Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
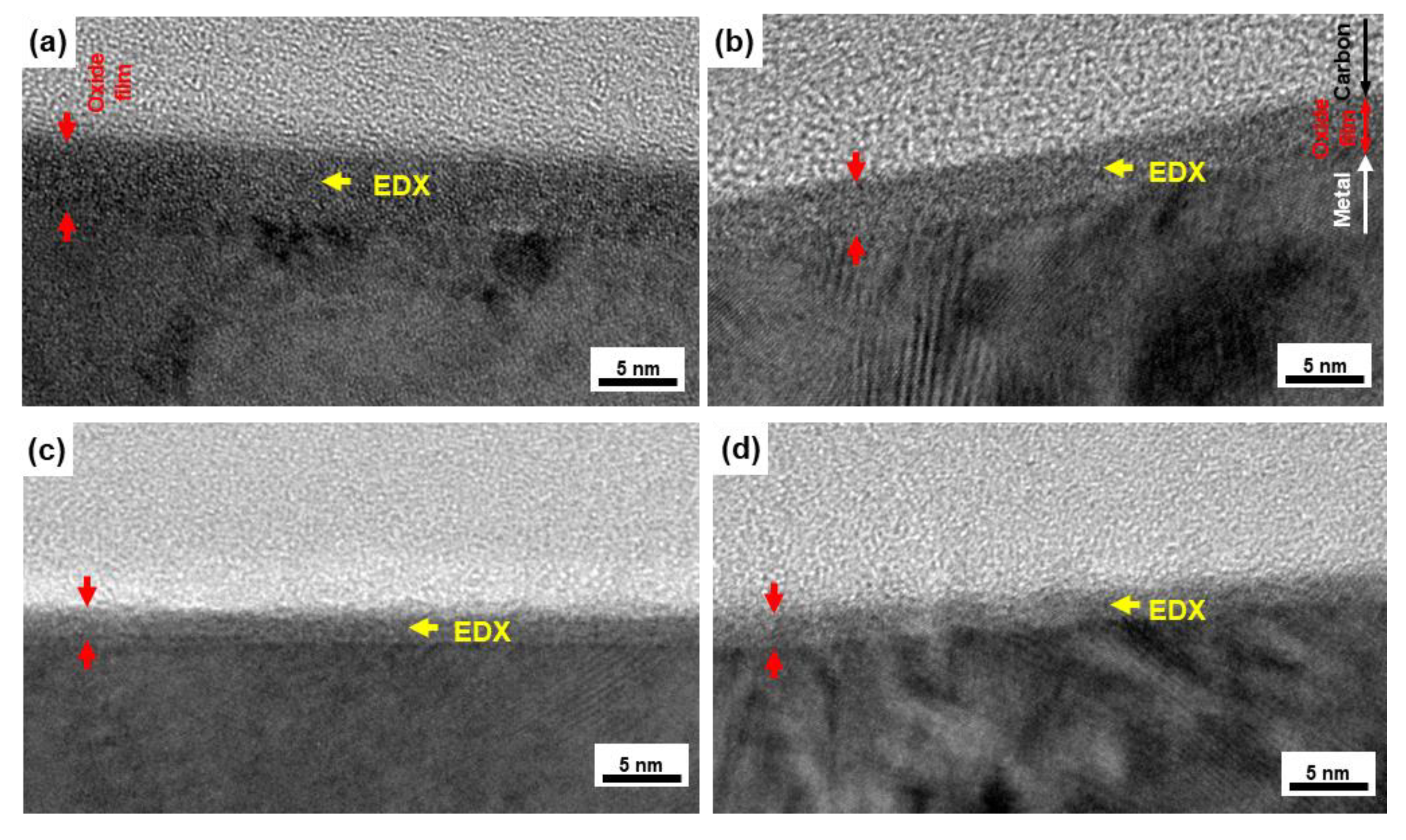
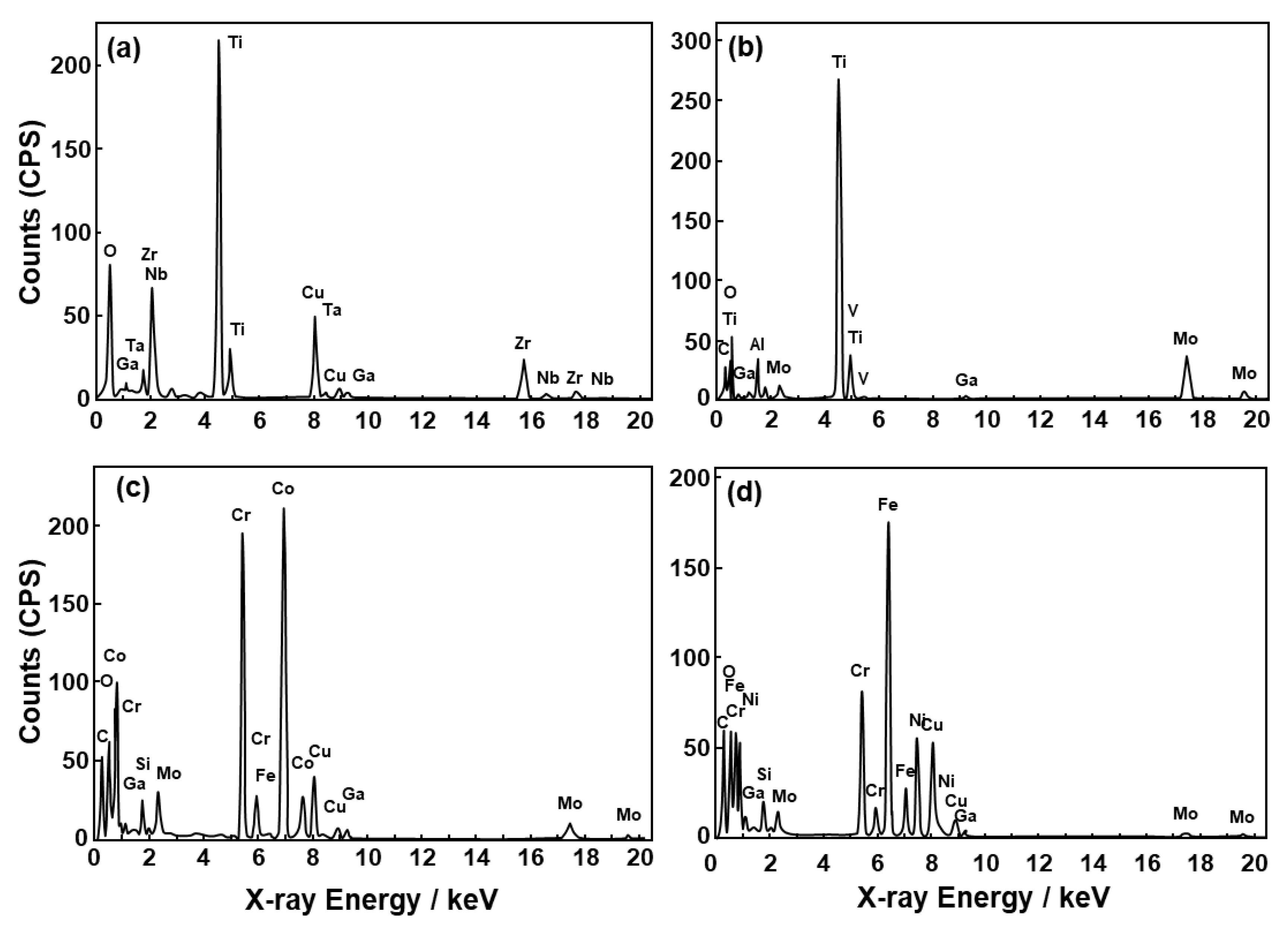
2.2. FE-TEM Observation of Oxide Film
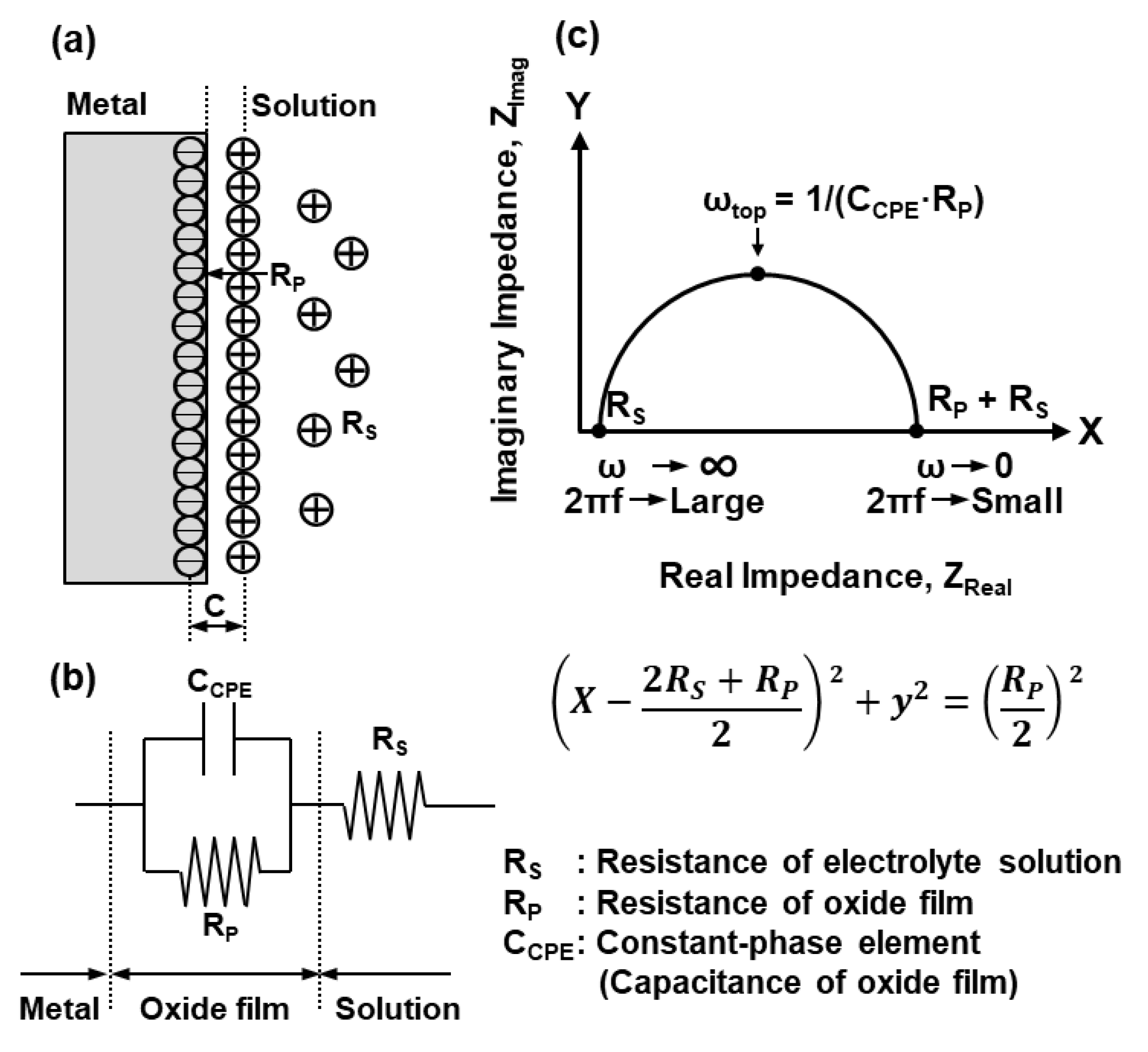
2.3. Electrochemical Model of Oxide Film
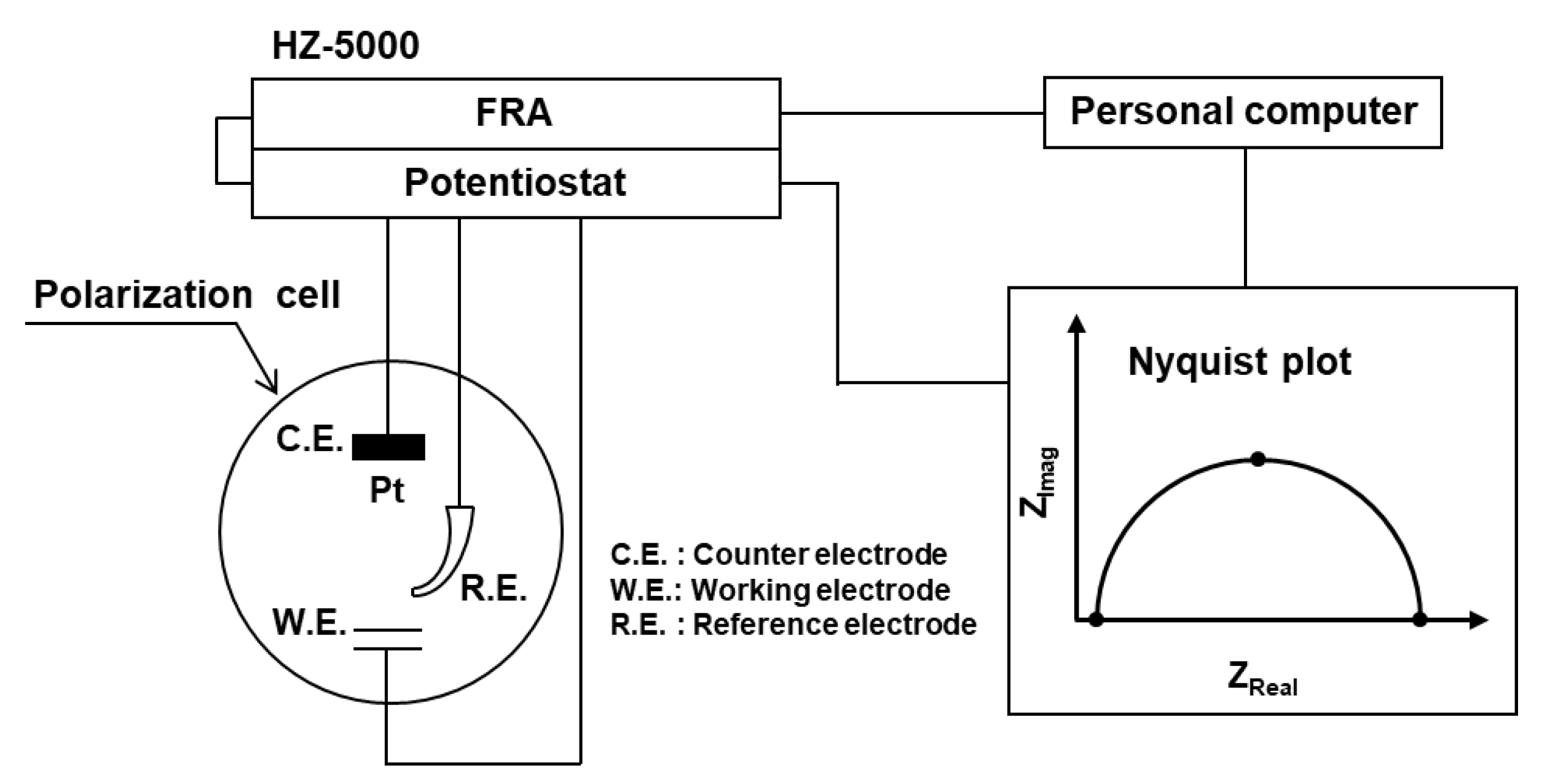
2.4. EIS Measurements
3. Results and Discussion
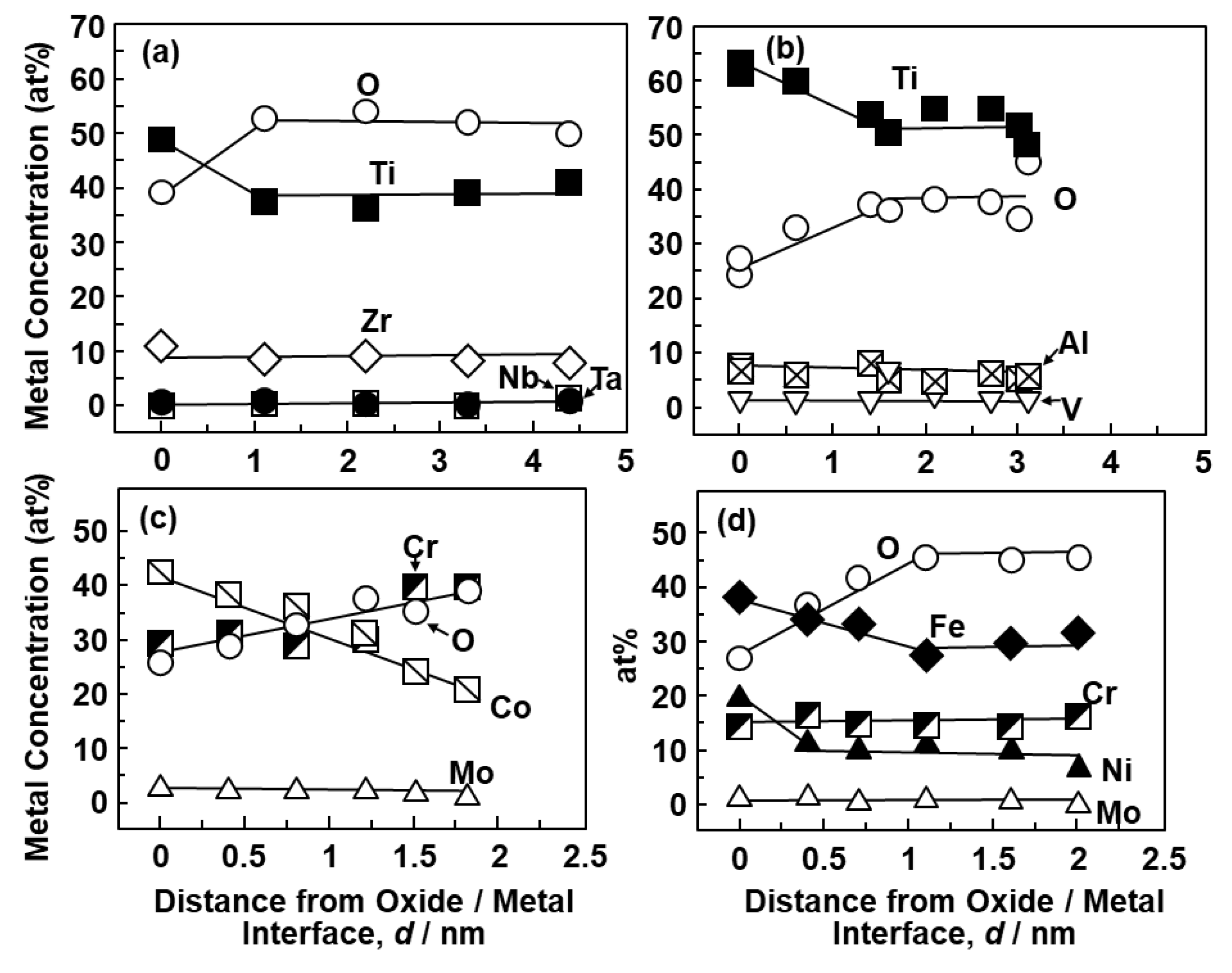
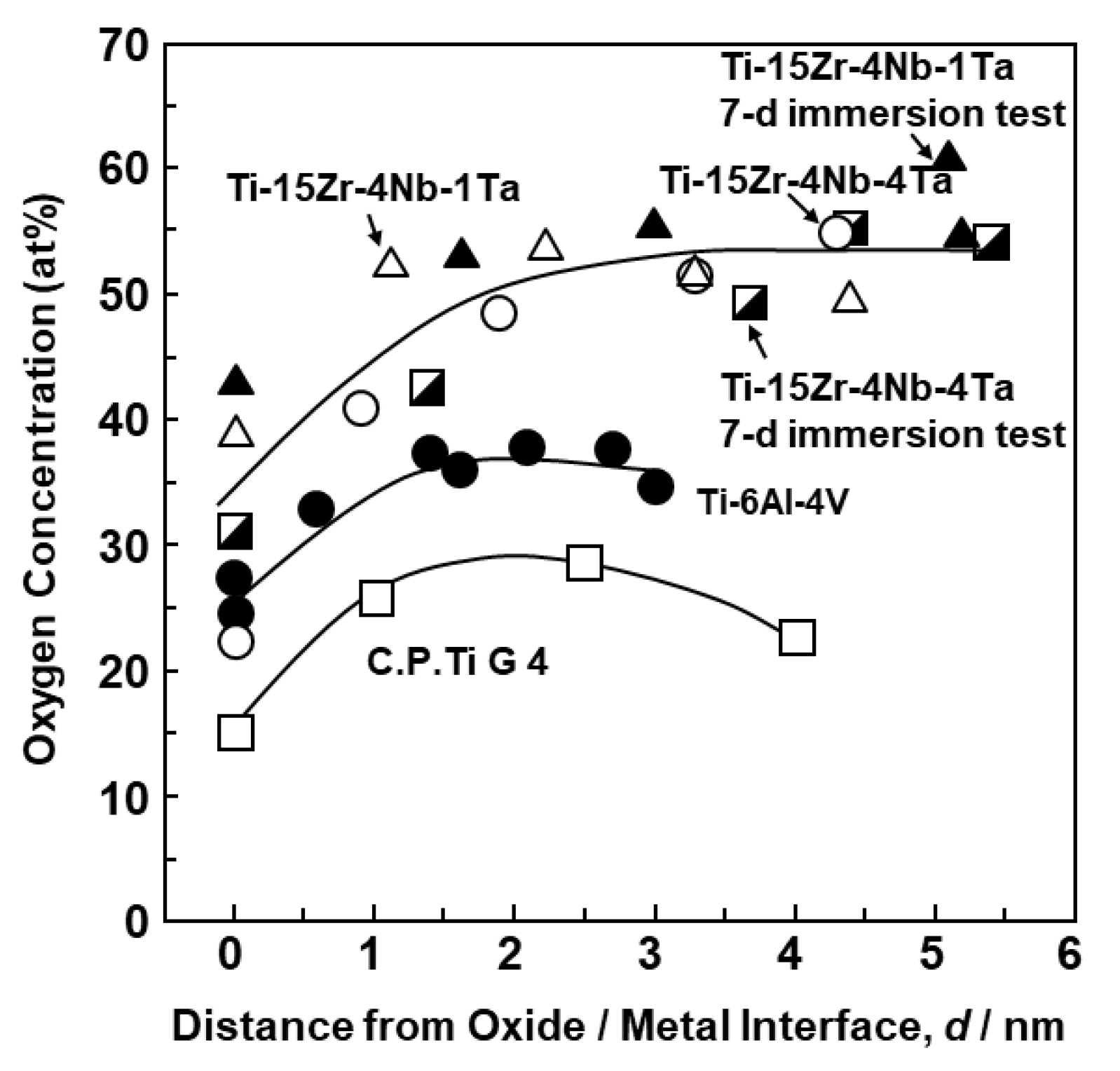
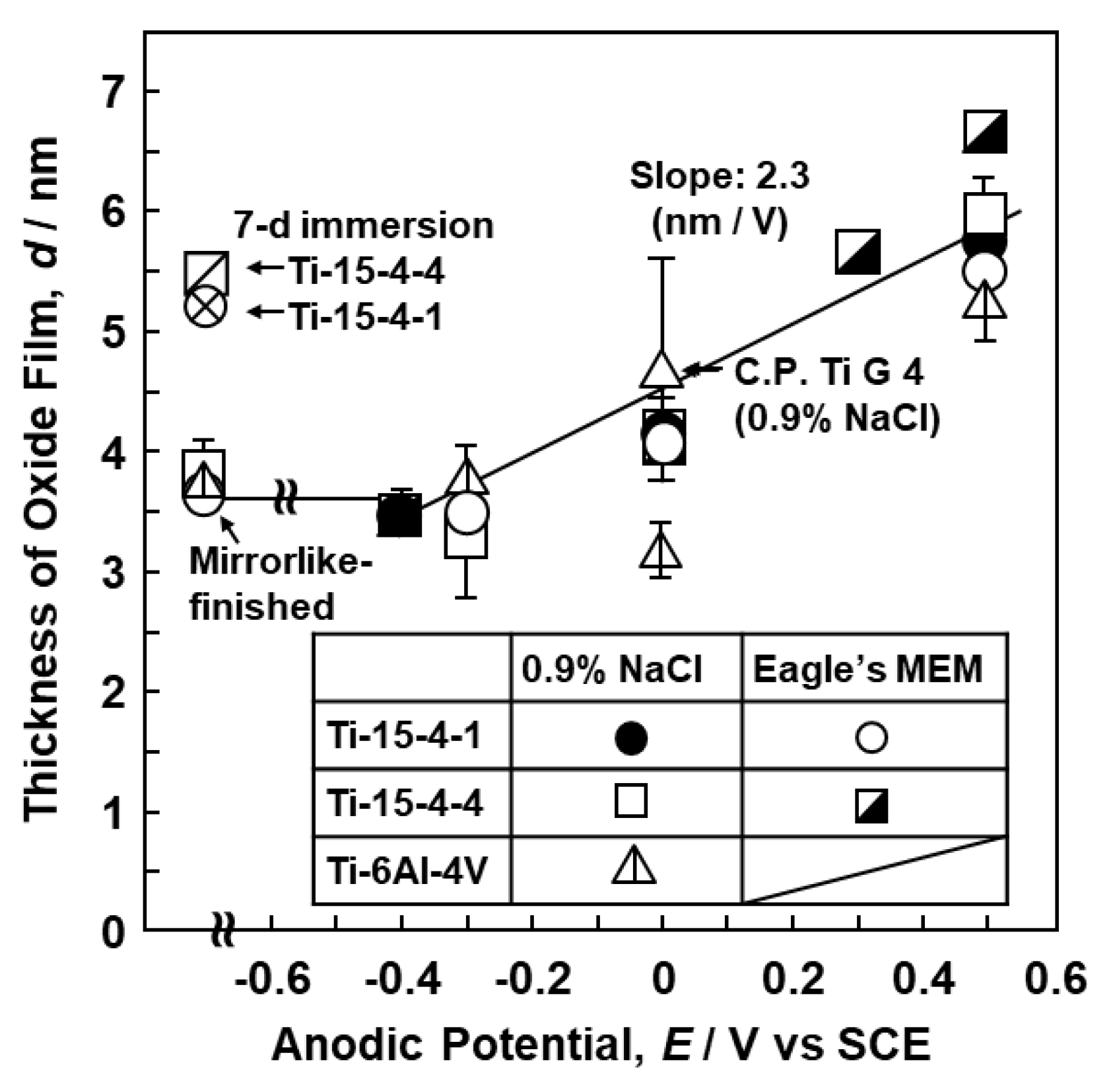
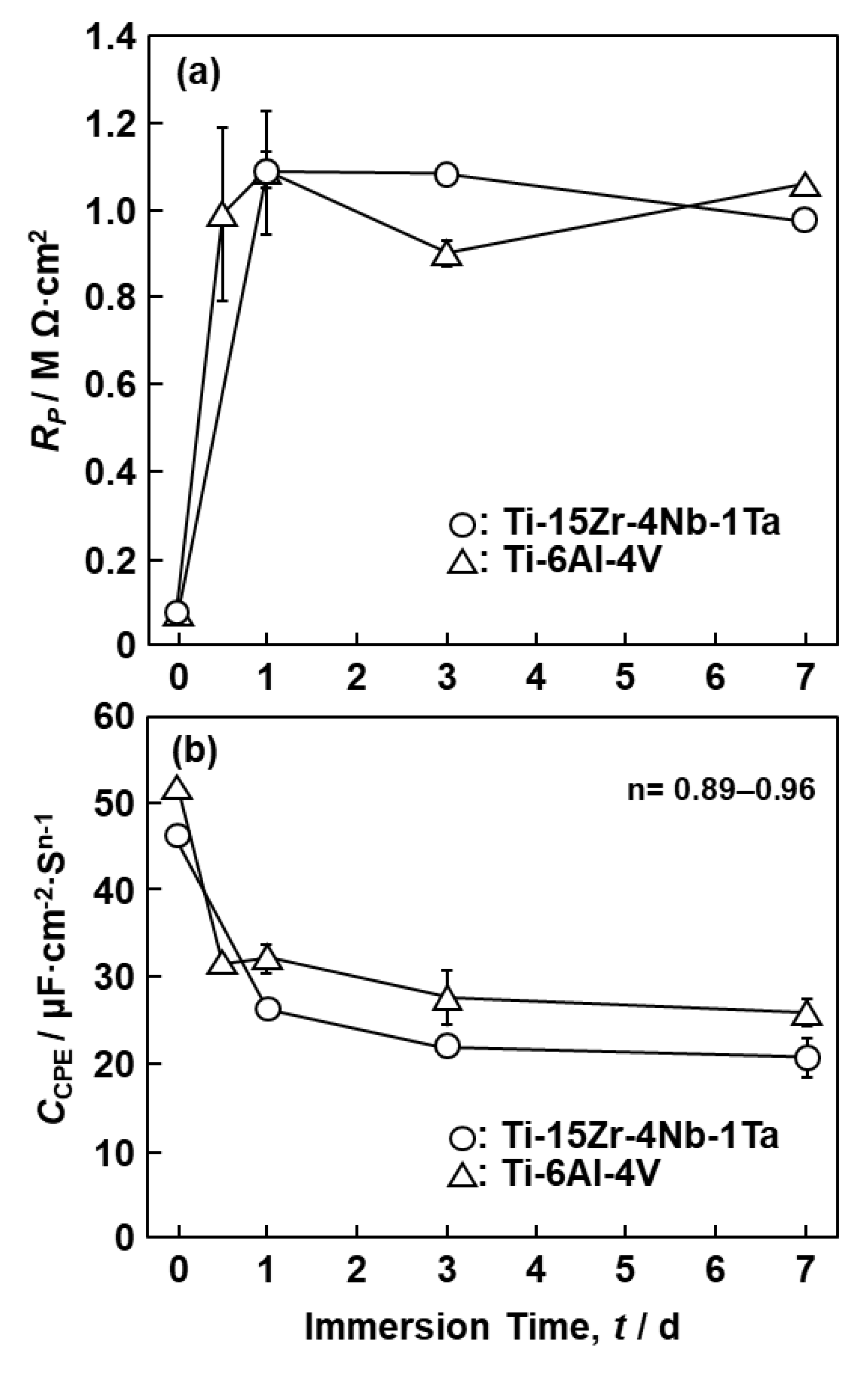
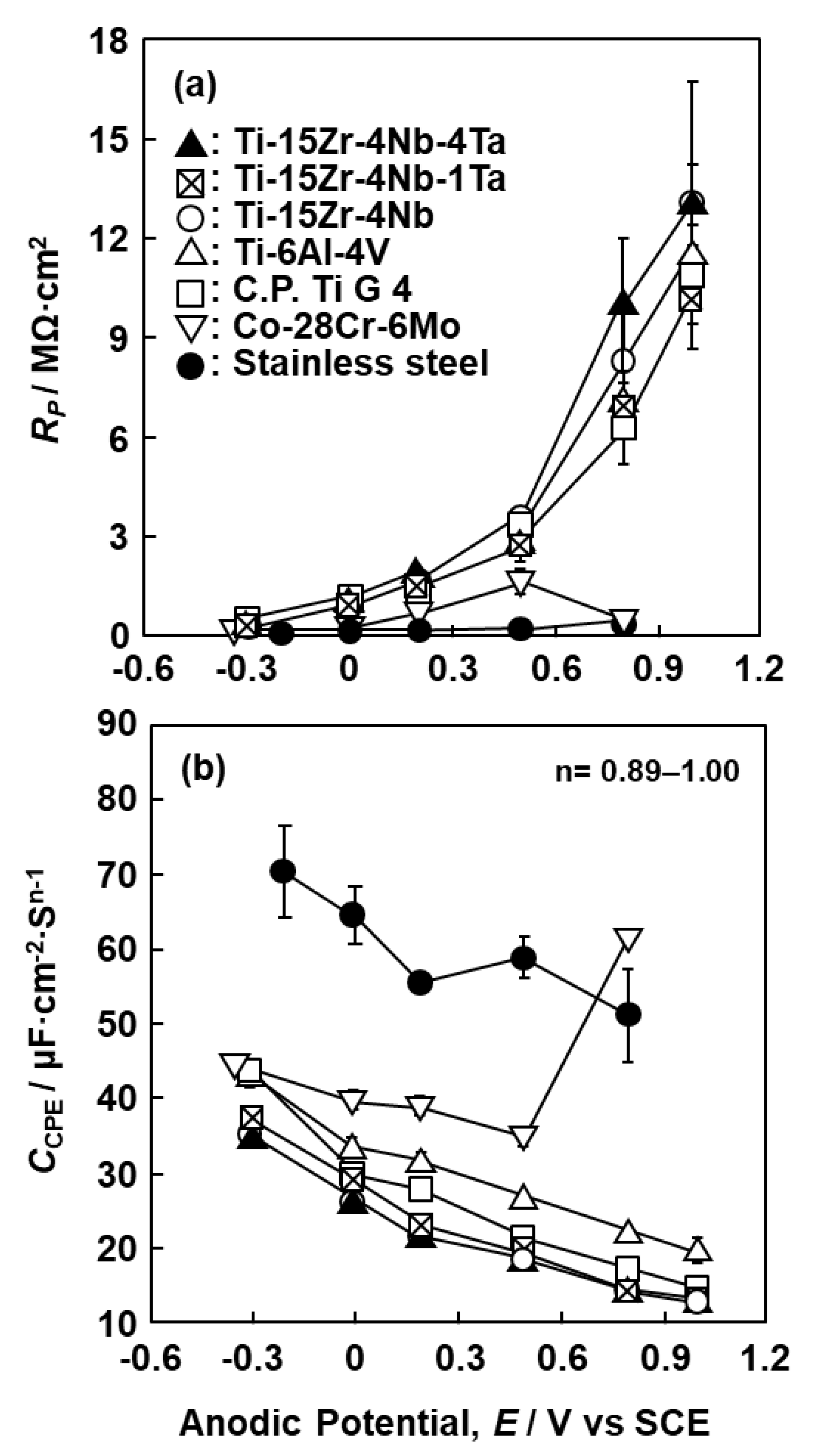
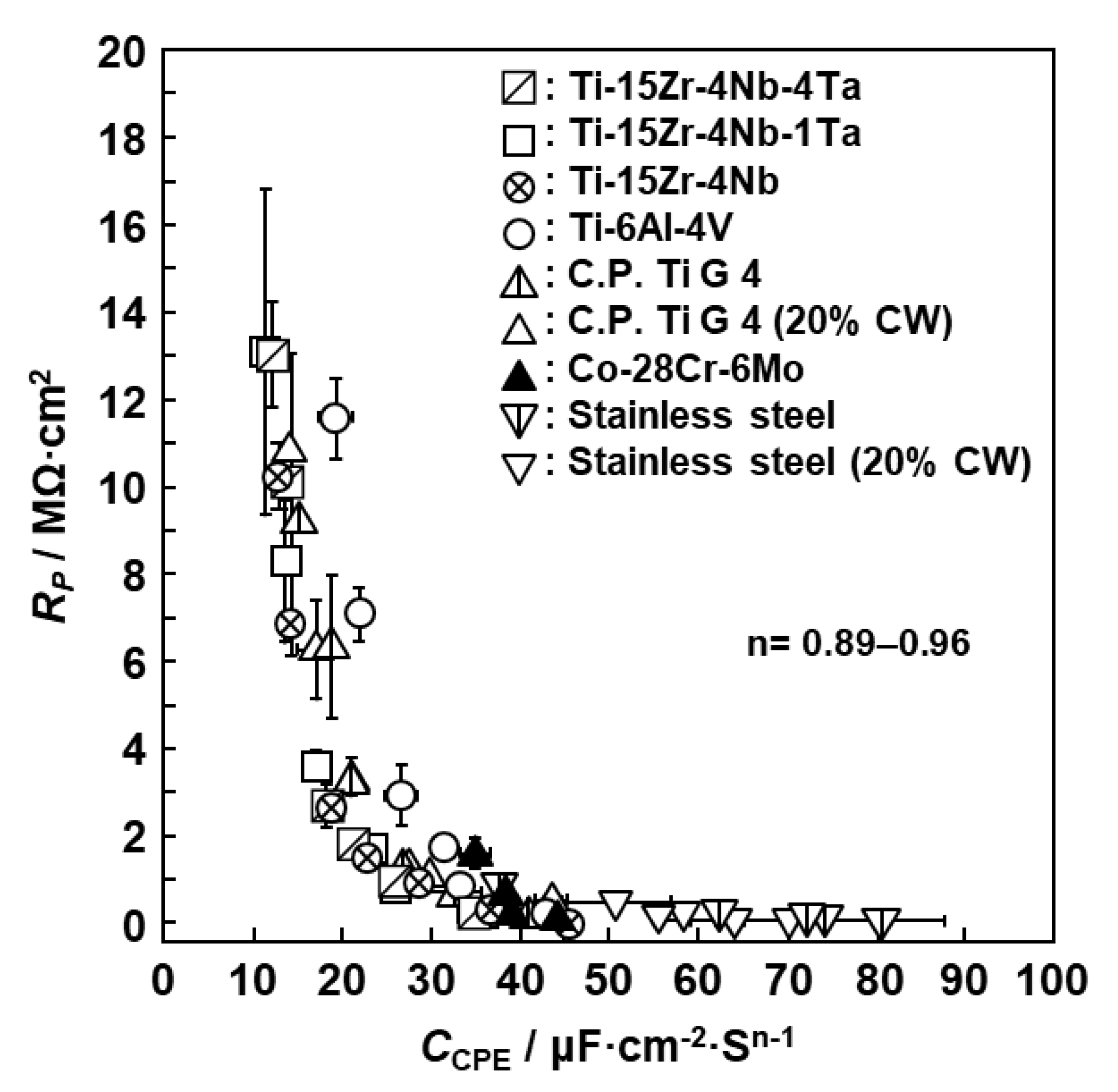
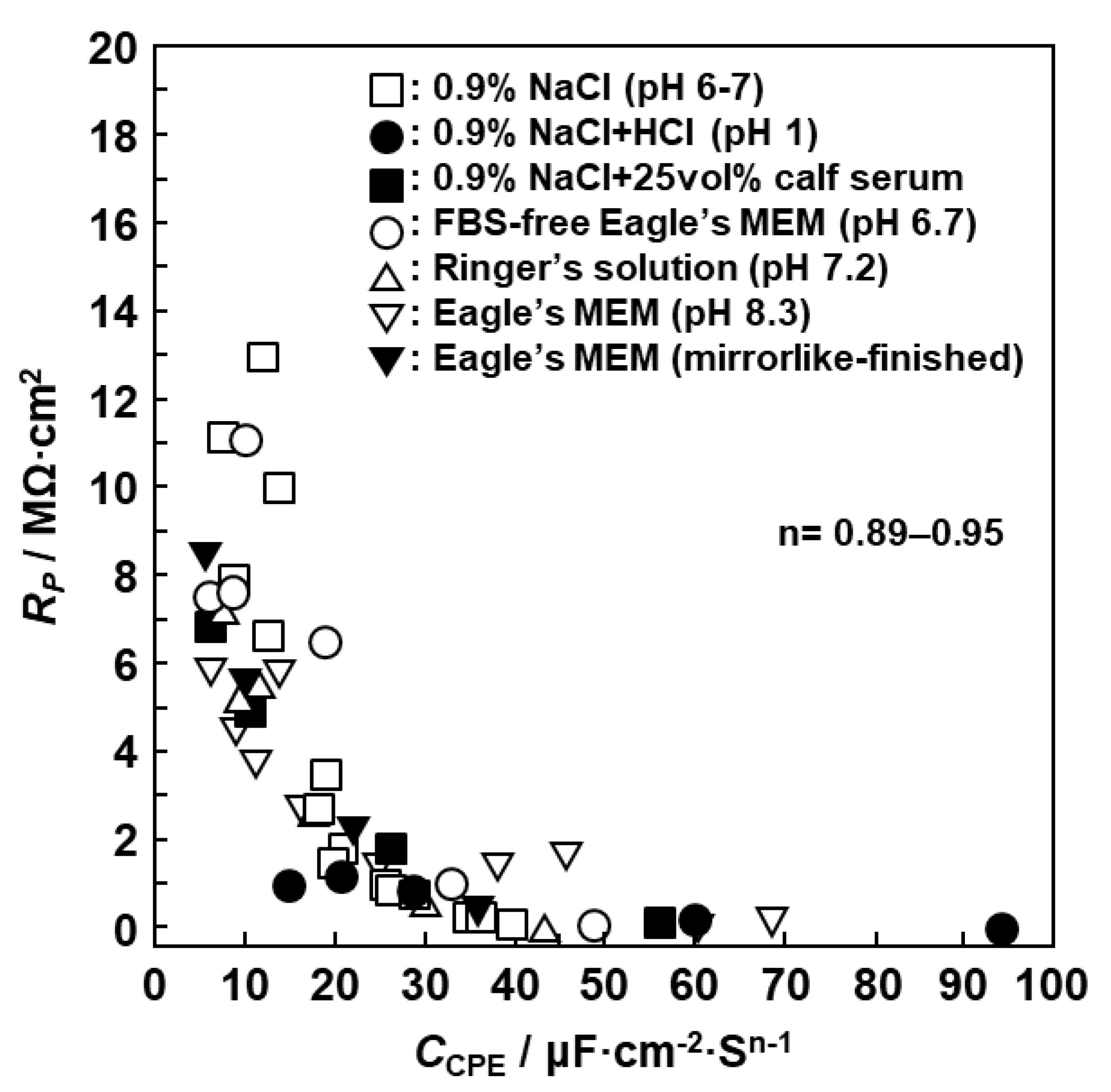
3.1. FE-TEM Analysis of Oxide Films
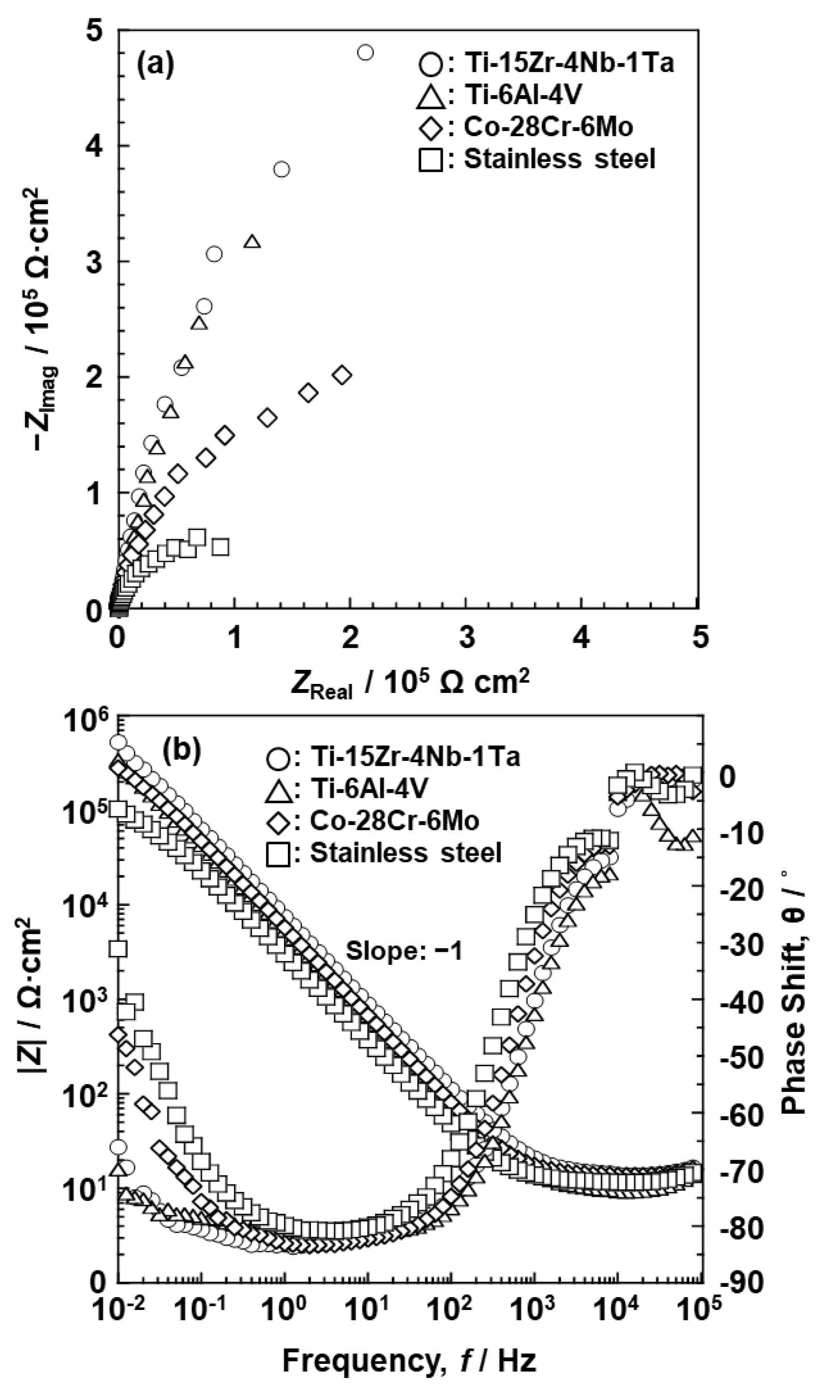
3.2. EIS Analysis of Oxide Films
4. Conclusions
Funding
Conflicts of Interest
References
- Okazaki, Y. Comparison of fatigue properties and fatigue crack growth rates of various implantable metals. Materials 2012, 5, 2981–3005. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of fatigue strengths of biocompatible Ti–15Zr–4Nb–4Ta alloy and other titanium materials. Mater. Sci. Eng. C 2011, 33, 325–333. [Google Scholar] [CrossRef]
- Okazaki, Y. On the effects of hot forging and hot rolling on the microstructural development and mechanical response of a biocompatible Ti alloy. Materials 2012, 5, 1439–1461. [Google Scholar] [CrossRef]
- Okazaki, Y. Development of low-cost manufacturing process and effects of adding small amounts of Ta, O, and N on the physical and mechanical properties of highly biocompatible Ti alloys. Mater. Trans. 2019, 60, 1769–1778. [Google Scholar] [CrossRef]
- JIS T 0304, Testing Method for Metal Release from Metallic Biomaterials; Japanese Standards Association: Tokyo, Japan, 2002.
- JIS T 0302, Testing Method for Corrosion Resistance of Metallic Biomaterials by Anodic Polarization Measurement; Japanese Standards Association: Tokyo, Japan, 2000.
- ISO 16428, Implants for Surgery–Test Solutions and Environmental Conditions for Static and Dynamic Corrosion Tests on Implantable Materials and Medical Devices; International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 16429, Implants for Surgery–Measurements of Open-Circuit Potential to Assess Corrosion Behavior of Metallic Implantable Materials and Medical Devices Over Extended Time Periods; International Organization for Standardization: Geneva, Switzerland, 2005.
- Okazaki, Y.; Nagata, H. Comparisons of immersion and electrochemical properties of highly biocompatible Ti–15Zr–4Nb–4Ta alloy and other implantable metals for orthopedic implants. Sci. Technol. Adv. Mater. 2012, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tian, J.; Dai, J.; Wang, X. Corrosion resistance of three-layer superhydrophobic composite coating on carbon steel in seawater. Electrochim. Acta 2013, 97, 409–419. [Google Scholar] [CrossRef]
- Fekry, A.M. The influence of chloride and sulphate ions on the corrosion behavior of Ti and Ti–6Al–4V alloy in oxalic acid. Electrochim. Acta 2009, 54, 3480–3489. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti–Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef]
- Hsu, R.W.-W.; Yang, C.-C.; Huang, C.-A.; Chen, Y.-S. Electrochemical corrosion studies on Co–Cr–Mo implant alloy in biological solutions. Mater. Chem. Phys. 2005, 93, 531–538. [Google Scholar] [CrossRef]
- Chelariu, R.; Bolat, G.; Izquierdo, J.; Mareci, D.; Gordin, D.M.; Gloriant, T.; Souto, R.M. Metastable beta Ti–Nb–Mo alloys with improved corrosion resistance in saline solution. Electrochim. Acta 2014, 137, 280–289. [Google Scholar] [CrossRef]
- Yadav, A.P.; Nishikata, A.; Tsuru, T. Electrochemical impedance study on galvanized steel corrosion under cyclic wet-dry conditions-influence of time of wetness. Corros. Sci. 2004, 46, 169–181. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, B.; Zhao, Y.; Pehkonen, S.O. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria. Corros. Sci. 2013, 74, 353–366. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co–28Cr–6Mo, and Ti–6Al–4V alloys in phosphate buffered saline solutions. Corros. Sci. 2011, 53, 3262–3272. [Google Scholar] [CrossRef]
- Woldemedhin, M.T.; Raabe, D.; Hassel, A.W. Characterization of thin anodic oxides of Ti–Nb alloys by electrochemical impedance spectroscopy. Electrochim. Acta 2012, 82, 324–332. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Momeni, M.; Kosari, A.; Zakeri, M.; Moayed, M.H. A comparative study of critical pitting temperature (CPT) of stainless steels by electrochemical impedance spectroscopy (EIS), potentiodynamic and potentiostatic techniques. Corros. Sci. 2012, 59, 96–102. [Google Scholar] [CrossRef]
- Wallinder, D.; Pan, J.; Leygraf, C.; Delblance-Bauer, A. EIS and XPS study of surface modification of 316LVM stainless steel after passivation. Corros. Sci. 1999, 41, 275–289. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Criado, M.; Bastidas, J.M. Electrochemical study on the corrosion behaviour of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides. Electrochim. Acta 2014, 129, 160–170. [Google Scholar] [CrossRef]
- Ehrensberger, M.T.; Gilbert, J.L. The effect of scanning electrochemical potential on the short-term impedance of commercially pure titanium in simulated biological conditions. J. Biomed. Mater. Res. Part A 2010, 94, 781–789. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Metal release from stainless steel, Co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros. Sci. 2008, 50, 3429–3438. [Google Scholar] [CrossRef]
- Okazaki, Y. Effects of heat treatment and hot forging on microstructure and mechanical properties of Co–Cr–Mo alloy for surgical implants. Mater. Trans. 2008, 49, 817–823. [Google Scholar] [CrossRef]
- Okazaki, Y. Effects of solution treatment and cold rolling on microstructure and mechanical properties of stainless steel for surgical implants. Mater. Trans. 2008, 49, 1423–1427. [Google Scholar] [CrossRef]
- Pound, B.G. Electrochemical behavior of cobalt-chromium alloys in a simulated physiological solution. J. Biomed. Mater. Res. A 2010, 94, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films. J. Electrochem. Soc. 2010, 157, C452–C457. [Google Scholar] [CrossRef]
- Bozzini, B.; Carlino, P.; D’Urzo, L.; Pepe, V.; Mele, C.; Venturo, F. An electrochemical impedance investigation of the behaviour of anodically oxidised titanium in human plasma and cognate fluids, relevant to dental applications. J. Mater. Sci.: Mater. Med. 2008, 19, 3443–3453. [Google Scholar] [CrossRef]
- Shukla, A.K.; Balasubramaniam, R.; Bhargava, S. Properties of passive film formed on CP titanium, Ti–6Al–4V and Ti–13.4Al–29Nb alloys in simulated human body conditions. Intermetallics 2005, 13, 631–637. [Google Scholar] [CrossRef]
- Takeuchi, M.; Itoh, T.; Nagasaka, H. Dielectric properties of sputtered TiO2 films. Thin Solid Films 1978, 51, 83–88. [Google Scholar] [CrossRef]
- Berberich, L.J.; Bell, M.E. The dielectric properties of the rutile form of TiO2. J. Appl. Phys. 1940, 11, 681–692. [Google Scholar] [CrossRef]

| Alloy | Zr | Nb | Ta | Al | V | Pd | Fe | O | N | H | C | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti-15-4-4 | 16.55 | 4.0 | 3.9 | − | − | < 0.01 | 0.04 | 0.28 | 0.09 | 0.0012 | 0.007 | Bal. |
| Ti-15-4-1 | 17.24 | 3.97 | 1.67 | − | − | 0.02 | 0.036 | 0.29 | 0.096 | 0.0027 | 0.011 | Bal. |
| Ti-15-4 | 17.1 | 4.2 | − | − | − | < 0.01 | 0.026 | 0.28 | < 0.01 | < 0.001 | < 0.01 | Bal. |
| C.P. Ti G-4 | − | − | − | − | − | − | 0.197 | 0.275 | 0.003 | 0.0069 | 0.011 | Bal. |
| Ti-6-4 | − | − | − | 6.4 | 4.4 | − | 0.10 | 0.07 | 0.02 | 0.0027 | 0.025 | Bal. |
| Cr | Mo | Ni | Cu | C | N | Mn | Si | P | Be | Fe | Co | |
| Stainless steel | 18.33 | 3.48 | 14.56 | < 0.01 | 0.021 | 0.157 | 1.77 | 0.79 | 0.016. | 0.0014 | Bal. | − |
| Co-Cr-Mo | 27.82 | 6.23 | 0.51 | − | 0.26 | − | 0.6 | 0.6 | − | − | 0.68 | Bal. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, Y. Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy. Materials 2019, 12, 3466. https://doi.org/10.3390/ma12213466
Okazaki Y. Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy. Materials. 2019; 12(21):3466. https://doi.org/10.3390/ma12213466
Chicago/Turabian StyleOkazaki, Yoshimitsu. 2019. "Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy" Materials 12, no. 21: 3466. https://doi.org/10.3390/ma12213466
APA StyleOkazaki, Y. (2019). Characterization of Oxide Film of Implantable Metals by Electrochemical Impedance Spectroscopy. Materials, 12(21), 3466. https://doi.org/10.3390/ma12213466






