VXC-72R/ZrO2/GCE-Based Electrochemical Sensor for the High-Sensitivity Detection of Methyl Parathion
Abstract
:1. Introduction
2. Materials and Methods
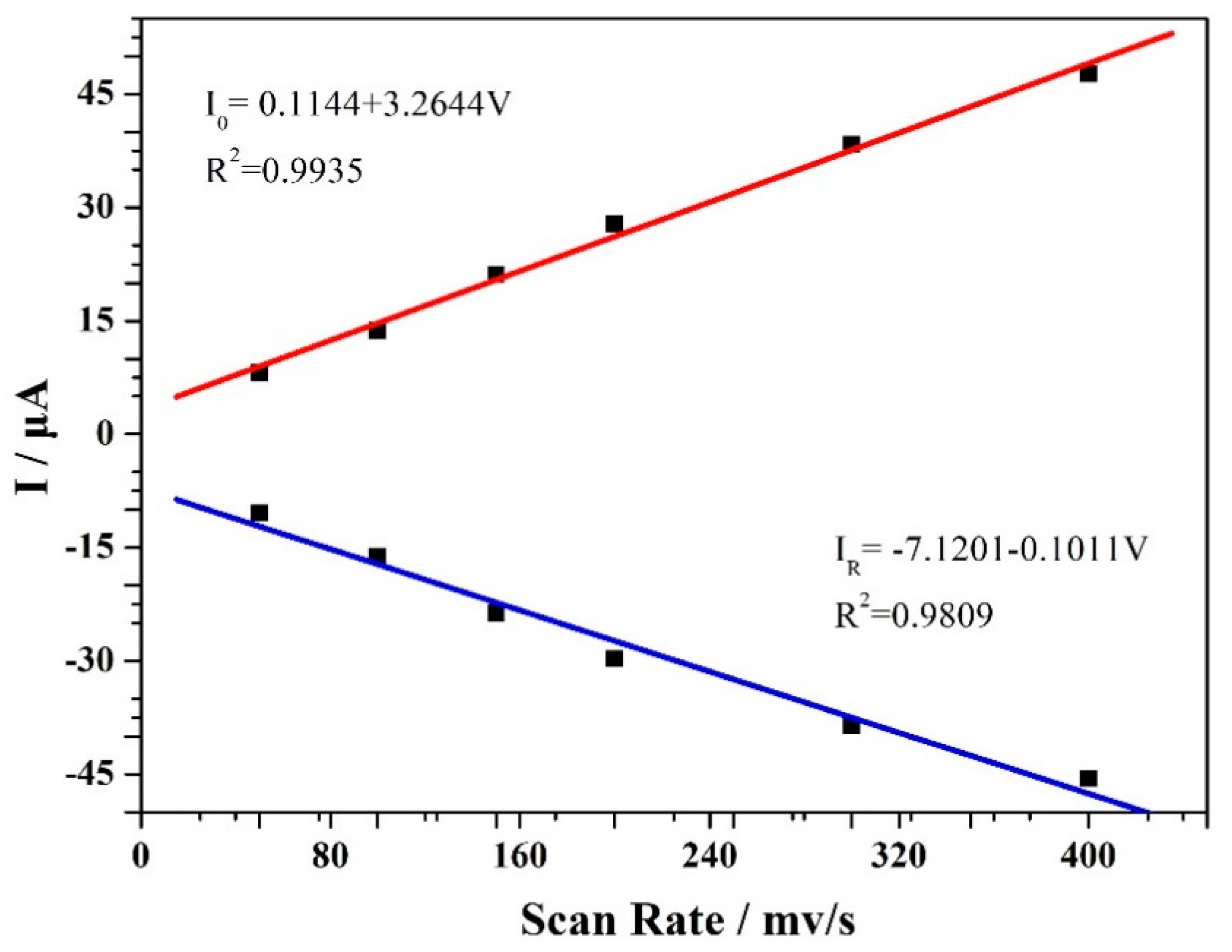
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akhtar, M.; Iqbal, S.; Bhanger, M.I.; Zia-Ul-Haq, M.; Moazzam, M. Sorption of organophosphorous pesticides onto chickpea husk from aqueous solutions. Coll. Surf. B Biointerfaces 2009, 69, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Muttray, A.; Spelmeyer, U.; Degirmenci, M.; Jung, D.; Backer, G.; Hill, G. Acute effects of low doses of methyl parathion on human EEG. Environ. Toxicol. Phar. 2005, 19, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Madhusudana Reddy, T.; Kumara Swamy, B.E.; Chandrashekar, B.N.; Reddaiah, K.; Sreedhar, M. Development of AChE biosensor for the determination of methyl parathion and monocrotophos in water and fruit samples: A cyclic voltammetric study. J. Electroanal. Chem. 2012, 665, 76–82. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.M.; Chen, T.W.; Sundramoorthy, A.K. Methyl parathion detection in vegetables and fruits using silver@graphene nanoribbons nanocomposite modified screen printed electrode. Sci. Rep. UK 2017, 7, 46471. [Google Scholar] [CrossRef]
- Sbaï, M.; Essis-Tome, H.; Gombert, U.; Breton, T.; Pontié, M. Electrochemical stripping analysis of methyl-parathion (MPT) using carbon fiber microelectrodes (CFME) modified with combinations of poly-NiTSPc and Nafion® films. Sensor. Actuat. B Chem. 2007, 124, 368–375. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, D.; Yu, Y.; Zhou, T.; Shi, G. Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes-poly(acrylamide) nanocomposite film modified electrode. J. Hazard. Mater. 2012, 217, 315–322. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Li, D.; Dong, L.; Li, B.; Liu, B.; Ma, H.; Li, F.; Yin, X.; Chen, X. A Simple, Low-Cost and Efficient β-CD/MWCNTs/CP-based Electrochemical Sensor for the Rapid and Sensitive Detection of Methyl Parathion. Int. J. Electrochem. Sci. 2019, 14, 9785–9795. [Google Scholar] [CrossRef]
- Gannavarapu, K.P.; Ganesh, V.; Thakkar, M.; Mitra, S.; Dandamudi, R.B. Nanostructured Diatom-ZrO2 composite as a selective and highly sensitive enzyme free electrochemical sensor for detection of methyl parathion. Sensor. Actuat. B Chem. 2019, 288, 611–617. [Google Scholar] [CrossRef]
- Kumaravel, A.; Chandrasekaran, M. A novel nanosilver/nafion composite electrode for electrochemical sensing of methyl parathion and parathion. J. Electroanal. Chem. 2010, 638, 231–235. [Google Scholar] [CrossRef]
- Parham, H.; Rahbar, N. Square wave voltammetric determination of methyl parathion using ZrO2-nanoparticles modified carbon paste electrode. J. Hazard. Mater. 2010, 177, 1077–1084. [Google Scholar] [CrossRef]
- Shulga, O.; Kirchhoff, J.R. An acetylcholinesterase enzyme electrode stabilized by an electrodeposited gold nanoparticle layer. Electrochem. Commun. 2007, 9, 935–940. [Google Scholar] [CrossRef]
- Yue, X.; Pang, S.; Han, P.; Zhang, C.; Wang, J.; Zhang, L. Carbon nanotubes/carbon paper composite electrode for sensitive detection of catechol in the presence of hydroquinone. Electrochem. Commun. 2013, 34, 356–359. [Google Scholar] [CrossRef]
- Liang, H.; Miao, X.; Gong, J. One-step fabrication of layered double hydroxides/graphene hybrid as solid-phase extraction for stripping voltammetric detection of methyl parathion. Electrochem. Commun. 2012, 20, 149–152. [Google Scholar] [CrossRef]
- Gong, J.; Miao, X.; Wan, H.; Song, D. Facile synthesis of zirconia nanoparticles-decorated graphene hybrid nanosheets for an enzymeless methyl parathion sensor. Sensor. Actuat. B Chem. 2012, 162, 341–347. [Google Scholar] [CrossRef]
- Gao, N.; He, C.; Ma, M.; Cai, Z.; Zhou, Y.; Chang, G.; Wang, X.; He, Y. Electrochemical co-deposition synthesis of Au-ZrO2-graphene nanocomposite for a nonenzymatic methyl parathion sensor. Anal. Chim. Acta 2019, 1072, 25–34. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, W.; Li, T.; Yue, X.; Liu, T.; Zhang, W.; Yu, S.; Zhang, D.; Wang, J. Facile green synthesis of graphene-Au nanorod nanoassembly for on-line extraction and sensitive stripping analysis of methyl parathion. Electrochim. Acta 2014, 146, 419–428. [Google Scholar] [CrossRef]
- Wu, S.; Lan, X.; Cui, L.; Zhang, L.; Tao, S.; Wang, H.; Han, M.; Liu, Z.; Meng, C. Application of graphene for preconcentration and highly sensitive stripping voltammetric analysis of organophosphate pesticide. Anal. Chim. Acta 2011, 699, 170–176. [Google Scholar] [CrossRef]
- Du, D.; Chen, W.; Zhang, W.; Liu, D.; Li, H.; Lin, Y. Covalent coupling of organophosphorus hydrolase loaded quantum dots to carbon nanotube/Au nanocomposite for enhanced detection of methyl parathion. Biosens. Bioelectron. 2010, 25, 1370–1375. [Google Scholar] [CrossRef]
- Saleh Ahammad, A.J.; Lee, J.J.; Rahman, M.A. Electrochemical sensors based on carbon nanotubes. Sensors Basel 2009, 9, 2289–2319. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly sensitive electrochemical stripping analysis of methyl parathion at MWCNTs–CeO2–Au nanocomposite modified electrode. Sensor. Actuat. B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- De Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The use of activated biochar for development of a sensitive electrochemical sensor for determination of methyl parathion. J. Electroanal. Chem. 2017, 799, 602–608. [Google Scholar] [CrossRef]
- Bazuła, P.A.; Lu, A.-H.; Nitz, J.-J.; Schüth, F. Surface and pore structure modification of ordered mesoporous carbons via a chemical oxidation approach. Micropor. Mesopor. Mat. 2008, 108, 266–275. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z. Nano-composite ZrO2/Au film electrode for voltammetric detection of parathion. Sensor. Actuat. B Chem. 2008, 133, 607–612. [Google Scholar] [CrossRef]
- Wang, H.; Su, Y.; Kim, H.; Yong, D.; Wang, L.; Han, X. A Highly Efficient ZrO2Nanoparticle Based Electrochemical Sensor for the Detection of Organophosphorus Pesticides. Chin. J. Chem. 2015, 33, 1135–1139. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Yuan, C.; Zhang, F.; Ma, L.; Qin, D.; Shan, D.; Lu, X. A novel electrochemical sensor based on zirconia/ordered macroporous polyaniline for ultrasensitive detection of pesticides. Analyst 2015, 140, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhu, G.; Shang, X.; Zhu, T.; Yang, J.; Liu, J. Electrospun zirconia-embedded carbon nanofibre for high-sensitive determination of methyl parathion. Electrochem. Commun. 2017, 81, 14–17. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, Z.; Deng, C.; Li, Y.; Zhang, X.; Yang, P. Hydrophilic polydopamine-coated graphene for metal ion immobilization as a novel immobilized metal ion affinity chromatography platform for phosphoproteome analysis. Anal. Chem. 2013, 85, 8483–8487. [Google Scholar] [CrossRef]
- Du, D.; Liu, J.; Zhang, X.; Cui, X.; Lin, Y. One-step electrochemical deposition of a graphene-ZrO2 nanocomposite: Preparation, characterization and application for detection of organophosphorus agents. J. Mater. Chem. 2011, 21, 8032. [Google Scholar] [CrossRef]
- ReddyPrasad, P.; Naidoo, E.B.; Sreedhar, N.Y. Electrochemical preparation of a novel type of C-dots/ZrO2 nanocomposite onto glassy carbon electrode for detection of organophosphorus pesticide. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Thota, R.; Ganesh, V. Selective and sensitive electrochemical detection of methyl parathion using chemically modified overhead projector sheets as flexible electrodes. Sensor. Actuat. B Chem. 2016, 227, 169–177. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.-D.; Chen, C.-H.; Yu, Y.-X. Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim. Acta 2010, 171, 57–62. [Google Scholar] [CrossRef]
- Kang, T.-F.; Wang, F.; Lu, L.-P.; Zhang, Y.; Liu, T.-S. Methyl parathion sensors based on gold nanoparticles and Nafion film modified glassy carbon electrodes. Sensor. Actuat. B Chem. 2010, 145, 104–109. [Google Scholar] [CrossRef]
- Pan, D.; Ma, S.; Bo, X.; Guo, L. Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon. Microchim. Acta 2011, 173, 215–221. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Shen, C.; Cheong, L.Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@MOF nanofibers-based biosensor. Talanta 2019, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
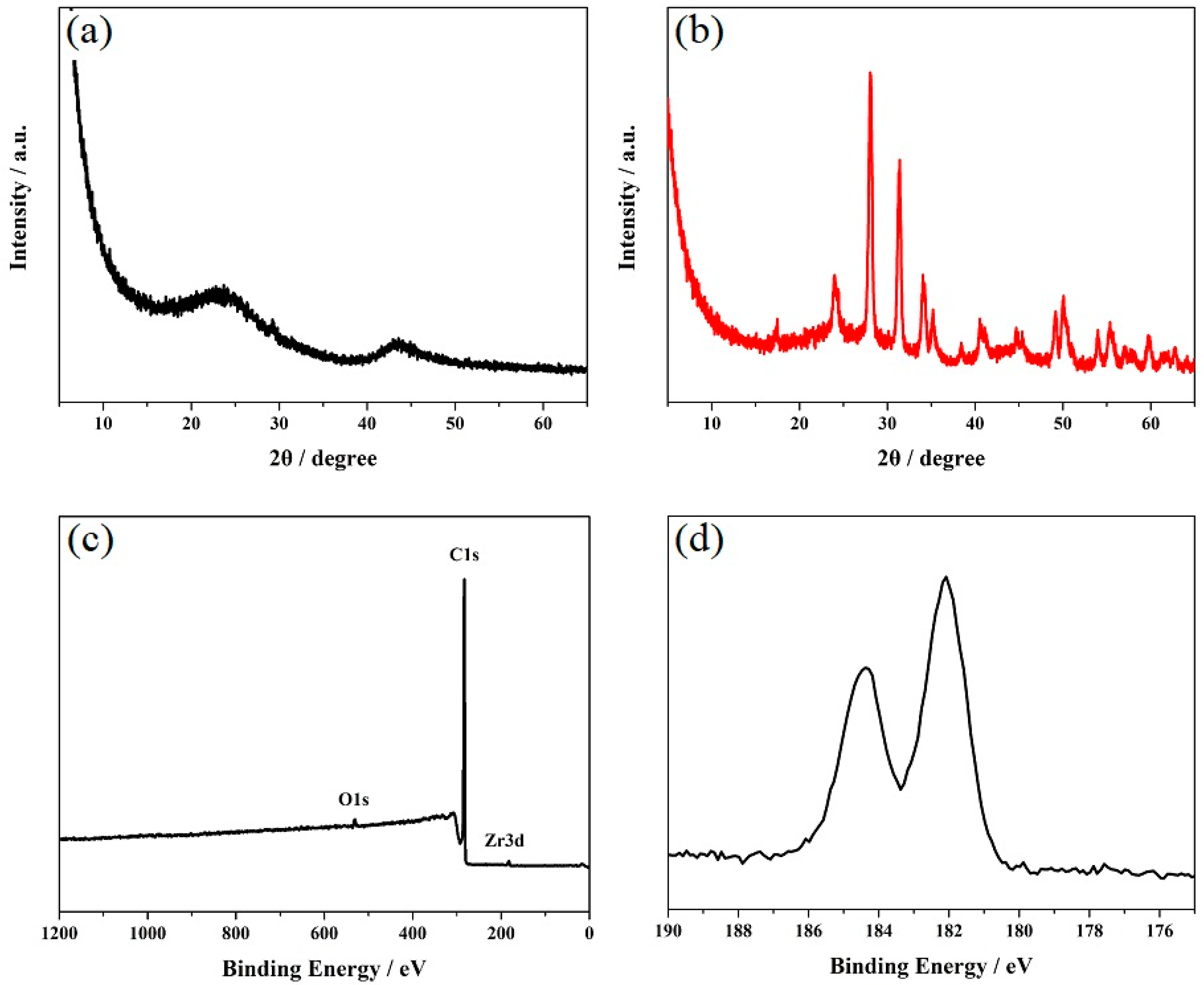
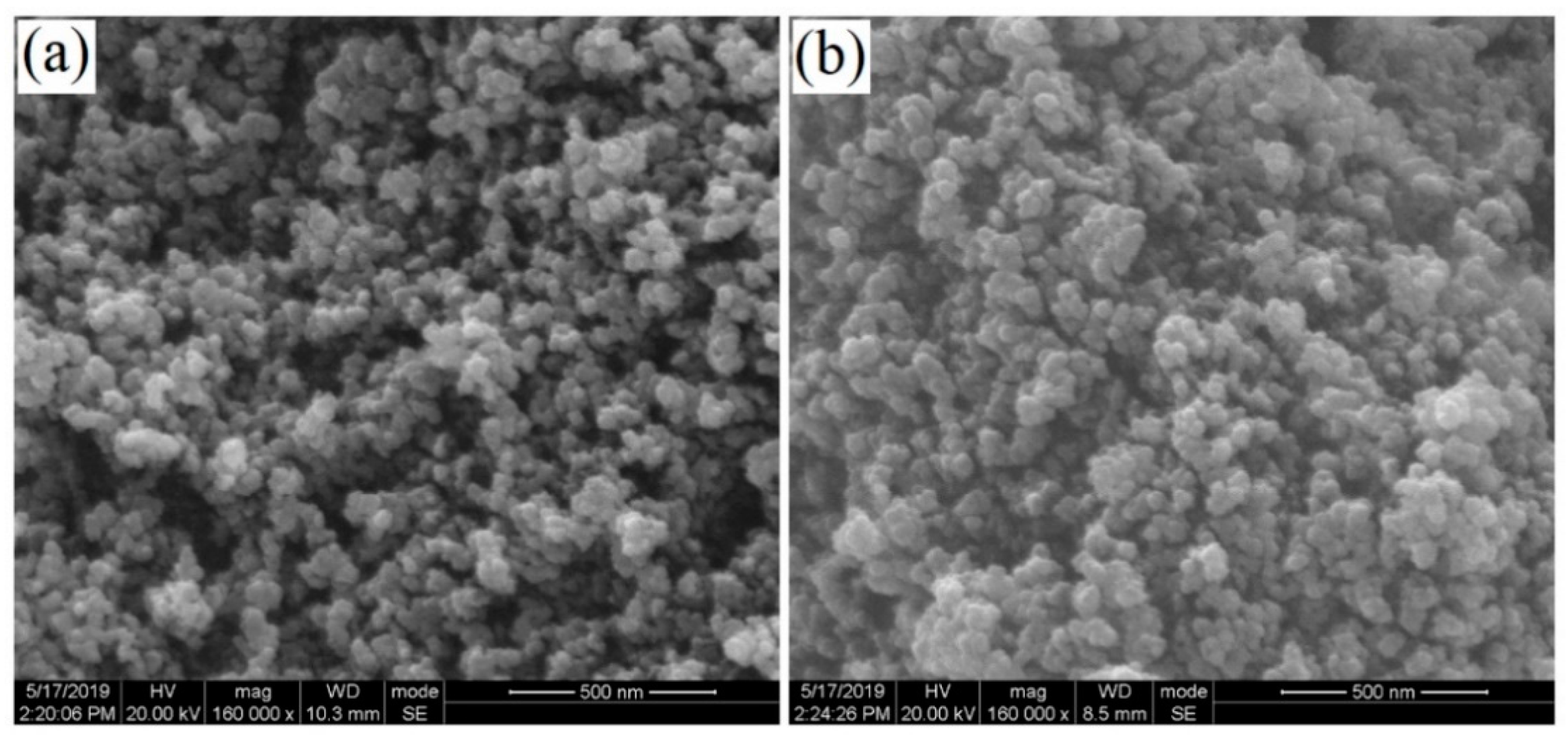


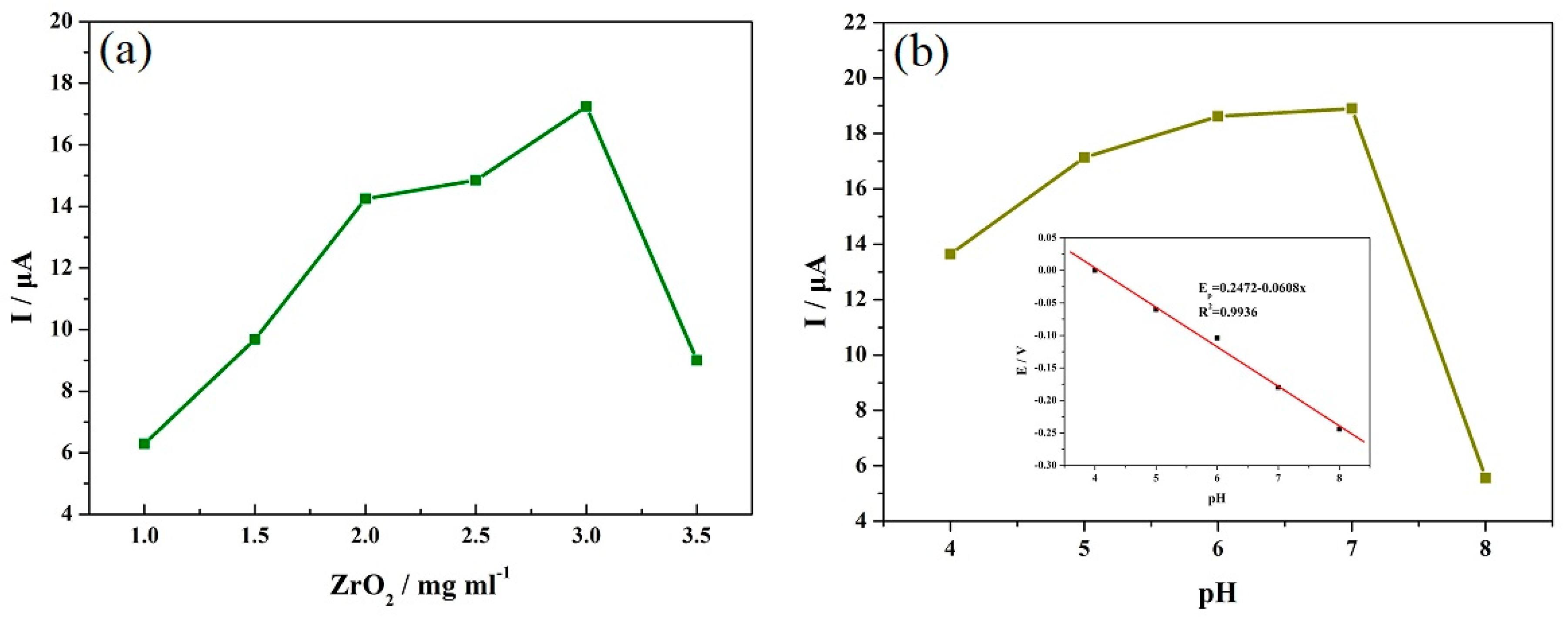
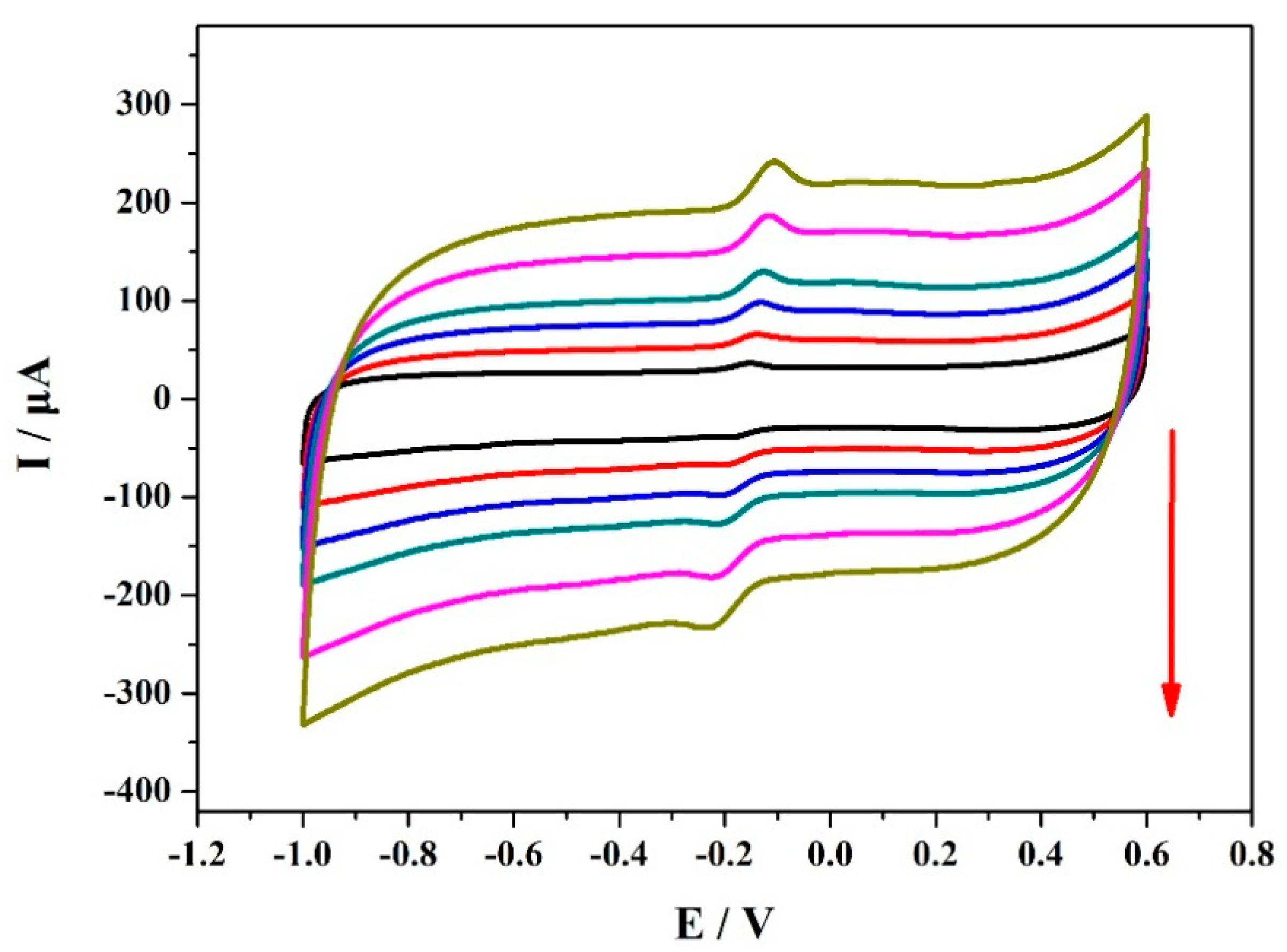


| Electrode | Analytical Method | Detection Limit (μM) | Linear Range (μM) | Reference |
|---|---|---|---|---|
| CPME–AB | DPAdSV | 3.9 × 104 | 0.1–70 | [21] |
| AuNPs/Nafion/GCE | SWV | 0.1 | 0.5–120 | [32] |
| OMC/GCE | LSV | 7.6 × 103 | 0.09–61 | [33] |
| Pd/MWCNTs | DPV | 0.19 | 0.38–53.2 | [31] |
| BCL@MOF/nanofibers/chitosan/GCE | DPV | 0.067 | 0.1–38 | [34] |
| ZrO2 NPs–GNs | SWV | 2.28 × 10−3 | 0.002–0.9 | [14] |
| ZrO2–Au nanocomposite | SWV | 0.011 | 0.02–0.140 | [23] |
| ZrO2–CNFs | DPV | 1.29 × 10−3 | 1 × 10-3–2 × 10−2 | [26] |
| VXC-72R/ZrO2/GCE | DPV | 0.053 | 1–100 | This work |
| Sample | MP Added (μM) | MP Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| River water 1 | 3.0 | 2.93 | 97.68 | 3.3 |
| River water 2 | 10 | 9.00 | 90.00 | 0.6 |
| River water 3 | 100 | 95.63 | 95.63 | 3.9 |
| Tap water 1 | 3.0 | 2.93 | 97.74 | 2.5 |
| Tap water 2 | 10 | 10.03 | 100.3 | 7.3 |
| Tap water 3 | 100 | 98.77 | 98.77 | 4.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Wang, Y.; Li, B.; Liu, B.; Ma, H.; Li, D.; Dong, L.; Li, F.; Chen, X.; Yin, X. VXC-72R/ZrO2/GCE-Based Electrochemical Sensor for the High-Sensitivity Detection of Methyl Parathion. Materials 2019, 12, 3637. https://doi.org/10.3390/ma12213637
Liu R, Wang Y, Li B, Liu B, Ma H, Li D, Dong L, Li F, Chen X, Yin X. VXC-72R/ZrO2/GCE-Based Electrochemical Sensor for the High-Sensitivity Detection of Methyl Parathion. Materials. 2019; 12(21):3637. https://doi.org/10.3390/ma12213637
Chicago/Turabian StyleLiu, Runqiang, Yashuang Wang, Bo Li, Binbin Liu, Huina Ma, Dongdong Li, Li Dong, Fang Li, Xiling Chen, and Xinming Yin. 2019. "VXC-72R/ZrO2/GCE-Based Electrochemical Sensor for the High-Sensitivity Detection of Methyl Parathion" Materials 12, no. 21: 3637. https://doi.org/10.3390/ma12213637
APA StyleLiu, R., Wang, Y., Li, B., Liu, B., Ma, H., Li, D., Dong, L., Li, F., Chen, X., & Yin, X. (2019). VXC-72R/ZrO2/GCE-Based Electrochemical Sensor for the High-Sensitivity Detection of Methyl Parathion. Materials, 12(21), 3637. https://doi.org/10.3390/ma12213637




