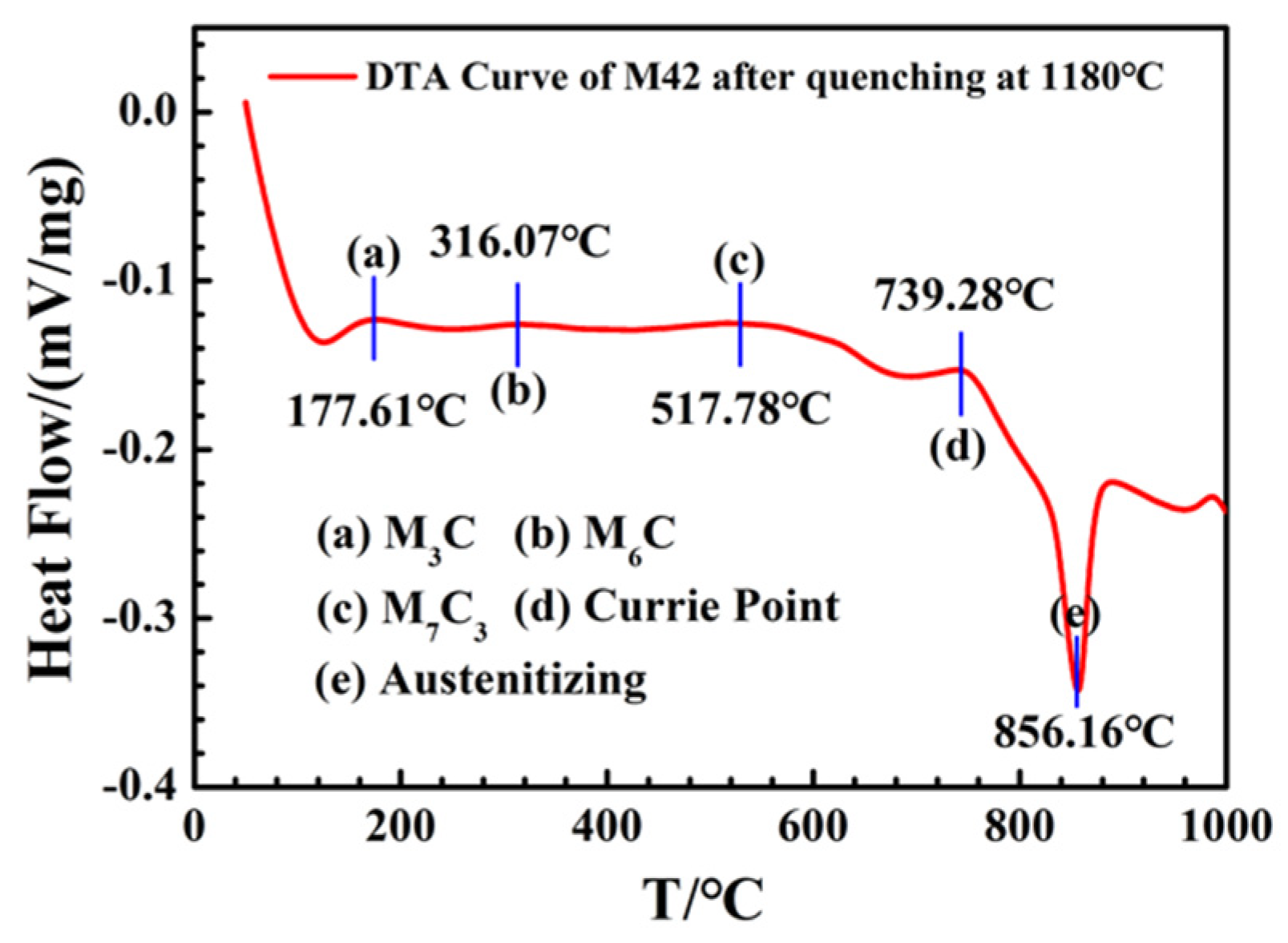
3.3. Evolution of Carbides
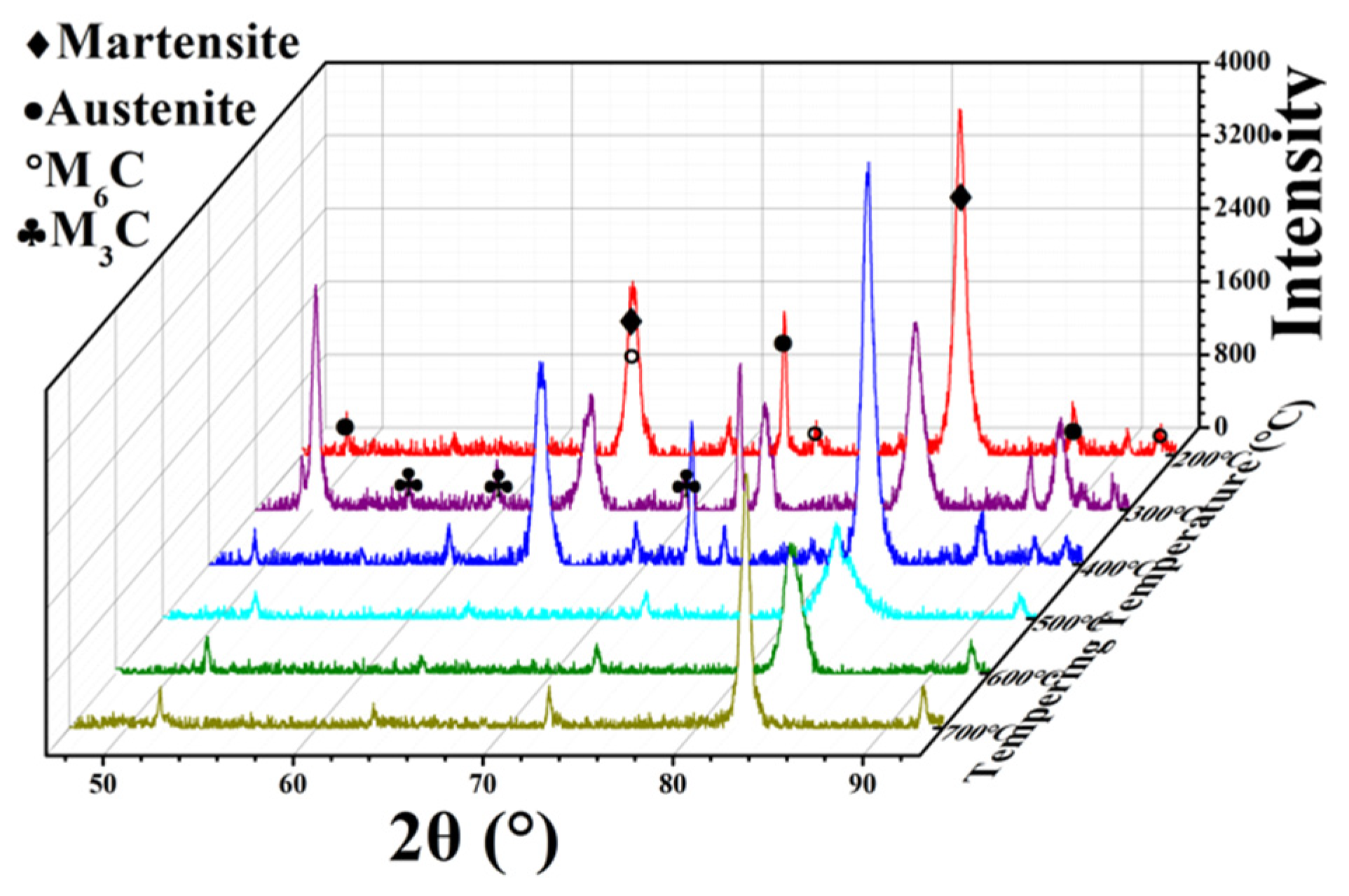
3.4. Effect of Tempering Conditions on Retained Austenite
4. Discussion
5. Conclusions
- (1)
- The tempering process has an impact on secondary hardening on spray-formed M42 HSS by refining martensite, reducing the retained austenite content, and controlling carbide phase composition and size. The best tempering process is three times at 540 °C for one hour each time. The Rockwell hardness of M42 HSS reaches 67.2 HRC and the bending strength reaches 3115 MPa, which is higher than the required values for cooperative enterprises (Rockwell hardness more than 65 HRC and bending strength more than 3000 MPa).
- (2)
- With the increase in tempering temperature, the martensite is refined, and the retained austenite is gradually transformed into martensite. The content of retained austenite is reduced from 27.99% at 200 °C to 10.04% at 700 °C, which increases the strength of M42 HSS. When tempering above 600 °C, the martensite matrix is gradually transformed into ferrite, and the strength of the M42 decreases.
- (3)
- Secondary hardening during tempering is closely related to the precipitation and transformation of carbide. With the increase in tempering temperature, the phase composition of carbides undergoes precipitation of M3C-M6C-M7C3, and the corresponding microhardness of the carbides first increases and then decreases.
Author Contributions
Funding
Conflicts of Interest
References
- Kwok, C.; Cheng, F.; Man, H. Microstructure and corrosion behavior of laser surface-melted high-speed steels. Surf. Coat. Technol. 2007, 202, 336–348. [Google Scholar] [CrossRef]
- Bedford, G.M.; Vitanov, V.I.; Voutchkov, I.I. On the thermo-mechanical events during friction surfacing of high speed steels. Surf. Coat. Technol. 2001, 141, 34–39. [Google Scholar] [CrossRef]
- Godec, M.; Pirtovsek, T.V.; Batic, B.S.; McGuiness, P.; Bujra, J.; Pdgornik, B. Surface and Bulk Carbide Transformations in High-Speed Steel. Sci. Rep. 2015, 5, 16202. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xi, X.; Luo, Y.; Mao, M.; Shi, X.; Guo, J.; Guo, H. Carbide Precipitation during Tempering and Its Effect on the Wear Loss of a High-Carbon 8 Mass% Cr Tool Steel. Materials 2018, 11, 2491. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, H.; Guo, J.; Yang, W.S. Gleeble-Simulated and Semi-Industrial Studies on the Microstructure Evolution of Fe-Co-Cr-Mo-W-V-C Alloy during Hot Deformation. Materials 2018, 11, 2577. [Google Scholar] [CrossRef]
- Sackl, S.; Leitner, H.; Clemens, H.; Primig, S. On the evolution of secondary hardening carbides during continuous versus isothermal heat treatment of high speed steel HS 6-5-2. Mater. Charact. 2016, 120, 323–330. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Zhang, J.; Lu, L.; Cui, H.; Zhang, J.S. The secondary precipitates of niobium-alloyed M3, 2 high speed steel prepared by spray deposition. Mater. Charact. 2015, 106, 245–254. [Google Scholar] [CrossRef]
- Hwang, K.; Lee, S.; Lee, H. Effects of alloying elements on microstructure and fracture properties of cast high speed steel rolls Part II. Fracture behavior. Mater. Sci. Eng. A 1998, 254, 296–304. [Google Scholar] [CrossRef]
- Hetzner, D.W. Refining carbide size distributions in M1 high speed steel by processing and alloying. Mater. Charact. 2001, 46, 175–182. [Google Scholar] [CrossRef]
- Boccalini, M.; Goldenstein, H. Solidification of high speed steels. Int. Mater. Rev. 2001, 46, 92–115. [Google Scholar] [CrossRef]
- Várez, A.; Portuondo, B.; Levenfeld, J.; Torralba, J.M. Processing of P/M T15 high speed steels by mould casting using thermosetting binders. Mater. Chem. Phys. 2001, 67, 43–48. [Google Scholar] [CrossRef]
- Zapata, W.C.; Costa, C.E.D.; Torralba, J.M. Wear and thermal behaviour of M2 high-speed steel reinforced with NbC composite. J. Mater. Sci. 1998, 33, 3219–3225. [Google Scholar] [CrossRef]
- Stolarz, S. Properties of powder metallurgy high speed steels. Met. Powder Rep. 1997, 52, 39. [Google Scholar]
- Massimo, P.; Anna, F. Mario. Spark Plasma Co-Sintering of Mechanically Milled Tool Steel and High Speed Steel Powders. Materials 2016, 9, 482–491. [Google Scholar]
- Lin, Y.; Mchugh, K.M.; Zhou, Y.; Lavernia, E.J. Microstructure and hardness of spray-formed chromium-containing steel tooling. Scr. Mater. 2006, 55, 581–584. [Google Scholar] [CrossRef]
- Cui, C.; Schulz, A.; Uhlenwinkel, V. Co-Spray Forming of Gradient Deposits from Two Sprays of Different Tool Steels Using Scanning Gas Atomizers. Steel Res. Int. 2013, 84, 1075–1084. [Google Scholar] [CrossRef]
- Serna, M.M.; Rossi, J.L. MC complex carbide in AISI M2 high-speed steel. Mater. Lett. 2009, 63, 691–693. [Google Scholar] [CrossRef]
- Mchugh, K.M.; Lin, Y.; Zhou, Y.; Lavernia, E.J. Influence of cooling rate on phase formation in spray-formed H13 tool steel. Mater. Sci. Eng. A (Struct. Mater. Prop. Microstruct. Process.) 2008, 477, 50–57. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Li, Y.; Qu, P.; Shen, L.; Wen, X.; Cui, H.; Zhang, J.S. Effect of Niobium on the Secondary Precipitates and Tempering Resistance of Spray-Formed M3, 2 High-Speed Steel. J. Mater. Eng. Perform. 2019, 28, 926–937. [Google Scholar] [CrossRef]
- García, C.; Romero, A.; Herranz, G.; Blanco, Y.; Martin, F. Effect of vanadium carbide on dry sliding wear behavior of powder metallurgy AISI M2 high speed steel processed by concentrated solar energy. Mater. Charact. 2016, 121, 175–186. [Google Scholar] [CrossRef]
- Rodenburg, C.; Rainforth, W.M. A quantitative analysis of the influence of carbides size distributions on wear behaviour of high-speed steel in dry rolling/sliding contact. Acta Mater. 2007, 55, 2443–2454. [Google Scholar] [CrossRef]
- Vilar, R.; Colaco, R.; Almeida, A. Laser surface treatment of tool steels. Opt. Quantum Electron. 2007, 55, 1273–1289. [Google Scholar]
- Jurci, P.; Dománková, M.; Ptacinová, J.; Pasak, M.; Martin, K.; Petre, P. Investigation of the Microstructural Changes and Hardness Variations of Sub-Zero Treated Cr-V Ledeburitic Tool Steel Due to the Tempering Treatment. J. Mater. Eng. Perform. 2018, 27, 1514–1529. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Sun, F. Tempering behavior of a semi-high speed steel containing nitrogen. Mater. Sci. Eng. A (Struct. Mater. Prop. Microstruct. Process.) 2006, 438–440, 1139–1142. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Feng, F.; Tu, Y.; Jiang, J. A New Approach for Refining Carbide Dimensions in M42 Super Hard High-speed Steel. J. Iron Steel Res. 2016, 23, 800–807. [Google Scholar] [CrossRef]
- Godec, M.; Batic, B.S.; Mandrino, D.; Nagode, A.; Vojteh, L.; Skapin, S.D.; Jenko, M. Characterization of the carbides and the martensite phase in powder-metallurgy high-speed steel. Mater. Charact. 2010, 61, 452–458. [Google Scholar] [CrossRef]
- Leskovsek, V.; Podgornik, B. Vacuum heat treatment, deep cryogenic treatment and simultaneous pulse plasma nitriding and tempering of P/M S390MC steel. Mate. Sci. Eng. A 2012, 531, 119–129. [Google Scholar] [CrossRef]
- Pavlina, E.J.; Speer, J.G.; Tyne, C.J.V. Equilibrium solubility products of molybdenum carbide and tungsten carbide in iron. Scr. Mater. 2012, 66, 243–246. [Google Scholar] [CrossRef]
- Alejandro, G.P.; Florentino, A.A.; Juan, A.L. Optimization of Quenching and Tempering Parameters for the Precipitation of M7C3 and MC Secondary Carbides and the Removal of the Austenite Retained in Vanadis 10 Tool Steel. Metals 2019, 9, 627. [Google Scholar]
- Chen, C.Y.; Yen, H.W.; Kao, F.H.; Li, W.C.; Huang, C.Y.; Yang, J.R.; Wang, S.H. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A (Struct. Mater. Prop. Microstruct. Process.) 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, H.; Chen, L.; Guo, S. Novel laser rapidly solidified medium-entropy high speed steel coatings with enhanced hot wear resistance. J. Alloy. Compd. 2019, 719–727. [Google Scholar] [CrossRef]
- Peng, H.; Hu, L.; Li, L.; Zhang, L.Y.; Zhang, X.L. Evolution of the microstructure and mechanical properties of powder metallurgical high-speed steel S390 after heat treatment. J. Alloy. Compd. 2017, 740, 766–773. [Google Scholar] [CrossRef]
- Liu, B.W.; Lu, X.; Pi, Z.Q.; Jia, C.C.; Zheng, W.; Wu, Z.L.; Zhang, Y.F. Effect of Quenching Temperature on Transformation Mechanism of Carbides of M42. Rare Met. Mater. Eng. 2017, 7, 27. [Google Scholar]


| C | Si | Mn | Mo | W | V | Co | Cr | Fe |
|---|---|---|---|---|---|---|---|---|
| 1.10 | 0.25 | 0.36 | 9.50 | 1.50 | 1.15 | 8.00 | 3.75 | Bal. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Qin, T.; Xu, W.; Jia, C.; Wu, Q.; Chen, M.; Liu, Z. Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel. Materials 2019, 12, 3714. https://doi.org/10.3390/ma12223714
Liu B, Qin T, Xu W, Jia C, Wu Q, Chen M, Liu Z. Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel. Materials. 2019; 12(22):3714. https://doi.org/10.3390/ma12223714
Chicago/Turabian StyleLiu, Bowen, Tian Qin, Wei Xu, Chengchang Jia, Qiuchi Wu, Mingying Chen, and Zhe Liu. 2019. "Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel" Materials 12, no. 22: 3714. https://doi.org/10.3390/ma12223714
APA StyleLiu, B., Qin, T., Xu, W., Jia, C., Wu, Q., Chen, M., & Liu, Z. (2019). Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel. Materials, 12(22), 3714. https://doi.org/10.3390/ma12223714





