Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties
Abstract
1. Introduction
2. Materials and Methods
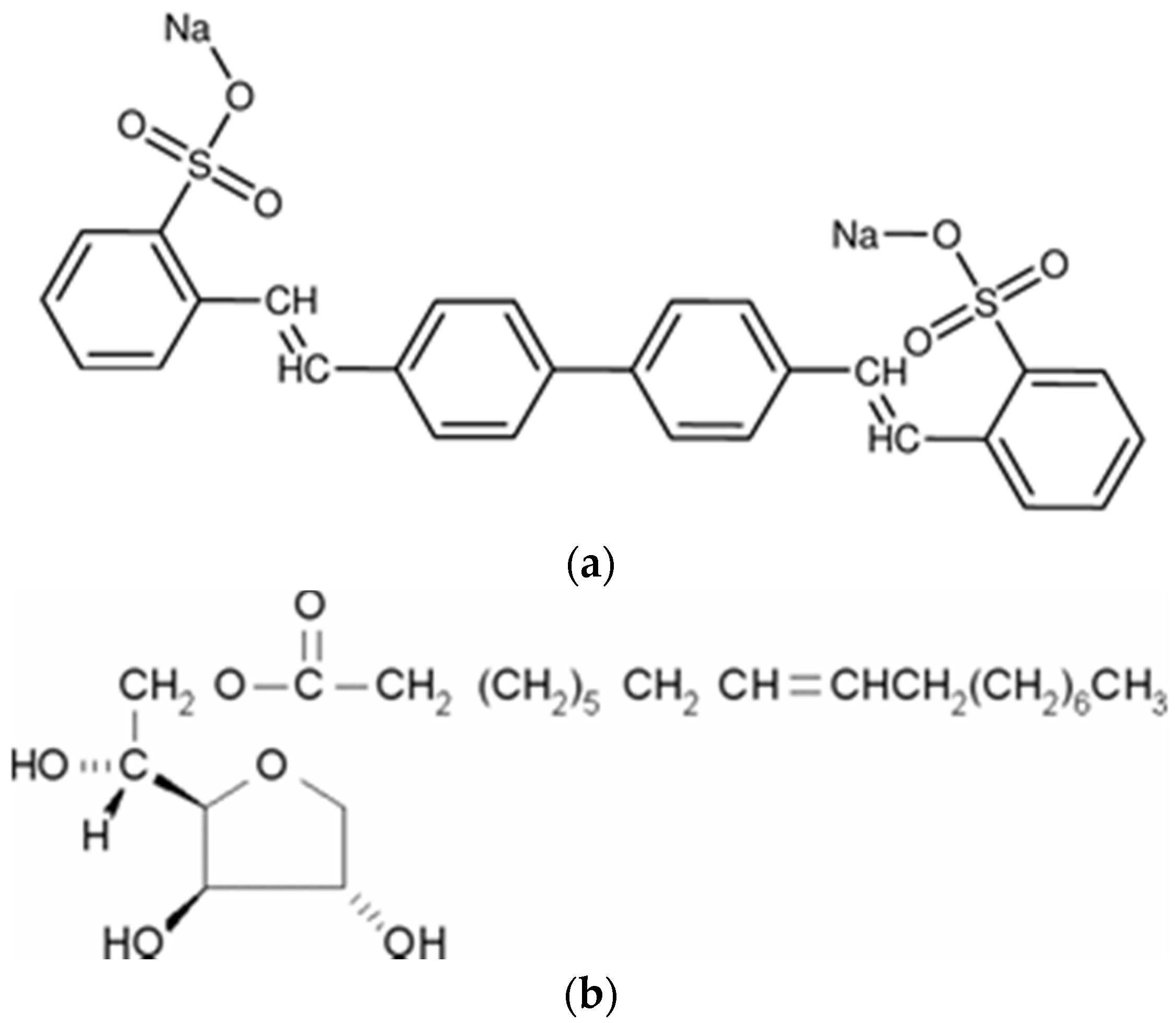
2.1. Materials
2.2. Ion Exchange Reaction
2.3. Preparation of Films
2.4. Characterization Techniques
3. Results
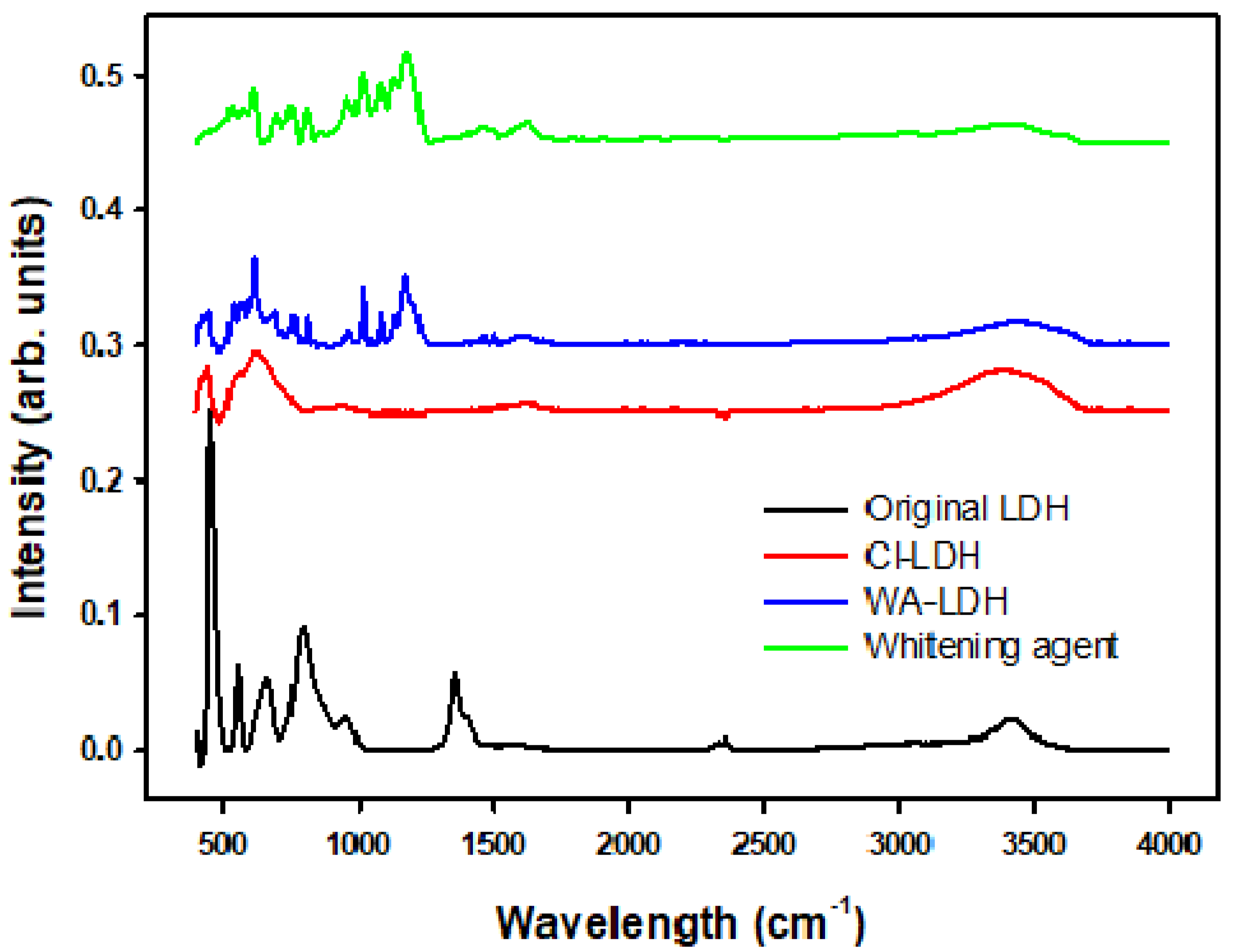
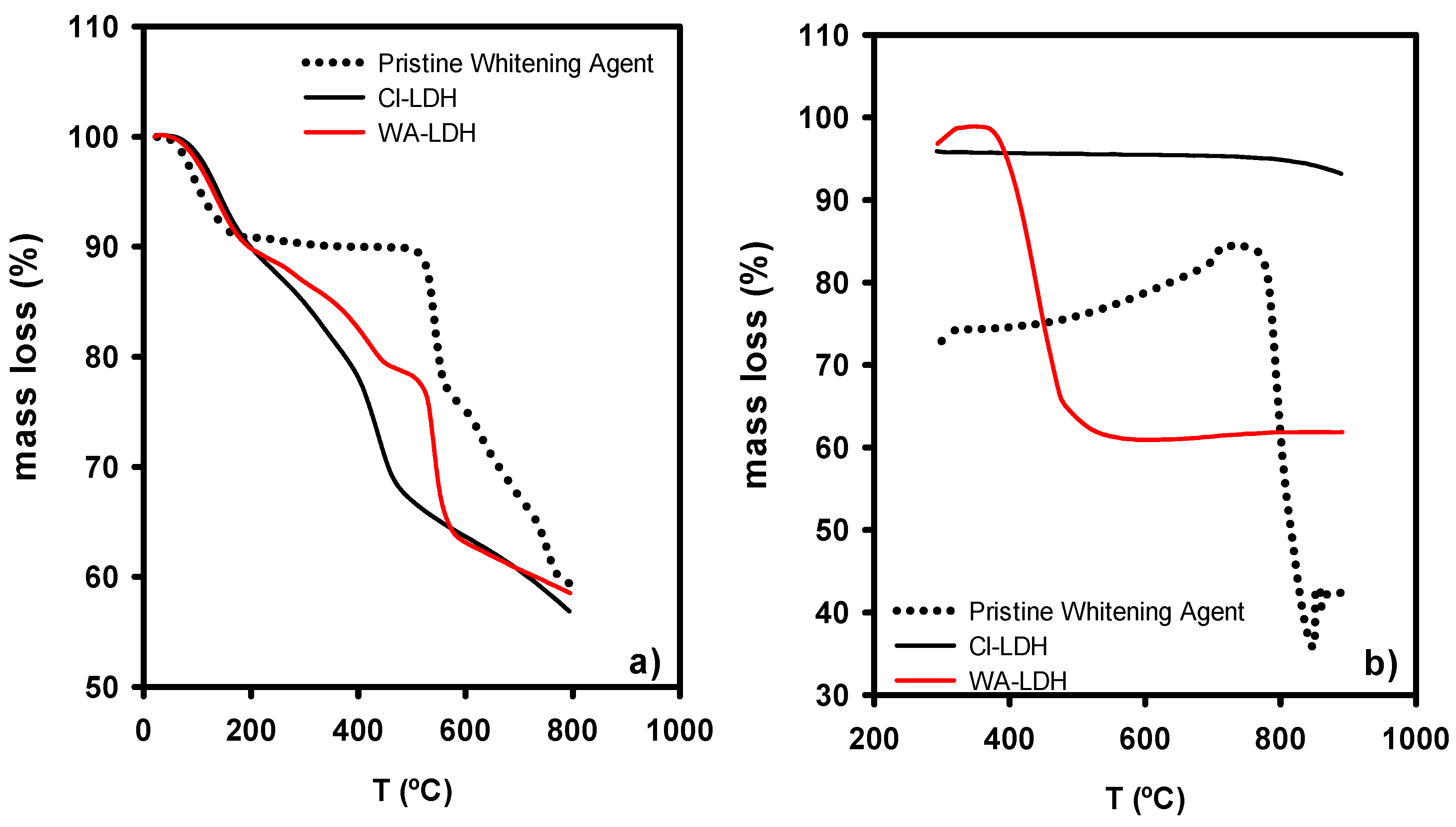
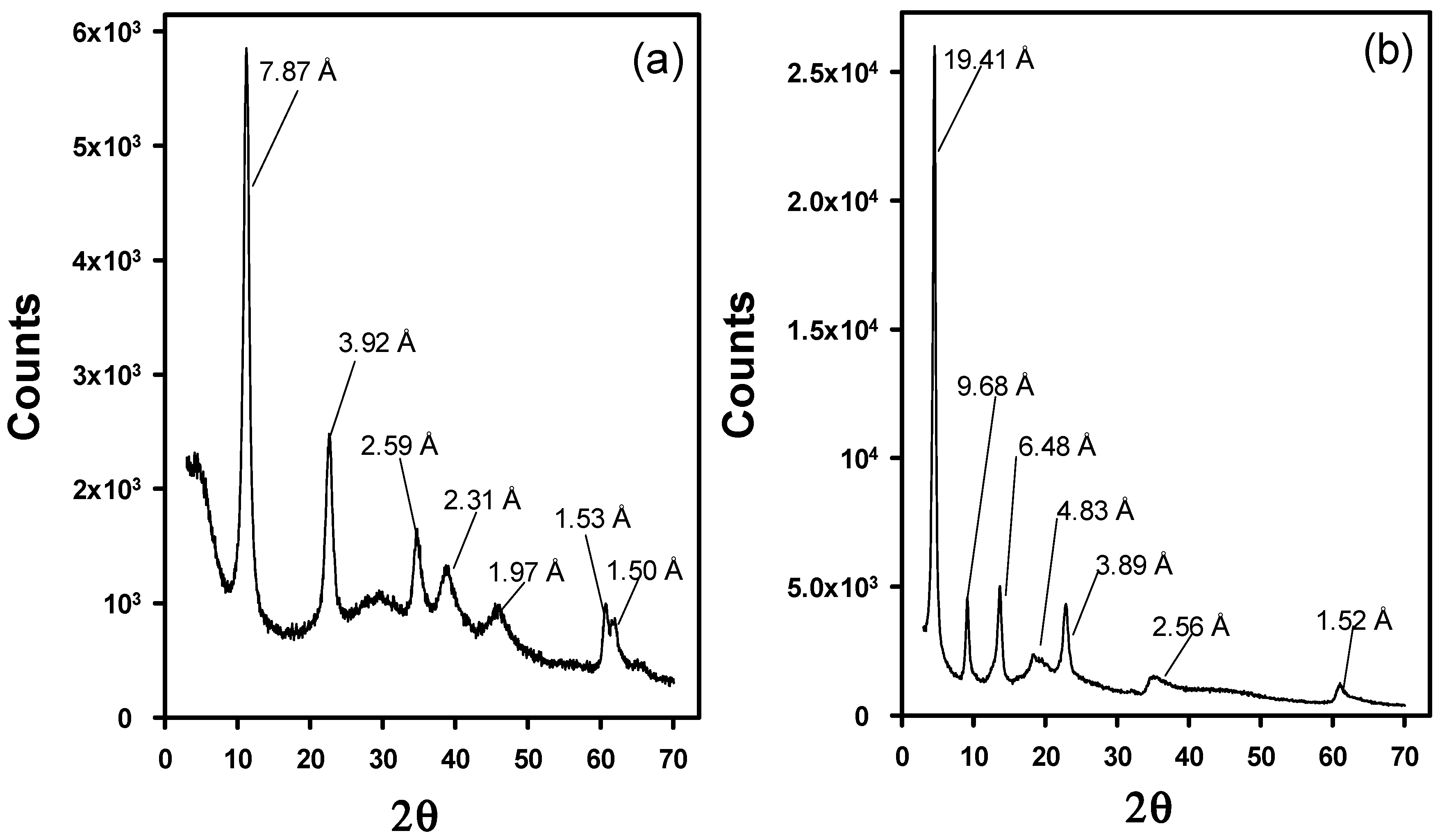
3.1. Characterization of the Modified LDHs with WA
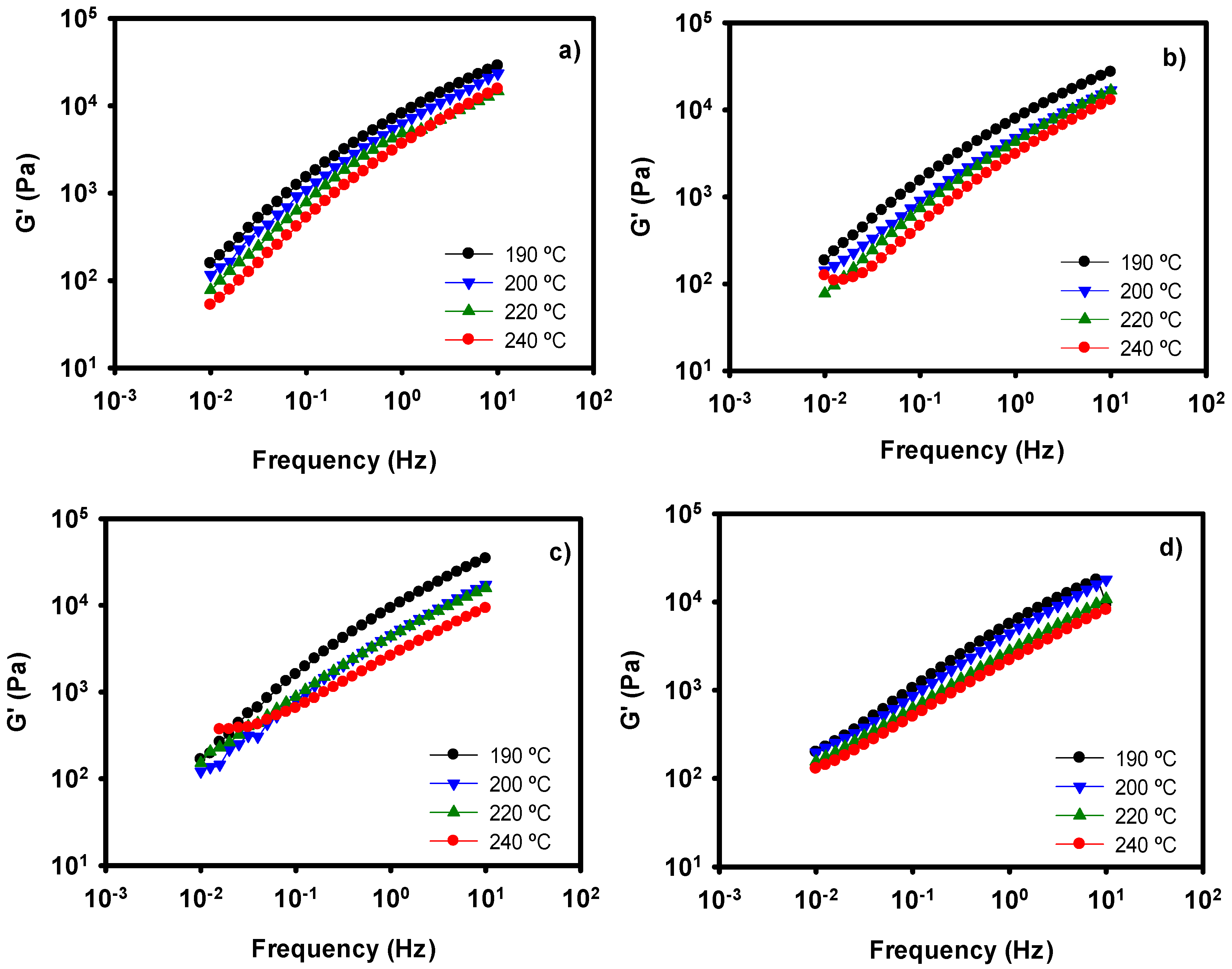
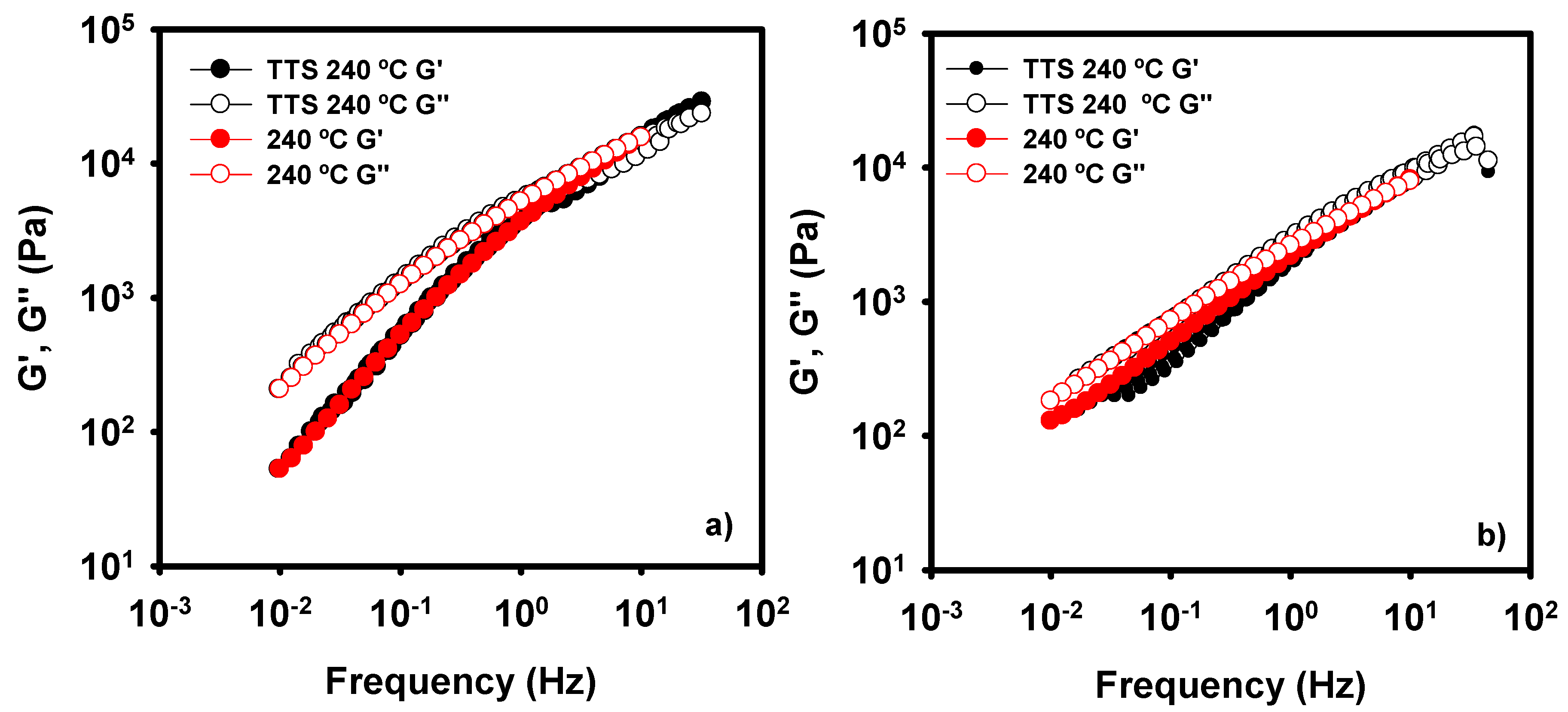
3.2. Rheology of LDH-Polyolefin Composites
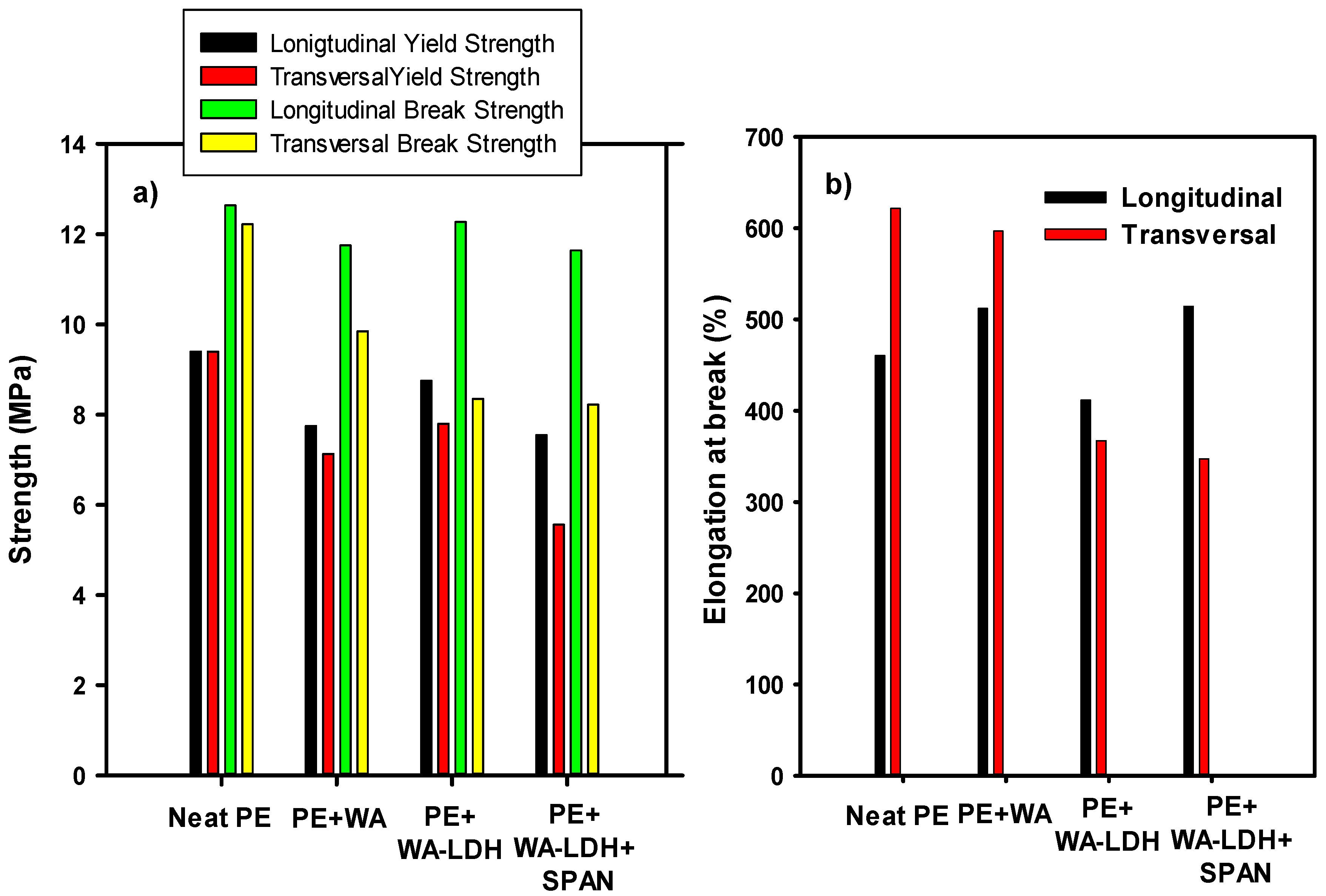
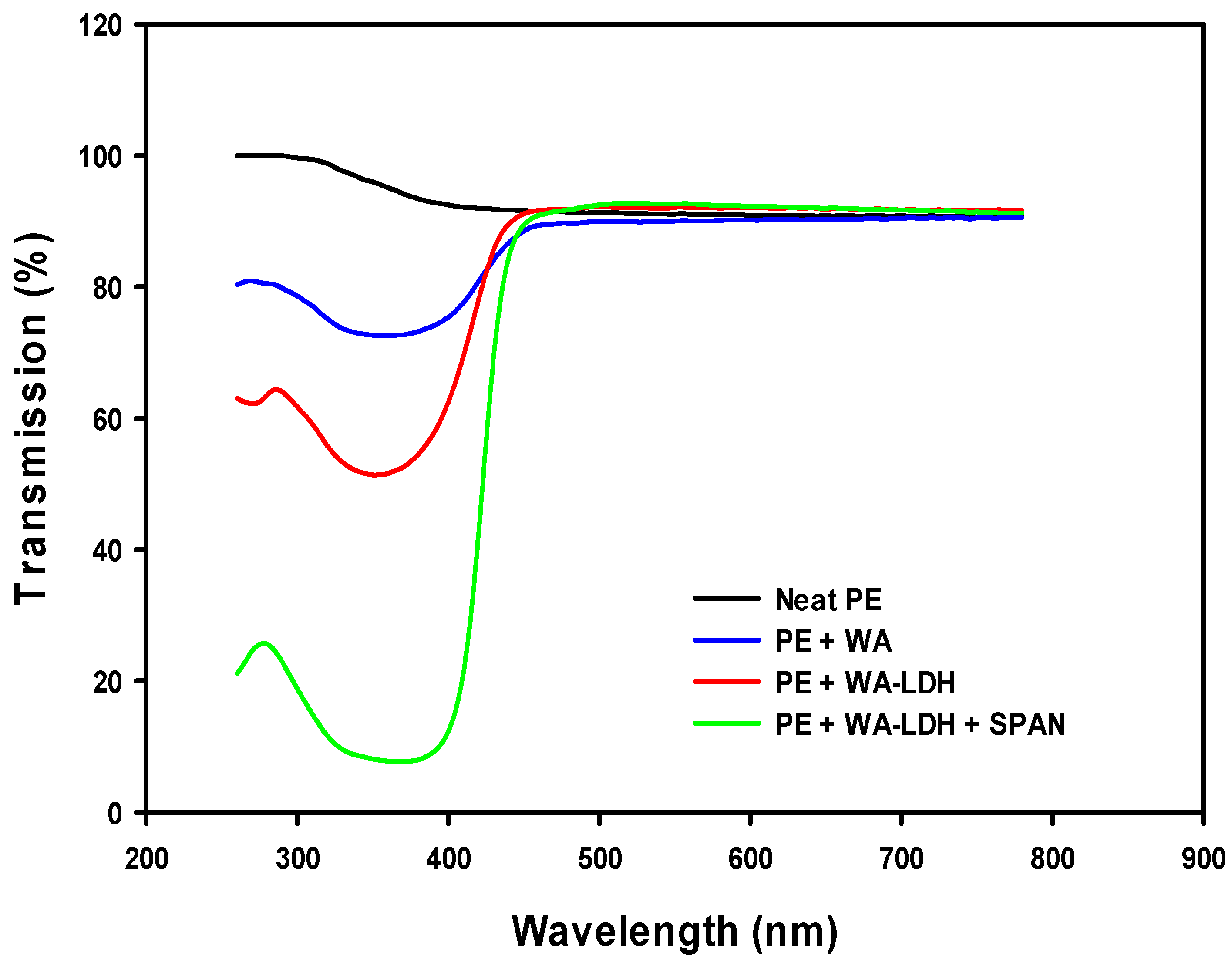
3.3. Mechanical and Optical Properties of the Composites
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
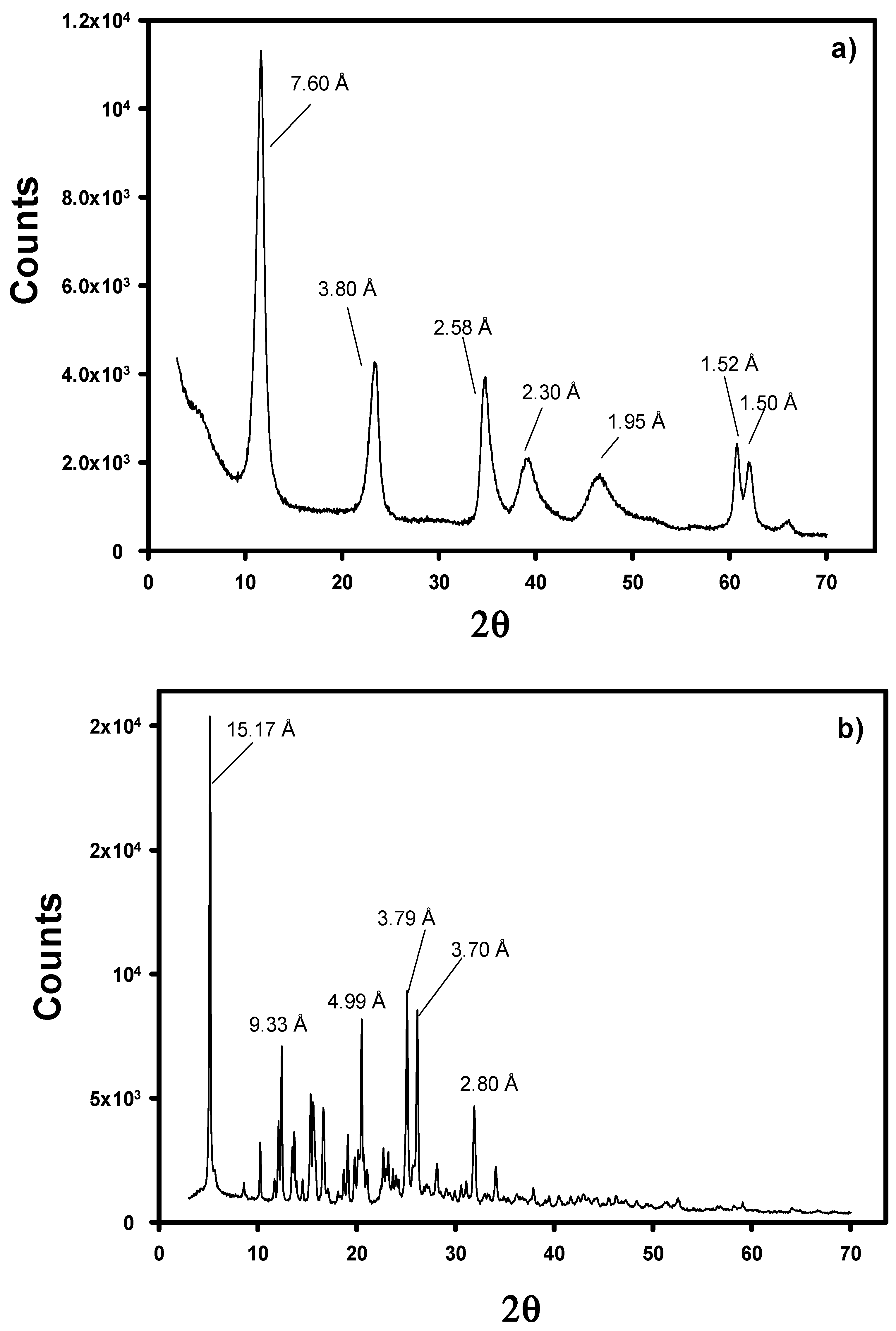
Appendix B

References
- Bartolomeo, P.; Irigoyen, M.; Aragon, E.; Frizzi, M.A.; Perrin, F.X. Dynamic mechanical analysis and Vickers micro hardness correlation for polymer coating UV ageing characterization. Polym. Degrad. Stabil. 2001, 72, 63–68. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.; Torikai, A. Effects of solar UV and climate change on materials. Photochem. Photobiol. Sci. 2011, 10, 292–300. [Google Scholar] [CrossRef]
- Gabrielle, B.; Lorthioir, C.; Laupretre, F. Thermal Aging of Interfacial Polymer Chains in Ethylene-Propylene-Diene Terpolymer/Aluminum Hydroxide Composites: Solid-State NMR Study. J. Phys. Chem. B 2011, 115, 12392–12400. [Google Scholar] [CrossRef] [PubMed]
- Poli, T.; Toniolo, L.; Sansonetti, A. Durability of Protective Polymers: The Effect of UV and Thermal Ageing. Macromol. Symp. 2006, 238, 78–83. [Google Scholar] [CrossRef]
- Bailey, D.; Vogl, O. Polymeric ultraviolet absorbers. J. Macromol. Sci. 1976, 14, 267–293. [Google Scholar] [CrossRef]
- Wang, G.; Xu, S.; Xia, C.; Yan, D.; Lin, Y.; Weia, M. Fabrication of Host–Guest UV-Blocking Materials by Intercalation of Fluorescent Anions into Layered Double Hydroxides. RSC Adv. 2015, 5, 23708–23714. [Google Scholar] [CrossRef]
- Jones, W.; Chidwe, M. Pillared Layered Structures—Current Trends and Applications; Mithecll, I.V., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; p. 67. [Google Scholar]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers: Hauppage, NY, USA, 2001. [Google Scholar]
- Ma, S.; Fan, C.; Du, L.; Huang, G.; Yang, X.; Tang, W.; Makita, Y.; Ooi, K. Synthesis, Anion Exchange, and Delamination of Co−Al Layered Double Hydroxide: Assembly of the Exfoliated Nanosheet/Polyanion Composite Films and Magneto-Optical Studies. Chem. Mater. 2009, 21, 3602–3610. [Google Scholar] [CrossRef]
- Chen, T.; Tang, P.; Feng, Y.; Li, D. Facile Color Tuning, Characterization, and Application of Acid Green 25 and Acid Yellow 25 Co-intercalated Layered Double Hydroxides. Ind. Eng. Chem. Res. 2017, 56, 5495–5504. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Petersen, P.A.D.; Gonçalves, M.B.; Petrilli, H.M.; Taviot-Gueho, C.; Leroux, F.; Temperini, M.L.A.; Constantino, V.R.L. Structural, Spectroscopic (NMR, IR, and Raman), and DFT Investigation of the Self-Assembled Nanostructure of Pravastatin-LDH (Layered Double Hydroxides) Systems. Chem. Mater. 2012, 24, 1415–1425. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiró, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and application. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Miyata, S.; Kumura, T. Synthesis of new hydrotalcite-like compounds and their physico-chemical properties. Chem. Lett. 1973, 2, 843–848. [Google Scholar] [CrossRef]
- Meyn, M.; Beneke, K.; Lagaly, G. Anion-Exchange Reactions of Layered Double Hydroxides. Inorg. Chem. 1990, 29, 5201–5207. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Chao, G.Y.; Gault, R. Quintinite-2H, quintinite-3T, charmarite-2H, charmarite-3T and caresite-3T, a new group of carbonate minerals related to the hydrotalcite—Manasseite group. Can. Mineral. 1997, 35, 1541–1549. [Google Scholar]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.; Greenwell, H.C. Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4. Miner. Mag. 2019, 83, 269–280. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Comparative study of Mg/M(III) (M = Al, Ga, In) layered double hydroxides obtained by co-precipitation and the sol-gel method. Solid State Chem. 2002, 168, 156–161. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Ghanaym, E.E.; Sadek, H.E.H.; El Defrawy, S.A.; Ali, O.A.M. Synthesis, characterization and sinterability of pure and Ni-doped nano layered double hydroxides from aluminum dross. Ceram. Int. 2019, 45, 17598–17610. [Google Scholar] [CrossRef]
- Li, J.; Fan, Q.; Wu, Y.; Wang, X.; Chen, C.; Tang, Z.; Wang, X. Magnetic polydopamine decorated with Mg–Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes. J. Mater. Chem. A 2016, 4, 1737–1746. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Yan, D.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. A luminescent ultrathin film with reversible sensing toward pressure. Chem. Commun. 2016, 52, 4663–4666. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, P.; Matusik, J.; Leiviskä, T. Mg/Al LDH Enhances Sulfate removal and Clarification of AMD Wastewater in Precipitation Processes. Materials 2019, 14, 2334. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Jing, M.; Hou, H.; Banks, C.E.; Yang, Y.; Zhang, Y.; Ji, X. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M. Applications of layered double hydroxides based electrochemical sensors for determination of environmental pollutants: A review. Trends Environ. Anal. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Guan, S.; Liang, R.; Li, C.; Yan, D.; Wei, M.; Evans, D.G.; Duan, X. A layered drug nanovehicle toward targeted cancer imaging and therapy. J. Mater. Chem. B 2016, 4, 1331–1336. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Azeem, M.; Wang, Z.Y.; Ashraf, G.; Xiao, F.; Chen, X.D.; Liu, H.F. A review on electrochemical biosensing platform based on layered double hydroxides for small molecule biomarkers determination. Adv. Colloid Int. Sci. 2018, 262, 21–38. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z.P. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials 2014, 35, 3331–3339. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, Y.; Huang, Q.; Chen, S.; Zhang, F.; Tang, P.; Li, D. High antioxidative performance of layered double hydroxides/polypropylene composite with intercalation of low-molecular-weight phenolic antioxidant. Ind. Eng. Chem. Res. 2014, 53, 2287–2292. [Google Scholar] [CrossRef]
- Yan, L.; Gonca, S.; Zhu, G.Y.; Zhang, W.J.; Chen, X.F. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. J. Mater. Chem. B 2019, 7, 5583–5601. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qu, B. Structural Characteristics and Thermal Properties of PE-g-MA/MgAl-LDH Exfoliation Nanocomposites Synthesized by Solution Intercalation. Chem. Mater. 2003, 15, 3208–3213. [Google Scholar] [CrossRef]
- Costa, F.R.; Abdel-Goad, M.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on polyethylene and Mg–Al layered double hydroxide. I. Synthesis and characterization. Polymer 2005, 45, 4447–4453. [Google Scholar] [CrossRef]
- Jia, L.; Ma, J.Z.; Gao, D.G.; Lv, B. Layered Double Hydroxides/Polymer Nanocomposites. Prog. Chem. 2018, 30, 295–303. [Google Scholar]
- Costa, F.R.; Wagenknecht, U.; Jehnichen, D.; Abdel-Goad, M.; Heinrich, G. Nanocomposites based on polyethylene and Mg–Al layered double hydroxide. Part II. Rheological characterization. Polymer 2006, 47, 1649–1660. [Google Scholar] [CrossRef]
- Iyi, N.; Matsumoto, T.; Kaneko, Y.; Kitamura, K. Deintercalation of Carbonate Ions from a Hydrotalcite-Like Compound: Enhanced Decarbonation Using Acid-Salt Mixed Solution. Chem. Mater. 2004, 16, 2926–2932. [Google Scholar] [CrossRef]
- ISO: Geneva, Switzerland. EN ISO 527-1:2012. Plastic-Determination of Thensile Properties—Part 1: General Principles. 2012. Available online: https://www.iso.org/standard/56045.html (accessed on 4 October 2019).
- ISO: Geneva, Switzerland. EN ISO 527-3:1996. Plastics-Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. 1996. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0013232 (accessed on 4 October 2019).
- Jobbágy, M.; Regazzoni, A.E. Dissolution of nano-size Mg–Al–Cl hydrotalcite in aqueous media. Appl. Clay Sci. 2011, 51, 366–369. [Google Scholar] [CrossRef]
- Theiss, F.; López, A.; Frost, R.L.; Scholz, R. Spectroscopic characterisation of the LDH mineral quintinite Mg4Al2(OH)12CO3·3H2O. Spectrochim. Acta A 2015, 50, 758–764. [Google Scholar] [CrossRef]
- Toyozo, U.; Akio, K.; Yutaka, S.; Katsunosuke, M. Normal Vibrations of Benzenesulfonate and Benzene-d5-sulfonate Ions. BCSJ 1975, 48, 2231–2235. [Google Scholar]
- Lin, Y.H.; Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T. Thermogravimetric analysis of hydrotalcites based on the takovite formula NixZn6-xAl2 (OH)16(CO3)·4H2O. J. Therm. Anal. Calorim. 2005, 81, 83–89. [Google Scholar] [CrossRef]
- Kloproggea, J.T.; Kristófb, J.; Frosta, R.L. Thermogravimetric analysis-mass spectrometry (TGA-MS) of hydrotalcites containing CO32−, NO3−, Cl−, SO42− or ClO4−. Clay Odyssey 2001, 1, 451. [Google Scholar]
- Crosby, S.; Tran, D.; Cocke, D.; Duraia, E.S.; Beall, G. Effect of isomorphous substitution on the thermal decomposition mechanism of hydrotalcites. Materials 2014, 7, 7048–7058. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, Anion Exchange, and Delamination of Co-Al Layered Double Hydroxide: Assembly of the Exfoliated Nanosheet/Polyanion Composite Films and Magneto-Optical Studies. JACS 2006, 128, 4872–4880. [Google Scholar] [CrossRef]
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int. J. Pharm 2015, 494, 623–642. [Google Scholar] [CrossRef]
- Aghjeh, M.D.; Mardani, E.; Rafiee, F.; Otadi, M.; Khonakdar, H.M.; Jafari, S.D. Analysis of dynamic oscillatory rheological properties of PP/EVA/organo-modified LDH ternary hybrids based on generalized Newtonian fluid and generalized linear viscoelastic approaches. Pol. Bull. 2017, 74, 465–482. [Google Scholar] [CrossRef]
- Coiai, S.; Passaglia, E.; Hermann, A.; Augier, S.; Pratelli, D.; Streller, R.C. The Influence of the Compatibilizer on the Morphology and Thermal Properties of Polypropylene-Layered Double Hydroxide Composites. Polym. Compos. 2010, 31, 744–754. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, Y.; Sun, S.; Zhao, G. Three-Dimensional Nonisothermal Simulation of Multi-Layer Extrusion Processes of Polymer Melts. Polym. Plast. Technol. 2006, 45, 1257–1262. [Google Scholar] [CrossRef]
- Zhu, H.; Feng, Y.; Tang, P.; Cui, G.; Evans, D.G.; Li, D.; Duan, X. Synthesis and UV Absorption Properties of Aurintricarboxylic Acid Intercalated Zn-Al Layered Double Hydroxides. Ind. Eng. Chem. Res. 2011, 50, 13299–13303. [Google Scholar] [CrossRef]

| Feeding | Zone 1 | Zone 2 | Zone 3 | Zone 4 | Die |
|---|---|---|---|---|---|
| 35 | 200 | 208 | 209 | 185 | 190 |
| Elements | Concentration (wt.%) | Concentration (mol/100 g) | ||
|---|---|---|---|---|
| Cl-LDH | WA-LDH | Cl-LDH | WA-LDH | |
| Na | 2.03 | 0.15 | 0.09 | 0.006 |
| Mg | 13.55 | 7.45 | 0.56 | 0.31 |
| Al | 14.63 | 6.80 | 0.54 | 0.25 |
| S | 0.058 | 5.88 | 0.002 | 0.18 |
| C | 0.34 | 30.80 | 0.03 | 2.57 |
| Cl | 14.73 | 0.024 | 0.41 | 0.0007 |
| Wavenumber (cm−1) | Band Assignment | ||||
|---|---|---|---|---|---|
| Original LDH | Quintinite [42] | Cl-LDH | WA-LDH | WA | |
| 1370 | 1350 | - | - | - | CO32− ν3 antisymmetric stretching |
| - | - | - | 1174 | 1180 | SO3− ν3 antisymmetric stretching |
| 3415 | 3388 | 3395 | 3467 | - | OH− stretching vibration |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monzó, F.; Caparrós, A.V.; Pérez-Pérez, D.; Arribas, A.; Pamies, R. Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties. Materials 2019, 12, 3580. https://doi.org/10.3390/ma12213580
Monzó F, Caparrós AV, Pérez-Pérez D, Arribas A, Pamies R. Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties. Materials. 2019; 12(21):3580. https://doi.org/10.3390/ma12213580
Chicago/Turabian StyleMonzó, Fuensanta, Ana Vanessa Caparrós, Diego Pérez-Pérez, Alejandro Arribas, and Ramón Pamies. 2019. "Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties" Materials 12, no. 21: 3580. https://doi.org/10.3390/ma12213580
APA StyleMonzó, F., Caparrós, A. V., Pérez-Pérez, D., Arribas, A., & Pamies, R. (2019). Synthesis and Characterization of New Layered Double Hydroxide-Polyolefin Film Nanocomposites with Special Optical Properties. Materials, 12(21), 3580. https://doi.org/10.3390/ma12213580






