Review of Recent Advances in Polylactic Acid/TiO2 Composites
Abstract
1. Introduction
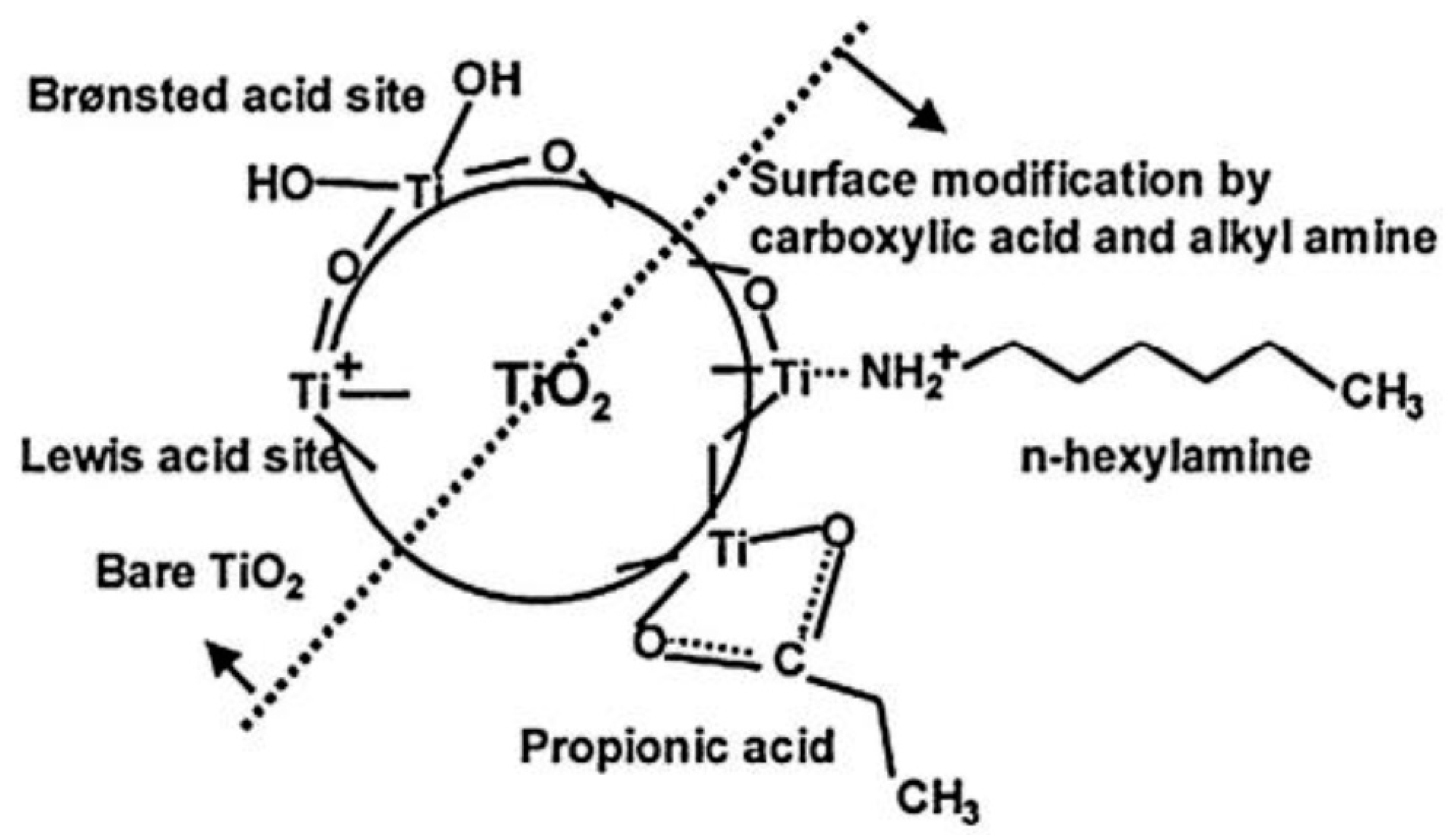
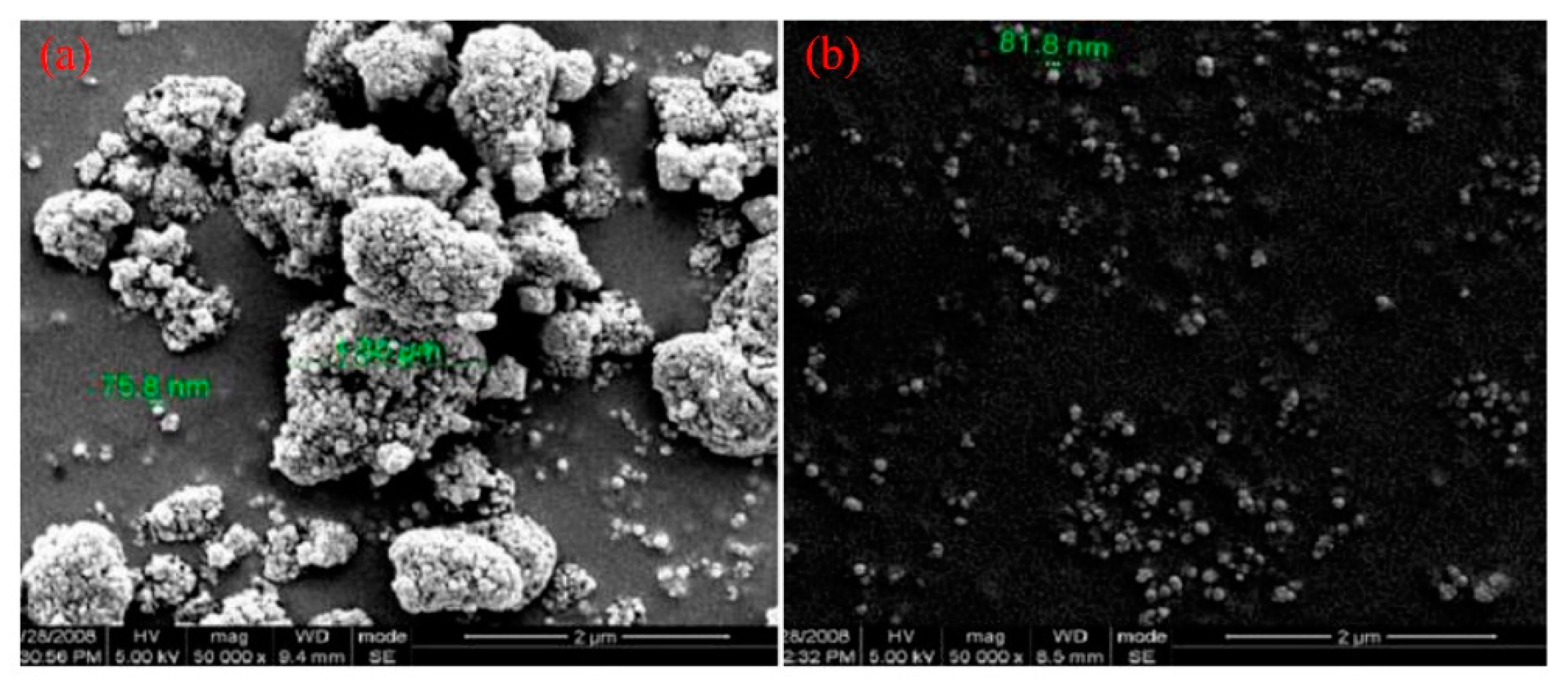
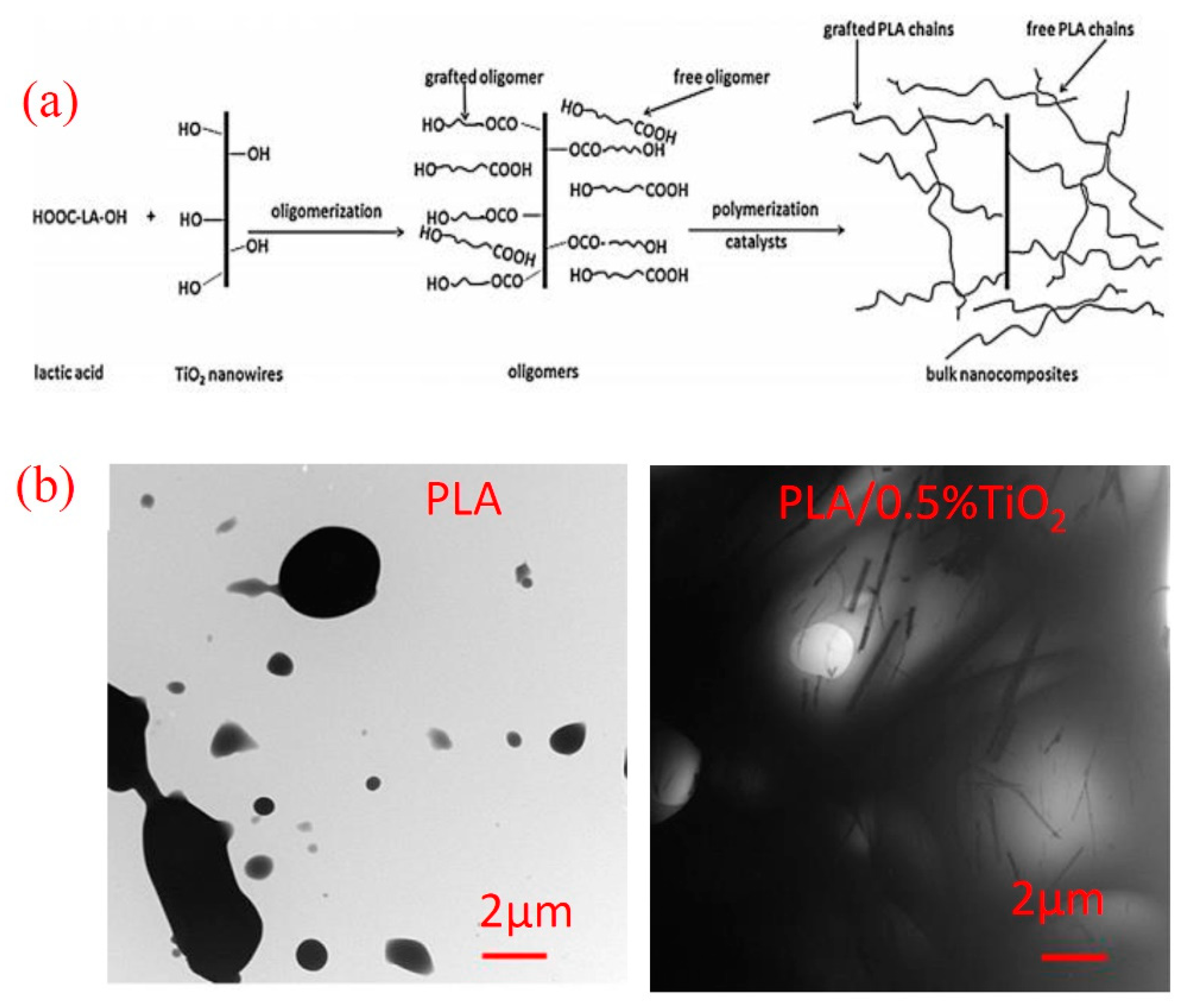
2. Improvement of TiO2 Dispersion in PLA Matrix
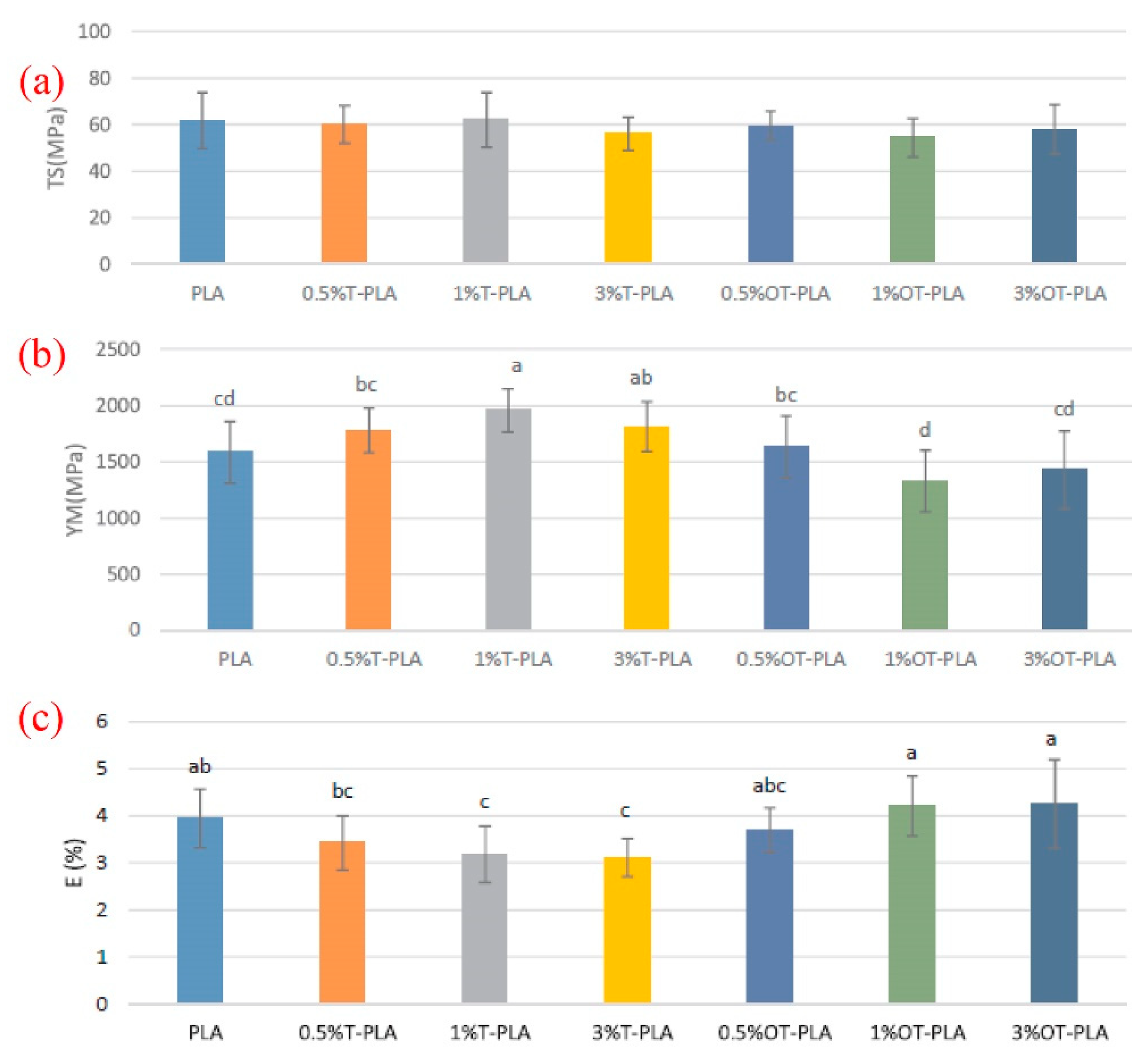
3. Mechanical Properties
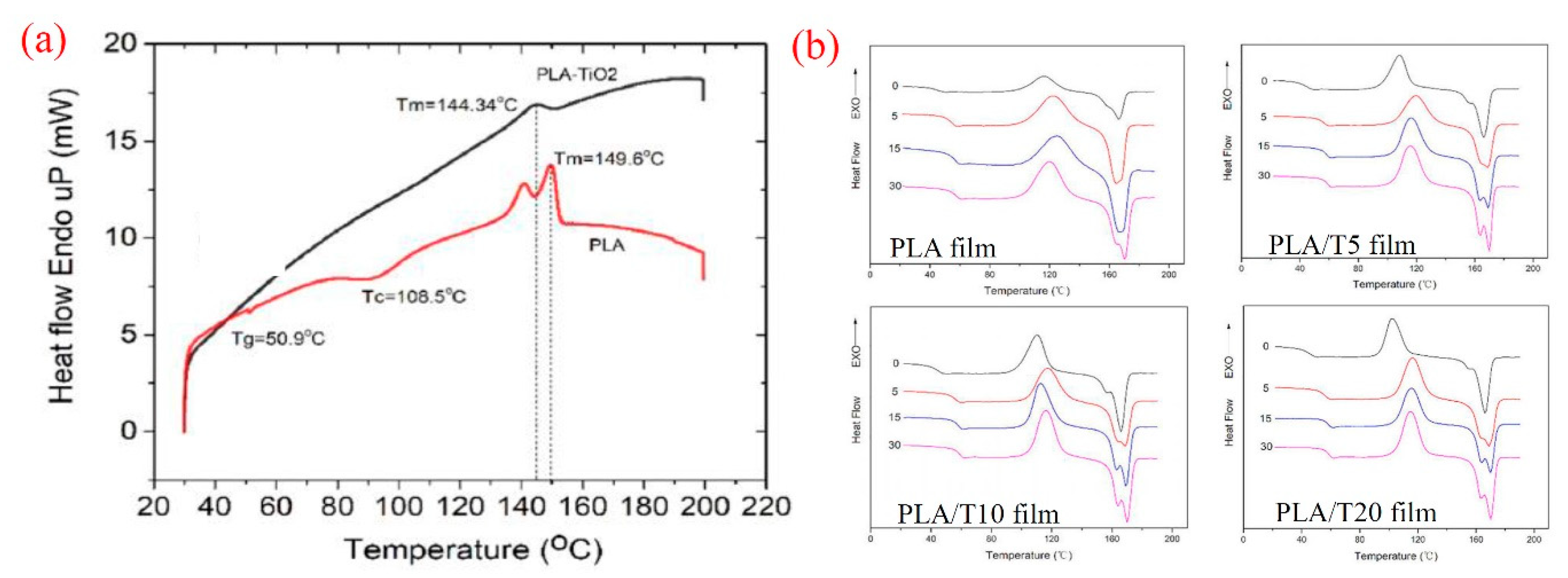
4. Thermal Properties
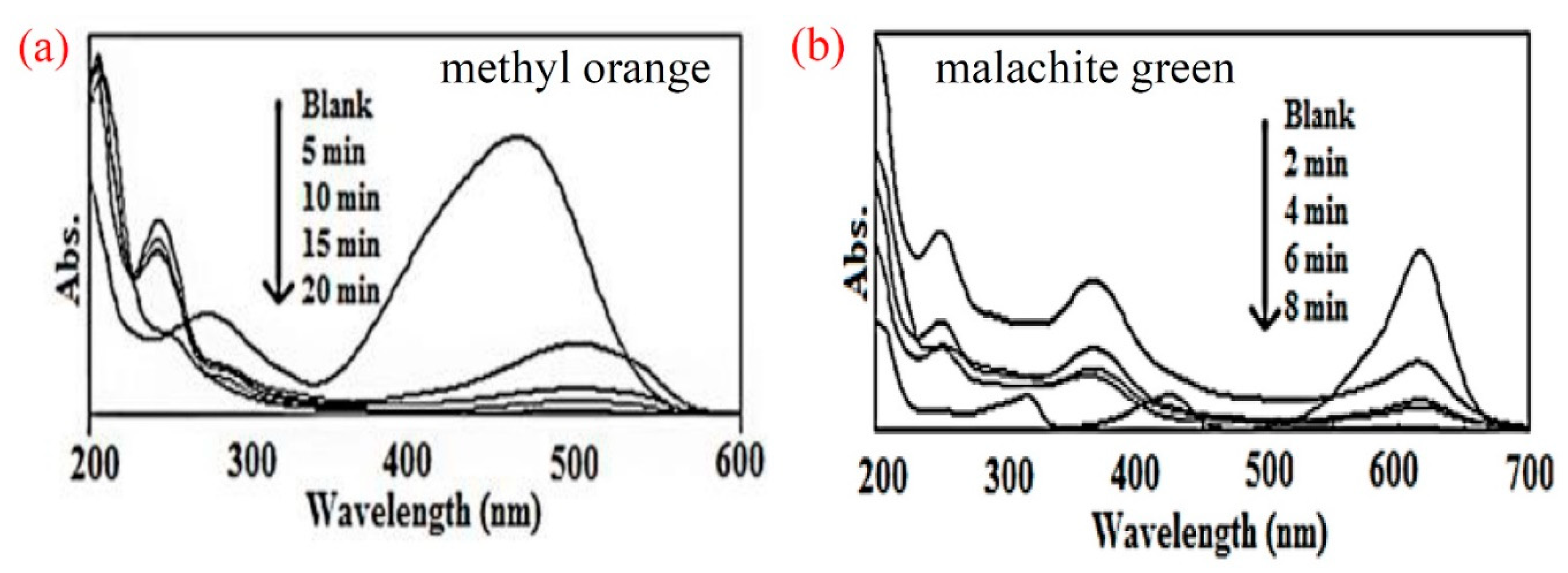
5. Photocatalytic Properties
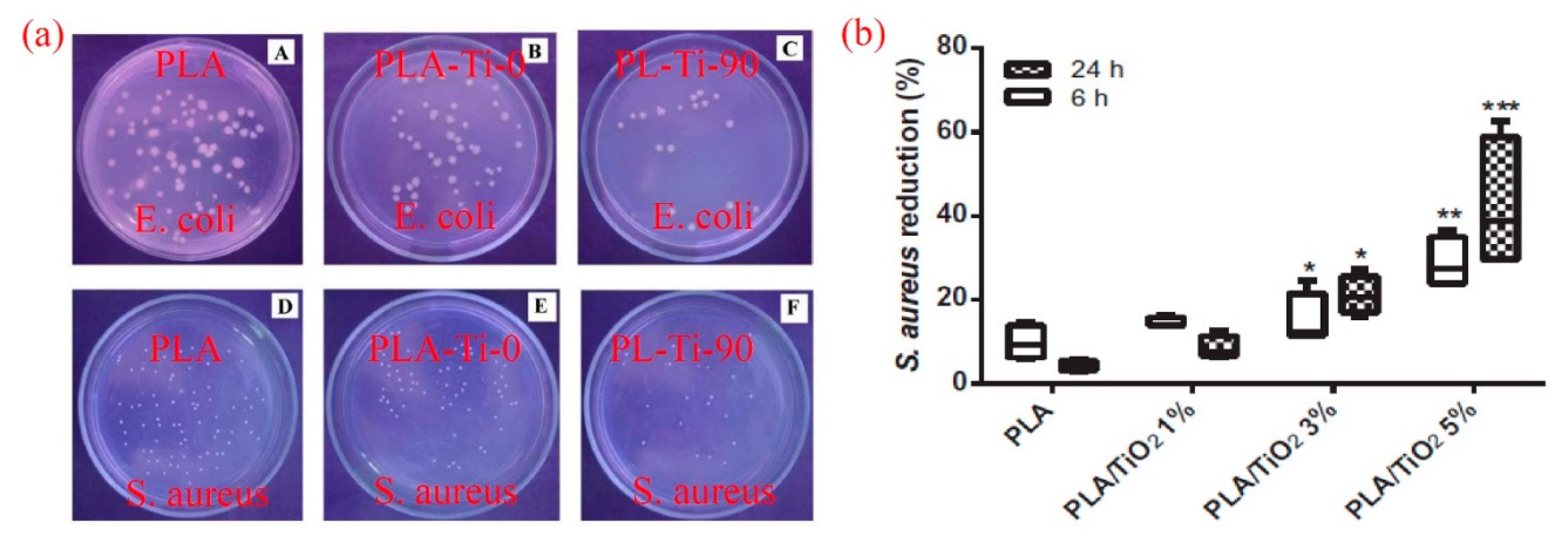
6. Antimicrobial Properties
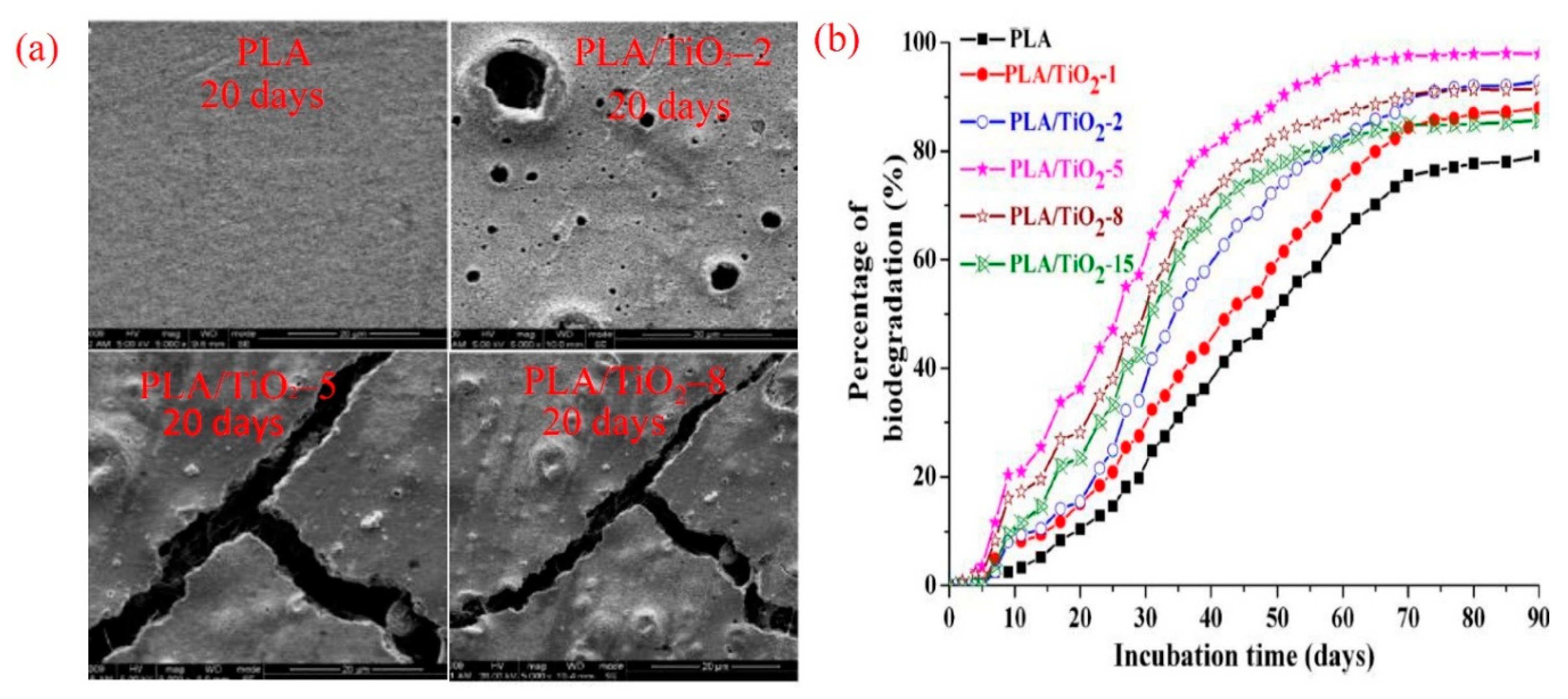
7. Degradation Behavior
8. Potential Applications of PLA/TiO2 Composites
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Docekal, B.; Vojtková, B. Determination of trace impurities in titanium dioxide by direct solid sampling electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 304–308. [Google Scholar] [CrossRef]
- Allodi, V.; Brutti, S.; Giarola, M.; Sgambetterra, M.; Navarra, M.A.; Panero, S.; Mariotto, G. Structural and spectroscopic characterization of a nanosized sulfated TiO2 filler and of nanocomposite nafion membranes. Polymers 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Palza, H.; Rabagliati, F.M. Novel antimicrobial polyethylene composites prepared by metallocenic “in-situ” polymerization with TiO2 based nanoparticles. J. Polym. Sci. A Polym. Chem. 2012, 50, 4055–4062. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, J.; Sun, X. Photoactivity of poly(lactic acid) nanocomposites modulated by TiO2 nanofillers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xie, G.; Li, G.; Zhnag, Z. Preparation and characterization of polyethylene/TiO2 nanocomposites. Compos. Interfaces 2006, 13, 623–632. [Google Scholar] [CrossRef]
- Althan, M.; Yildirim, H. Mechanical and Antibacterial Properties of Injection Molded Polypropylene/TiO2 Nano-Composites: Effects of Surface Modification. J. Mater. Sci. Technol. 2012, 28, 686–692. [Google Scholar] [CrossRef]
- Xuefeng, L.; Shijie, D.; Han, Y. Fabrication and properties of PVA-TiO2 hydrogel composites. Procedia Eng. 2012, 27, 1488–1491. [Google Scholar]
- Hanemann, T.; Szabo, D.V. Polymer-nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Basu, A.; Nazarkovsky, M.; Ghadi, R.; Khan, W.; Domb, A.J. Poly(lactic acid)-based nanocomposites. Polym. Adv. Technol. 2017, 28, 919–930. [Google Scholar] [CrossRef]
- Xu, N.; Shi, Z.; Fan, Y.; Dong, J.; Sji, J.; Hu, M.Z.C. Effects of particle size of TiO2 on photocatalytic degradation of methylene blue in aqueous suspensions. Ind. Eng. Chem. Res. 1999, 38, 373–379. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Doubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F.; Ko, Y.G. A review on recent researches on polylactic acid/carbon nanotube composites. Polym. Bull. 2017, 74, 2921–2937. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Wang, Y.Z. Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ko, Y.G. Fabrication and materials properties of polystyrene/carbon nanotube (PS/CNT) composites: A review. Eur. Polym. J. 2016, 79, 36–62. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of poly(l-lactic acid)/ TiO2 nanoparticle nanocomposite films with high transparency and efficient photodegradability. Polym. Degrad. Stabil. 2007, 92, 1255–1264. [Google Scholar] [CrossRef]
- Hojjati, B.; Sui, R.; Charpentier, P.A. Synthesis of TiO2/PAA nanocomposite by RAFT polymerization. Polymer 2007, 48, 5850–5858. [Google Scholar] [CrossRef]
- Luo, Y.B.; Li, W.D.; Wang, X.L.; Xu, D.Y.; Wang, Y.Z. Preparation and properties of nanocomposites based on poly (lactic acid) and functionalized Tioacta. Acta Mater. 2009, 57, 3182–3191. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, J.; Sun, X.S. Synthesis and characterization of bio-nanocomposites of poly(lactic acid) and TiO2 nanowires by in situ polymerization. Polymer 2011, 52, 2367–2375. [Google Scholar] [CrossRef]
- Lu, X. Nanocomposites of poly(L-lactide) and surface-grafted TiO2 nanoparticles: Synthesis and characterization, People’s Republic of China. Eur. Polym. J. 2008, 44, 2476–2481. [Google Scholar] [CrossRef]
- Tabriz, K.R.; Katbab, A.A. Preparation of modified-TiO2/PLA nanocomposite films: Micromorphology, photo-degradability and antibacterial studies. AIP Conf. Proc. 2017, 1914, 070009. [Google Scholar]
- Alberton, J.; Martelli, S.M.; Fakhouri, F.M.; Soldi, V. Mechanical and moisture barrier properties of titanium dioxide nanoparticles and halloysite nanotubes reinforced polylactic acid (PLA). IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 01201. [Google Scholar] [CrossRef]
- Xiu, H.; Bai, H.W.; Huang, C.M.; Xu, C.L.; Li, X.Y.; Fu, Q. Selective localization of titanium dioxide nanoparticles at the interface and its effect on the impact toughness of poly(L-lactide)/poly(ether)urethane blends. Express. Polym. Lett. 2013, 7, 261–271. [Google Scholar] [CrossRef]
- Athanasoulia, I.G.; Mikropoulou, M.; Karapati, S.; Tarantili, P.; Trapalis, C. Study of thermomechanical and antibacterial properties of TiO2/poly(lactic acid) nanocomposites. Mater. Today Proc. 2018, 5, 27553–27562. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, D.; Zhang, H.; Su, G.; Li, G. Preparation and properties of poly(lactic acid)/sesbania gum/nano-TiO2 composites. Polym. Bull. 2018, 75, 623–635. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.Y.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Baek, N.; Kim, Y.T.; Marcy, J.E.; Duncan, S.E.; O’Keefe, S.F. Physical properties of nanocomposite polylactic acid films prepared with oleic acid modified titanium dioxide. Food. Packag. Shelf. 2018, 17, 30–38. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, J.; Zhang, J.H.; Hu, B.X.; Shen, J. Preparation characterization and properties of TiO2/PLA nanocomposites by in situ polymerization. Polym. Compos. 2009, 30, 1074–1080. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Kujundziski, A.P.; Chamovska, D.; Duraccio, D. Preparation and characterization of nanocomposites based on PLA and TiO2 nanoparticles functionalized with fluorocarbons. Polym. Bull. 2017, 74, 3027–3041. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Touati, F.; Bhadra, J.; Shakoor, R.A.; Al-Thani, N.J. PLA-TiO2 nanocomposites: Thermal, morphological, structural, and humidity sensing properties. Ceram. Int. 2018, 44, 16507–16513. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Wang, J.; Qin, Y. Structural changes and nano-TiO2 migration of poly(lactic acid)-based food packaging film contacting with ethanol as food simulant. Int. J. Biol. Macromol. 2019, 139, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Nomai, J.; Suksut, B.; Schlab, A.K. Crystallization behavior of poly(lactic acid)/titanium dioxide nanocomposites. Int. J. Appl. Sci. Technol. 2015, 8, 251–258. [Google Scholar] [CrossRef][Green Version]
- Farhoodi, M.; Daddashi, S.; Mohammad, M.A.; Mousavi, A.; Djomeh, Z. Influence of TiO2 Nanoparticle Filler on the Properties of PET and PLA Nano composites. Polymer (Korean) ISSN 2012, 36, 745–755. [Google Scholar]
- Zhang, H.; Huang, J.; Yang, L.; Chen, R.; Zou, W.; Lin, X.; Qu, J. Preparation, characterization and properties of PLA/TiO2 nanocomposites based on a novel vane extruder. RSC Adv. 2015, 5, 4639–4647. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(l-lactic acid)/TiO2 nanocomposites: Thermal properties and degradation. J. Appl. Polym. Sci. 2011, 123, 2187–2193. [Google Scholar] [CrossRef]
- Athanasoulia, I.G.I.; Tarantili, P.A. Thermal transitions and stability of melt mixed TiO2/poly(L-lactic acid) nanocomposites. Polym. Eng. Sci. 2019, 59, 704–713. [Google Scholar] [CrossRef]
- Buzarovska, A. PLA Nanocomposites with Functionalized TiO2 Nanoparticles. Polym. Plast. Technol. Eng. 2013, 52, 280–286. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Wang, X.J.; Huang, Z.; Wei, M.Y.; Lu, T.; Nong, D.D.; Zhao, J.X.; Gao, X.Y.; Teng, L.J. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly (lactic acid) and isoconversional kinetic analysis. Thermochim. Acta. 2019, 672, 14–24. [Google Scholar] [CrossRef]
- Martín-Alfonsoa, J.E.; Urbanob, J.; Cuadria, A.A.; Franco, J.M. The combined effect of H2O2 and light emitting diodes (LED) process assisted by TiO2 on the photooxidation behavior of PLA. Polym. Test. 2019, 73, 268–275. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Rathore, A.; Kaur, H. Poly (lactic acid) grafting of TiO2 nanoparticles: A shift in dye degradation performance of TiO2 from UV to solar light. Chem. Select 2017, 2, 6901–6908. [Google Scholar]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M.; Ambrosio, L. Poly(lactic acid)/titanium dioxide nanocomposite films: Influence of processing procedure on dispersion of titanium dioxide and photocatalytic activity. Polym. Compos. 2011, 32, 519–528. [Google Scholar] [CrossRef]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M. Photocatalytic activity of PLA/TiO2 nanocomposites and TiO2-active multilayered hybrid coatings. Ital. J. Food. Sci. 2012, 24, 102–106. [Google Scholar]
- Hou, X.B.; Cai, Y.B.; Mushtaq, M.; Song, X.; Yang, Q.; Huang, F.; Wei, Q. Deposition of TiO2 nanoparticles on porous polylactic acid fibrous substrates and its photocatalytic capability. J. Nanosci. Nanotechnol. 2018, 18, 5617–5623. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends. Food. Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Lumyong, S.; Leejarkpai, T.; Punyodom, W. Biodegradable nanocomposite blown films based on poly(lactic acid) containing silver-loaded kaolinite: A route to controlling moisture barrier property and silver ion release with a prediction of extended shelf life of dried longan. Polymer. 2014, 55, 6776–6788. [Google Scholar] [CrossRef]
- Lantano, C.; Alfieri, I.; Cavazza, A.; Corradini, C.; Lorenzi, A.; Zucchetto, N.; Montenero, A. Natamycin based sol-gel antimicrobial coatings on polylactic acid films for food packaging. Food. Chem. 2014, 165, 342–347. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food. Process. Pres. 2018, 42, e13362. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- De Falco, G.; Porta, A.; Petrone, A.M.; Del Gaudio, P.; El Hassanin, A.; Commodo, M.; Minutolo, P.; Squillace, A.; D’Anna, A. Antimicrobial activity of flame-synthesized nano-TiO2 coatings. Environ. Sci. Nano 2017, 4, 1095–1107. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Pascual, A.M.D.; Diez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Ahmed, S.; Liu, Y. Physico-mechanical and antibacterial properties of PLA/TiO2 composite materials synthesized via electrospinning and solution casting processes. Coatings 2019, 9, 525. [Google Scholar] [CrossRef]
- Gupta, K.K.; Mishra, P.K.; Srivastava, P.; Gangwar, M.; Nath, G.; Maiti, P. Hydrothermal in situ preparation of TiO2 particles onto poly(lactic acid) electrospun nanofibers. Appl. Surf. Sci. 2013, 264, 375–382. [Google Scholar] [CrossRef]
- Toniatto, T.V.; Rodrigues, B.V.M.; Marsi, T.C.O.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Nanostructured poly (lactic acid) electrospun fiber with high loadings of TiO2 nanoparticles: Insights into bactericidal activity and cell viability. Mater. Sci. Eng. C 2017, 71, 381–385. [Google Scholar] [CrossRef]
- Dural-Erem, A.; Erem, H.H.; Ozcan, G.; Skrifvars, M. Anatase titanium dioxide loaded polylactide membranous films: Preparation, characterization, and antibacterial activity assessment. J. Text. I. 2015, 106, 571–576. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation assessment of poly (lactic acid) filled with functionalized Titania nanoparticles (PLA/TiO2) under compost conditions. Nanoscale Res. Lett. 2019, 14, 56–65. [Google Scholar] [CrossRef]
- Williams, D.F. Enzymatic hydrolysis of polylactic acid. Eng. Med. 1981, 10, 5–7. [Google Scholar] [CrossRef]
- Oda, Y.; Yonetsu, A.; Urakami, T.; Tonomura, K. Degradation of polylactide by commercial proteases. J. Polym. Environ. 2000, 8, 29–32. [Google Scholar] [CrossRef]
- Luo, Y.B.; Cao, Y.Z.; Guo, G. Effects of TiO2 nanoparticles on the photodegradation of poly (lactic acid). J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Marra, A.; Cimmino, S.; Silvestre, C. Effect of TiO2 and ZnO on PLA degradation in various media. Adv. Mater. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, G.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Segura Gonzalez, E.A.; Olmos, D.; Angel Lorente, M.; Velaz, I.; Gonzalez-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2 for antibacterial packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Buzarovska, A.; Qualandi, C.; Parrilli, A.; Scandola, M. Effect of TiO2 nanoparticle loading on poly(L-lactic acid) porous scaffolds fabricated by TIPS. Compos. Part. B. Eng. 2015, 81, 189–195. [Google Scholar] [CrossRef]
- Buzarovska, A.; Dinescu, S.; Chitoiu, L.; Costache, M. Porous poly(L-lactic acid) nanocomposite scaffolds with functionalized TiO2 nanoparticles: Properties, cytocompatibility and drug release capability. J. Mater. Sci. 2018, 53, 11151–11166. [Google Scholar] [CrossRef]
- Song, M.; Pan, C.; Li, J.Y.; Wang, X.M.; Gu, Z.Z. Electrochemical study on synergistic effect of the blending of nano TiO2 and PLA polymer on the interaction of antitumor drug with DNA. Electroanalysis 2006, 18, 1995–2000. [Google Scholar] [CrossRef]
- Song, M.; Pan, C.; Chen, C.; Li, J.Y.; Wang, X.M.; Gu, Z.Z. The application of new nanocomposites: Enhancement effect of polylactide nanofibers/nano-TiO2 blends on biorecognition of anticancer drug daunorubicin. Appl. Surf. Sci. 2008, 255, 610–612. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of biocompatibility and bactericidal activity of hierarchically porous PLA-TiO2 nanocomposite films fabricated by breath-figure method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Lizundia, L.; Vilas, J.L.; Sangroniz, A.; Etxeberria, A. Light and gas barrier properties of PLLA/metallic nanoparticles composite films. Eur. Polym. J. 2017, 91, 10–20. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.J.; Wang, J.G.; Zhao, R.Z. A novel hierarchical structured poly(lactic acid)/titania fibrous membrane with excellent antibacterial activity and air filtration performance. J. Nanomater. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; Zhang, D.; Sun, Q.; Cao, K.; Zha, J.; Lu, Y.; Wang, B.; Cao, X.; Feng, Y.; et al. Significantly improved dielectric properties of polylactide nanocomposites via TiO2 decorated carbon nanotubes. Comp. Part A Appl. Sci. 2019, 127, 105650. [Google Scholar] [CrossRef]
- Barut, N.; Shaikh, T.; Kaur, H. A PLA–TiO2 particle brush as a novel support for CuNPs: A catalyst for the fast-sequential reduction and N-arylation of nitroarenes. New J. Chem. 2017, 41, 5347–5354. [Google Scholar] [CrossRef]
| PLA | PLA/TiO2-1 | PLA/TiO2-3 | PLA/TiO2-5 | PLA/TiO2-10 | |
|---|---|---|---|---|---|
| TiO2 content (%) | 0 | 1 | 3 | 5 | 10 |
| TS (MPa) | 9.37 | 9.45 | 17.2 | 10.5 | 3.35 |
| EB (%) | 245.3 | 250.0 | 261.8 | 178.6 | 39.4 |
| YM(MPa) | 12.3 | 138.3 | 287.5 | 253.5 | 202.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of Recent Advances in Polylactic Acid/TiO2 Composites. Materials 2019, 12, 3659. https://doi.org/10.3390/ma12223659
Kaseem M, Hamad K, Ur Rehman Z. Review of Recent Advances in Polylactic Acid/TiO2 Composites. Materials. 2019; 12(22):3659. https://doi.org/10.3390/ma12223659
Chicago/Turabian StyleKaseem, Mosab, Kotiba Hamad, and Zeeshan Ur Rehman. 2019. "Review of Recent Advances in Polylactic Acid/TiO2 Composites" Materials 12, no. 22: 3659. https://doi.org/10.3390/ma12223659
APA StyleKaseem, M., Hamad, K., & Ur Rehman, Z. (2019). Review of Recent Advances in Polylactic Acid/TiO2 Composites. Materials, 12(22), 3659. https://doi.org/10.3390/ma12223659






