Ultrasound Axicon: Systematic Approach to Optimize Focusing Resolution through Human Skull Bone
Abstract
1. Introduction
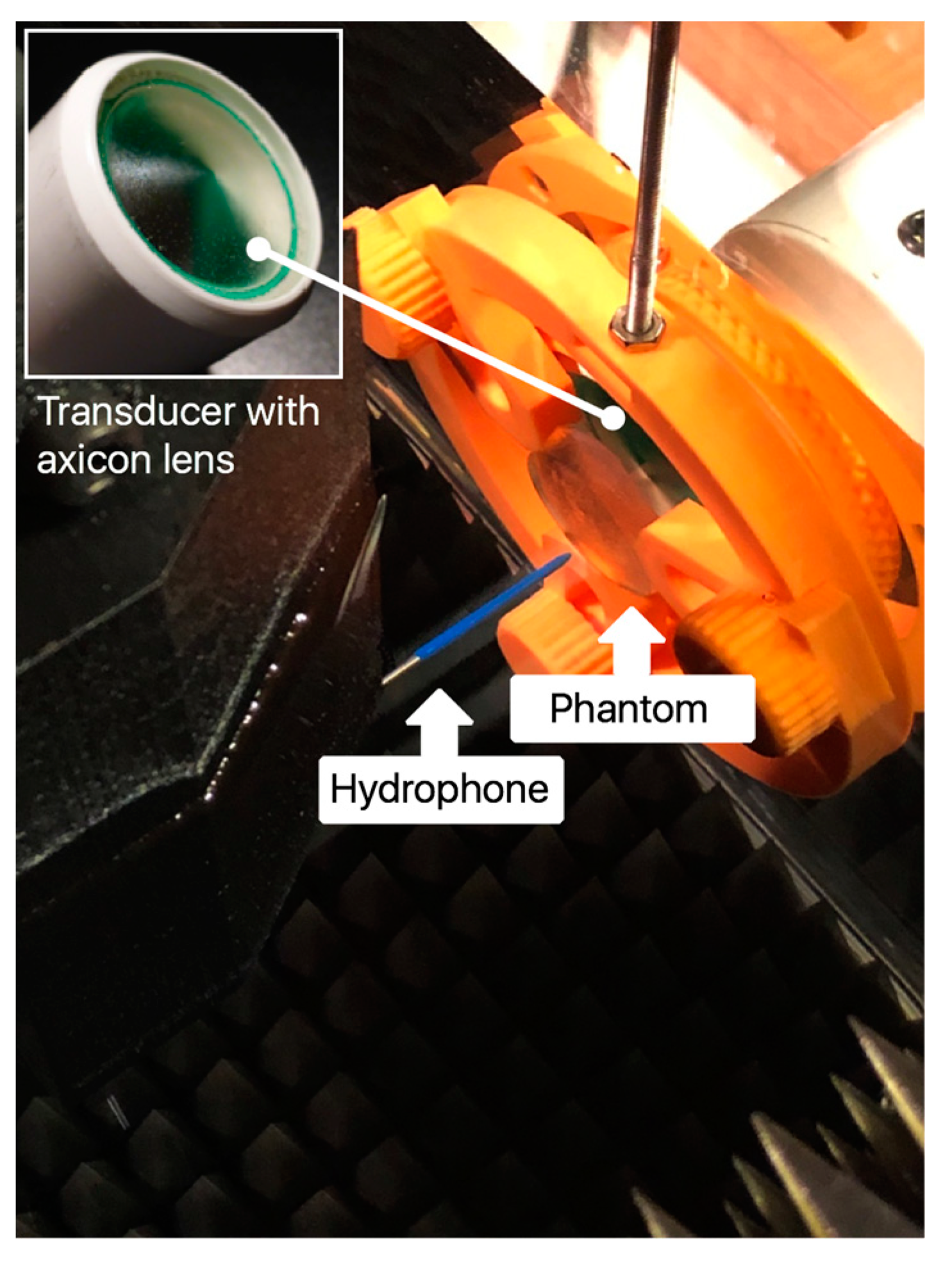
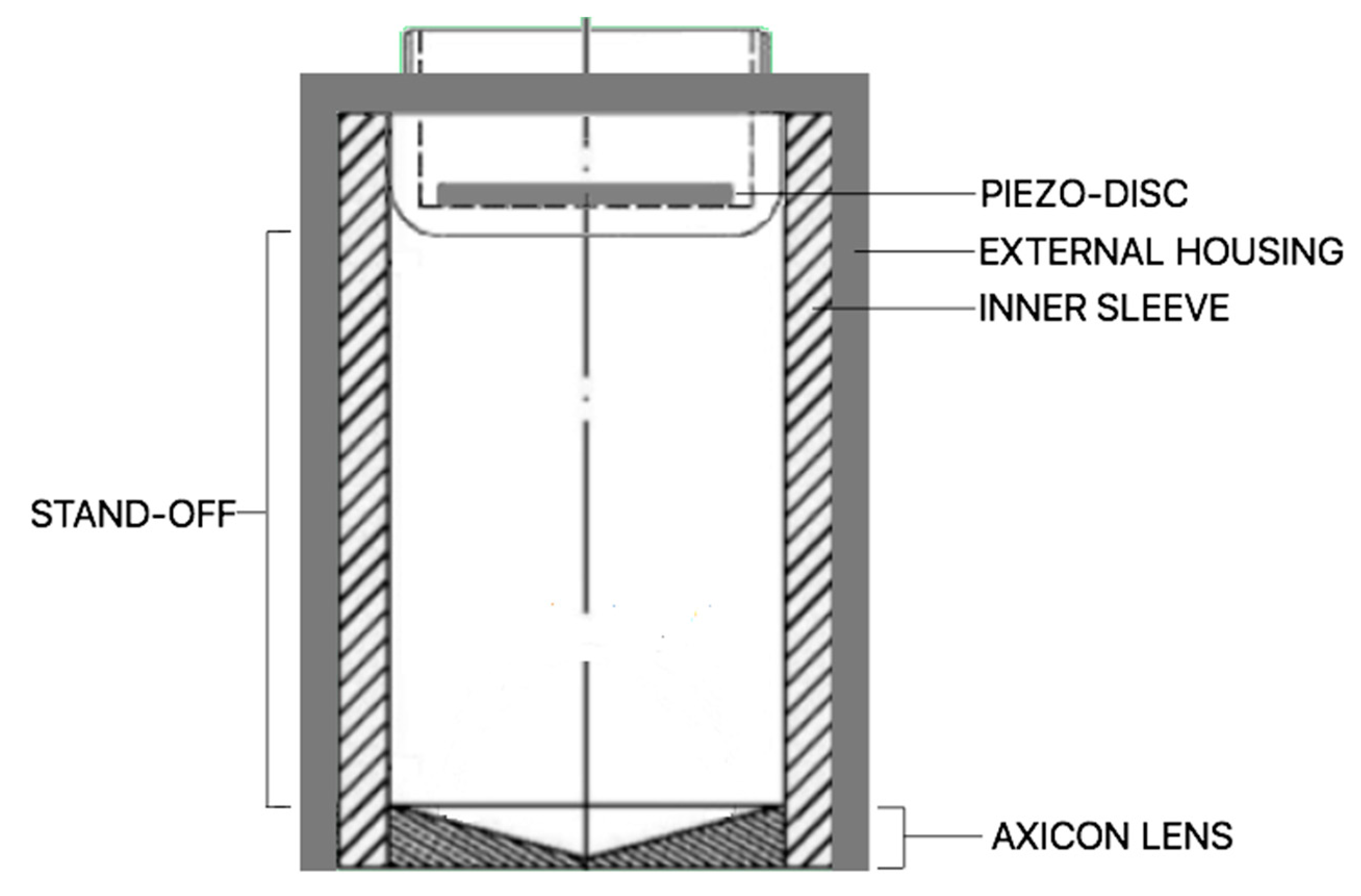
2. Materials and Methods
3. Results
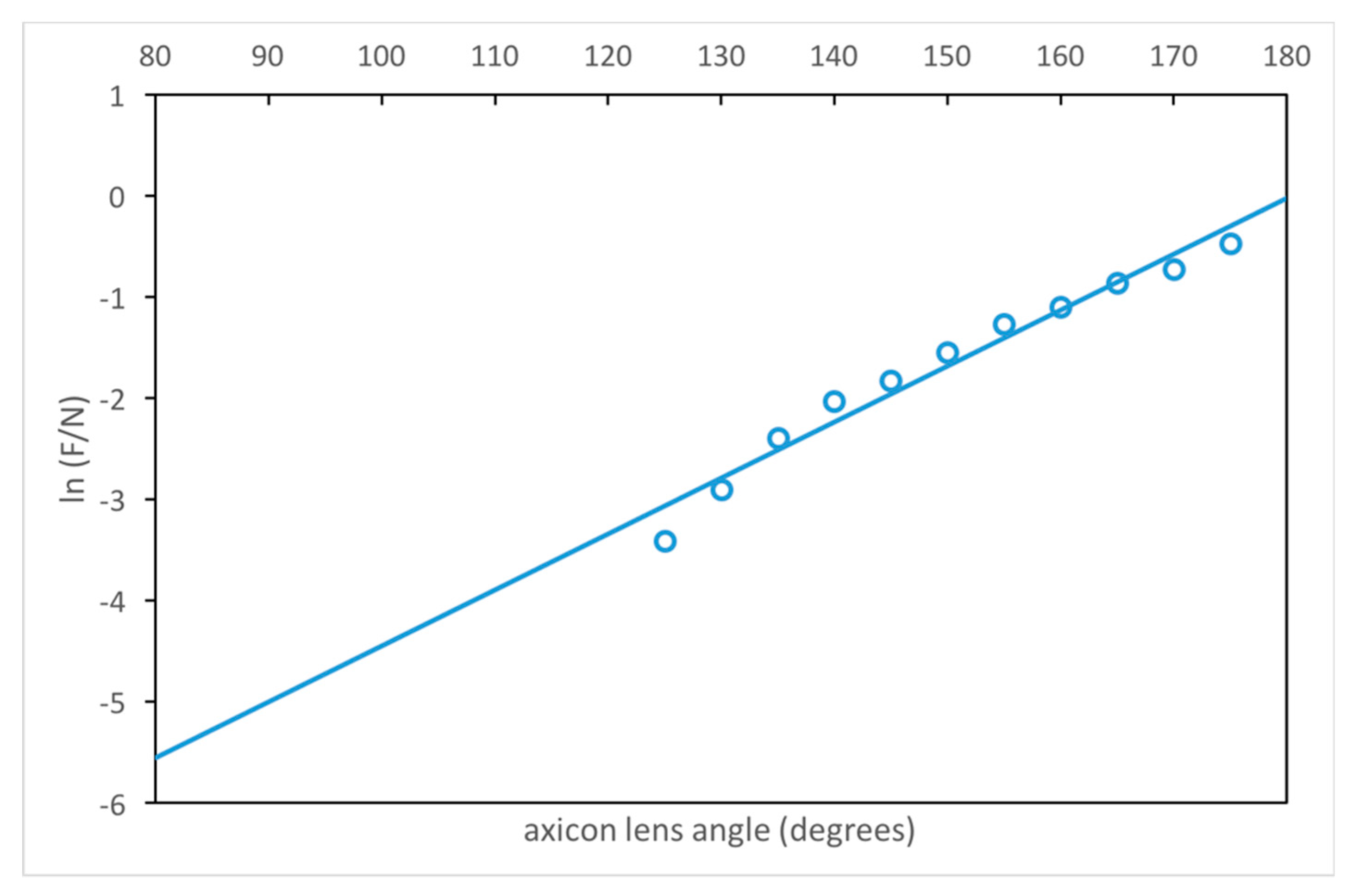
3.1. Estimation of the Axicon Lens Angle
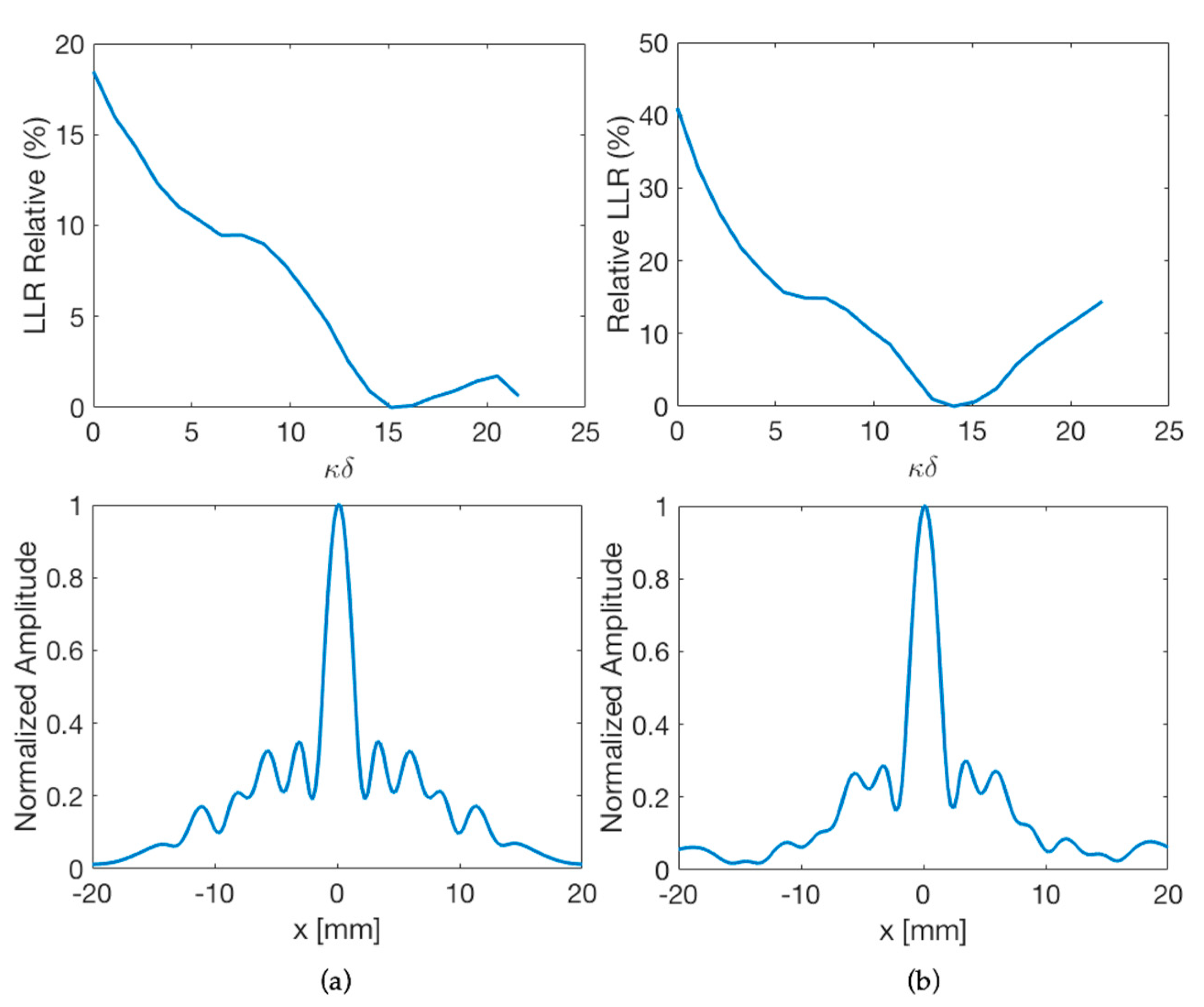
3.2. Reflectivity Effect on the Internal Walls of the Lens Housing
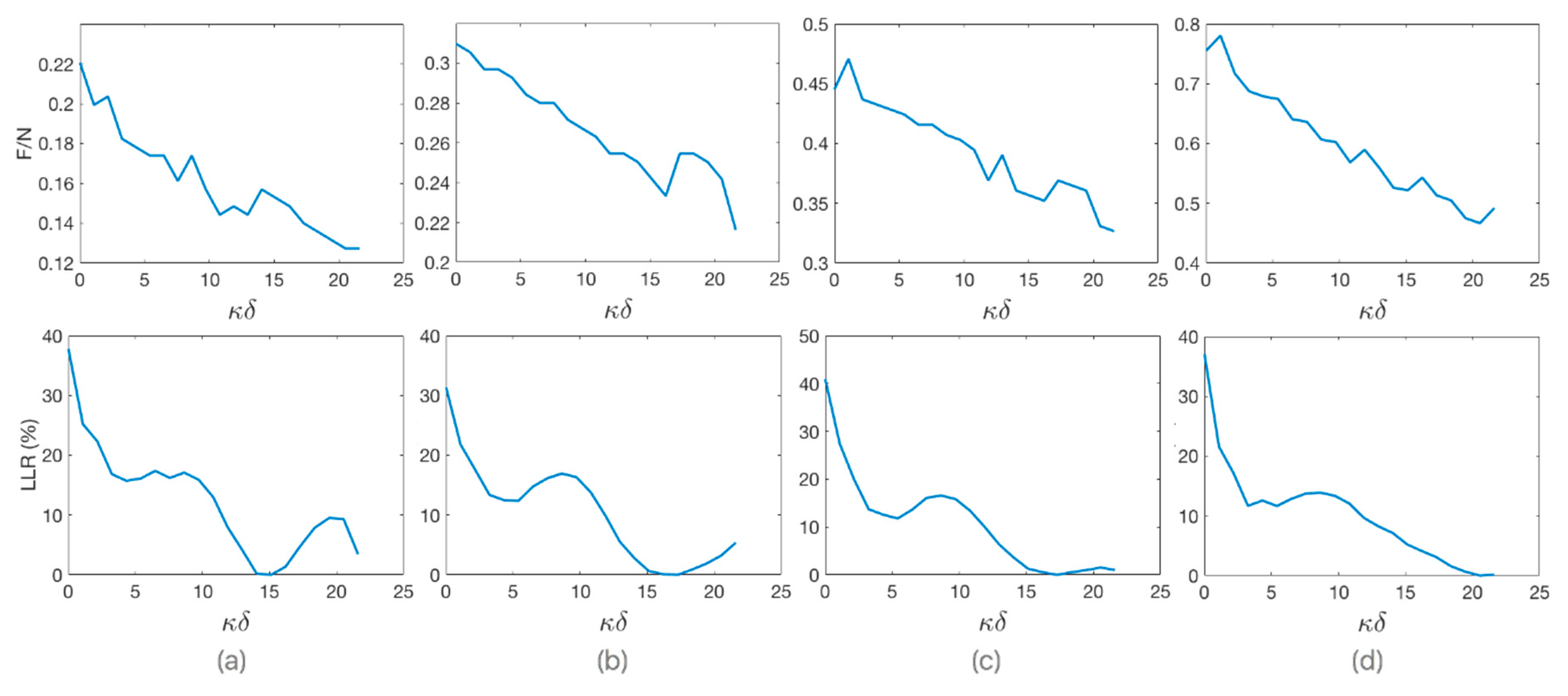
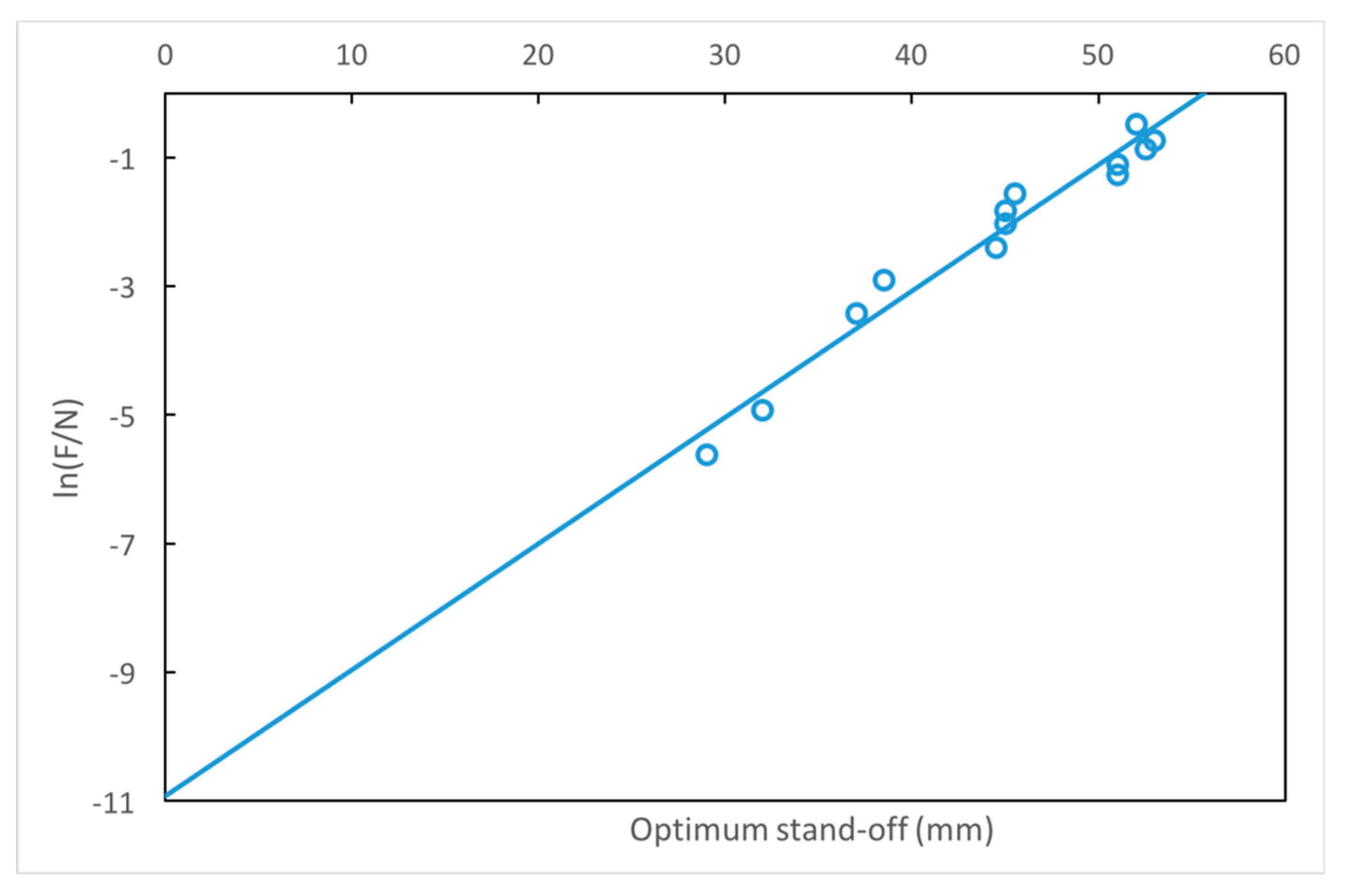
3.3. Estimation of the Optimum Stand-Off
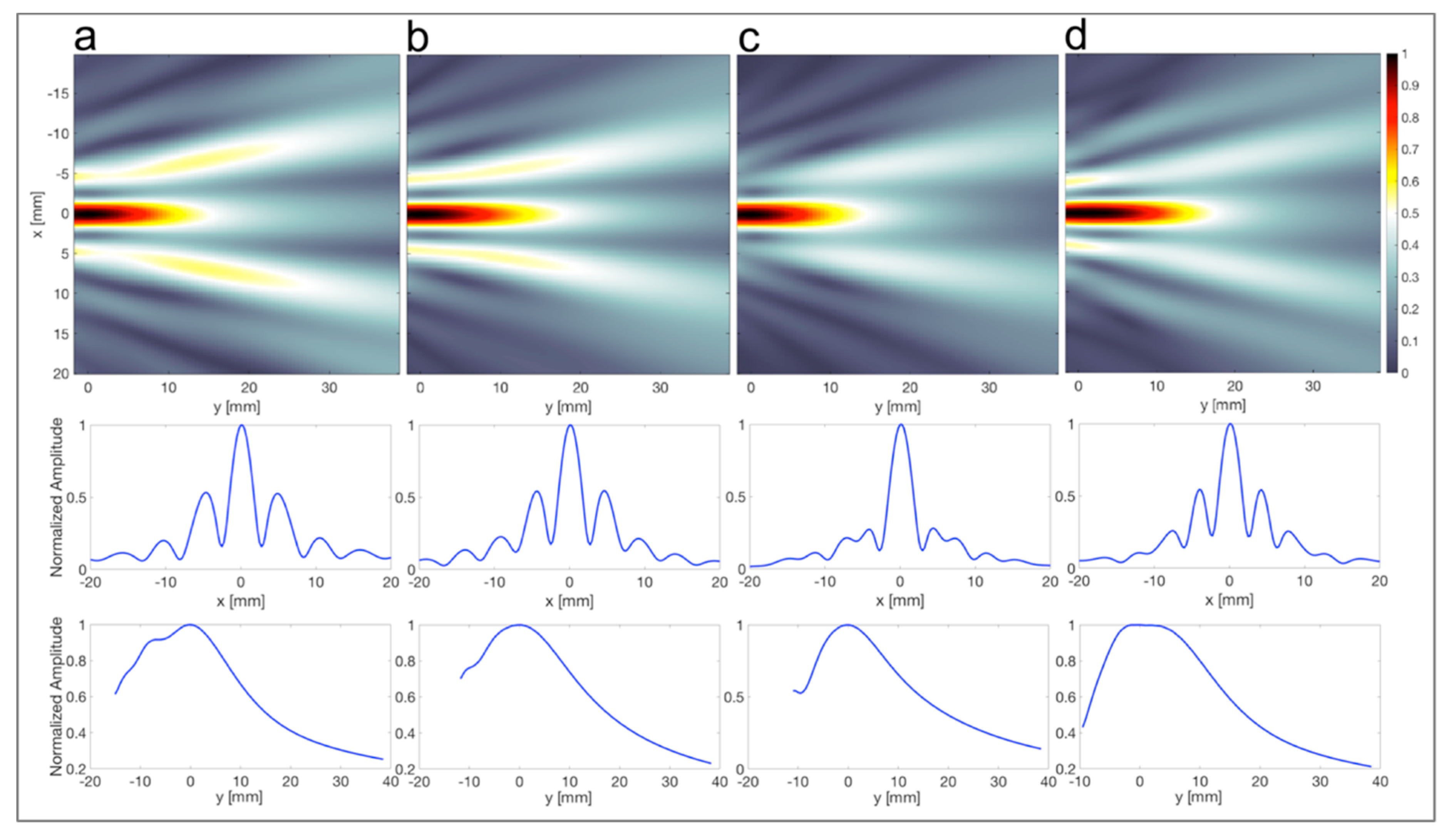
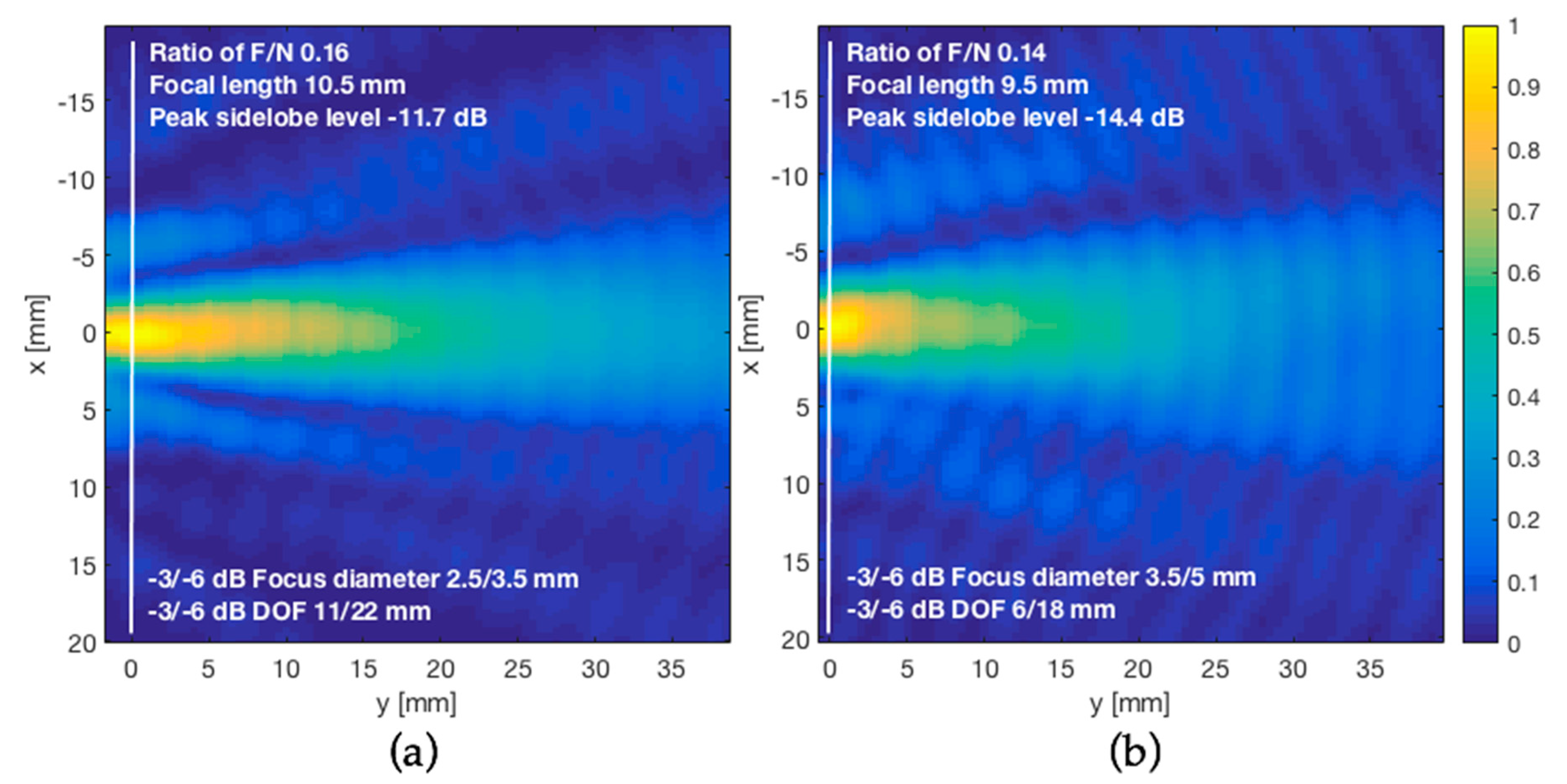
3.4. Acoustic-Field Experimental Scan
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
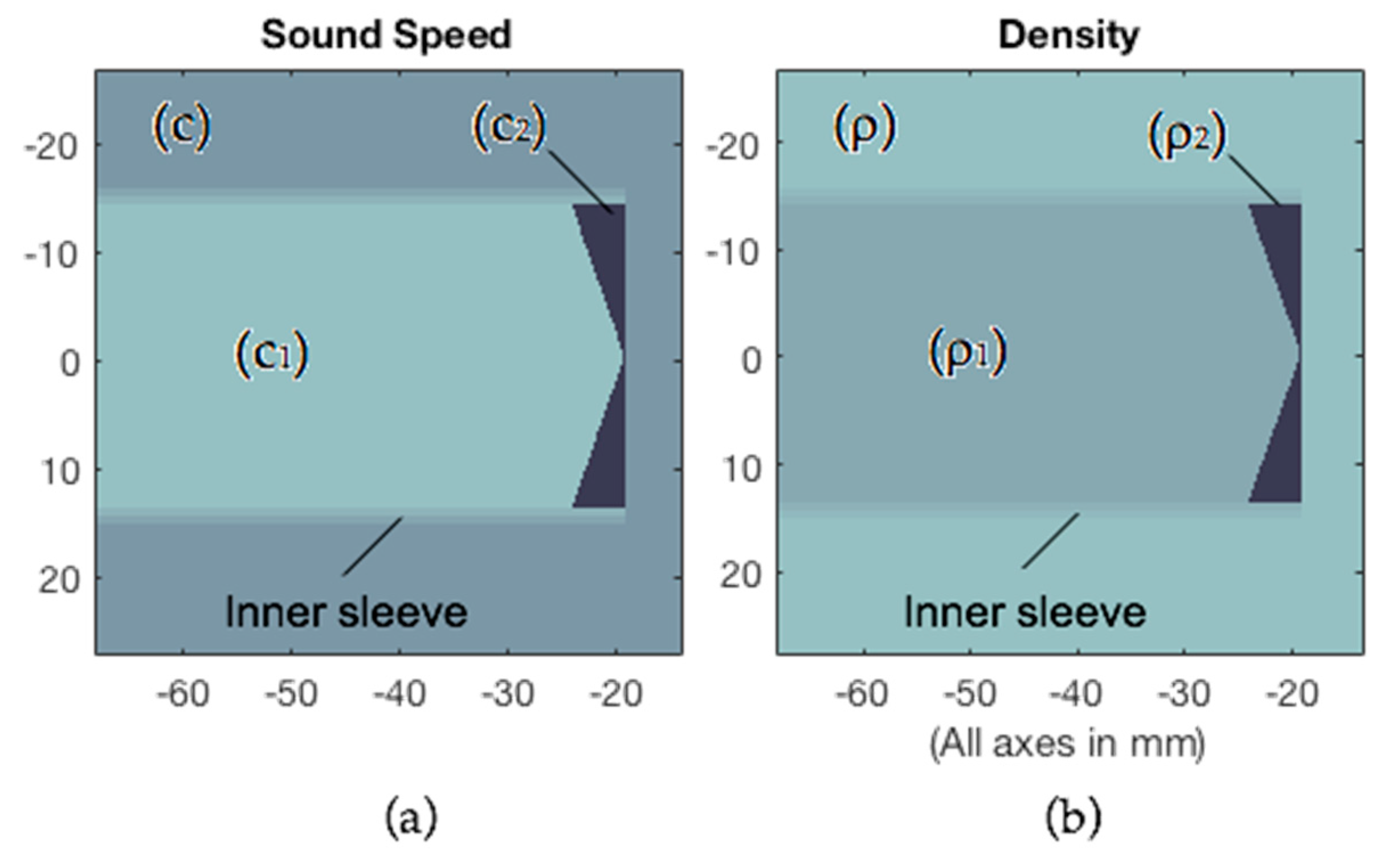
Appendix A. k-Wave Simulations
| Field | Value |
|---|---|
| Grid | |
| Number of grid points (NX) | 512 |
| Size of the domain (x) | 128 × 10−3 m |
| Grid point spacing (dX = x/NX) | 2.5 × 10−4 m |
| Number of grid points for the sensor mask | 512 × 512 |
| Perfect Match Layer thickness | 20 grid points |
| Transducer | |
| Sinusoidal transducer frequency (f) | 0.515 × 106 Hz |
| Radius of the disc transducer (D/2) | 28 × 10−3 m |
| Target medium (water) | |
| Sound speed (c) | 1480 m/s |
| Density (ρ) | 1000 kg/m3 |
| Stand-off and filling medium of the Axicon lens cavity (PDMS) | |
| Sound speed (c1) | 1030 m/s |
| Density (ρ1) | 1030 kg/m3 |
| Axicon lens cavity medium (epoxy resin) | |
| Sound speed (c2) | 2530 m/s |
| Density (ρ2) | 1170 kg/m3 |
| Inner sleeve | |
| Echo Reduction (ER) | −40 dB |
- % size of the computational grid
- Nx = 512; % number of grid points in the x (row) direction
- x = 128e-3; % size of the domain in the x direction [m]
- dx = x/Nx; % grid point spacing in the x direction [m]
- % create the computational grid
- kgrid = makeGrid(Nx, dx, Nx, dx);
- % create the time array
- [kgrid.t_array, dt] = makeTime(kgrid, medium.sound_speed);
- medium.alpha_power = 2; %[dB/(MHz^y cm)]
- medium.alpha_coeff = 2.17e-3; %[dB/(MHz^y cm)]
- medium.BonA = 4.96;
- % define a transducer element
- source.p_mask = makeLine(Nx, Ny, startpoint, pi/2, 112);
- % define a time varying sinusoidal source
- source_freq = 0.515e6; % [Hz]
- source_mag = 1.0; % [Pa]
- source.p = source_mag*sin(2*pi*source_freq*kgrid.t_array);
- % filter the source to remove any high frequencies not supported %by the grid
- source.p = filterTimeSeries(kgrid, medium, source.p);
- % create a sensor mask covering the entire computational domain
- % using the opposing corners of a rectangle
- sensor.mask = ones(Nx, Ny);
- % set the record mode to capture the final wave-field and the
- % statistics at each sensor point
- sensor.record = {‘p_final’, ‘p_max’, ‘p_rms’};
- % create a display mask to display the transducer
- display_mask = source.p_mask;
- % assign the input options
- input_args = {‘DisplayMask’, display_mask, ‘PMLInside’, false,… ‘DataCast’, ‘gpuArray-single’, ‘PlotPML’, true, ‘PlotLayout’, true};
- % run the simulation
- sensor_data = kspaceFirstOrder2D(kgrid, medium, source, sensor,… input_args{:});
| Angle φ | F (mm) | F/N (N = 68.2 mm) | δOptimum (mm) | κδOptimum (λ = 2 mm) | dF (mm @-6dB) | MILR (%) |
|---|---|---|---|---|---|---|
| 115° | 0.25 | 0.0037 | 29.0 | 14.50 | 1.50 | 65 |
| 120° | 0.50 | 0.0073 | 32.0 | 16.00 | 2.00 | 58 |
| 125° | 2.25 | 0.033 | 37.0 | 18.50 | 2.50 | 50 |
| 130° | 3.75 | 0.055 | 38.5 | 19.25 | 2.50 | 51 |
| 135° | 6.25 | 0.092 | 44.5 | 22.25 | 2.50 | 48 |
| 140° | 9.00 | 0.13 | 45.0 | 22.50 | 3.00 | 54 |
| 145° | 11.00 | 0.16 | 45.0 | 22.50 | 3.00 | 49 |
| 150° | 14.50 | 0.21 | 45.5 | 22.75 | 3.50 | 51 |
| 155° | 19.25 | 0.28 | 51.0 | 25.50 | 4.00 | 49 |
| 160° | 22.75 | 0.33 | 51.0 | 25.50 | 4.50 | 47 |
| 165° | 28.75 | 0.42 | 52.5 | 26.25 | 5.50 | 45 |
| 170° | 33.00 | 0.48 | 53.0 | 26.50 | 6.00 | 44 |
| 175° | 42.50 | 0.62 | 52.0 | 26.00 | 8.00 | 51 |
Appendix B. Phantom Design
Appendix C. Material Characterization
| Material | Velocity (mm/μs@2MHz) | Density (g/cm3) | Impedance (MRayl) | Loss (dB/cm@2MHz) |
|---|---|---|---|---|
| Epoxy (25 °C) | 2.53 | 1.17 | 2.96 | 6.8 |
| PDMS (25 °C) | 1.03 | 1.03 | 1.06 | 5.3 |
| Water (20 °C) | 1.48 | 1.00 | 1.48 | 0.08 |
Appendix D. Guideline for Axicon Lens Design
- (1)
- Calculate transducer near field, as:where D is transducer diameter, f is transducer frequency, and c is sound velocity of the material under inspection
- (2)
- Select the desired lens focus F and check that:Values of 0.1 are rarely used because it gives a very near focus. Values or 0.4 give a profile similar to the transducer but remove the N zone.
- (3)
- Calculate the angle of the axicon lens, as:
- (4)
- Calculate the optimum value of stand-off, as:
- (5)
- Focus diameter is:
- (6)
- Depth of focus is:

References
- Mehić, E.; Xu, J.M.; Caler, C.J.; Coulson, N.K.; Moritz, C.T.; Mourad, P.D. Increased anatomical Specificity of neuromodulation via modulated focused ultrasound. PLoS ONE 2014, 9, e86939. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Cox, B.T.; Jaros, J.; Treeby, B.E. Accurate simulation of transcranial ultrasound propagation for ultrasonic neuromodulation and stimulation. J. Acoust. Soc. Am. 2017, 141, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. Neuroimage 2011, 56, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Rezayat, E.; Toostani, G. A review on brain stimulation using low intensity focused ultrasound. Basic Clin. Neurosci. 2016, 7, 187–194. [Google Scholar] [PubMed]
- Fry, F.J.; Ades, H.W.; Fry, W.J. Production of reversible changes in the central nervous system by ultrasound. Science 1958, 127, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Acquaticci, F.; Guarracino, J.F.; Gwirc, S.N.; Lew, S.E. A polydimethylsiloxane-based axicon lens for focused ultrasonic brain stimulation techniques. Acoust. Sci. Technol. 2019, 40, 116–126. [Google Scholar] [CrossRef]
- Katchadjian, P.; Desimone, C.; Garcia, A.D. Application of axicon lenses in ultrasonic techniques. AIP Conf. Proc. 2010, 1211, 1043–1050. [Google Scholar]
- Murphy, R.V. Focussed ultrasonic probes for contact inspection. Mater. Eval. 1980, 38, 53–58. [Google Scholar]
- Treeby, B.E.; Jaros, J.; Rendell, A.P.; Cox, B.T. Modeling nonlinear ultrasound propagation in heterogeneous media with power law absorption using a k-space pseudospectral method. J. Acoust. Soc. Am. 2012, 131, 4324–4336. [Google Scholar] [CrossRef] [PubMed]
- Treeby, B.E.; Cox, B.T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 2010, 15, 021314. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Teoh, E.; Jaros, J.; Treeby, B.E. Modelling Nonlinear Ultrasound Propagation in Absorbing Media using the K-Wave Toolbox: Experimental Validation. In Proceedings of the 2012 IEEE International Ultrasonics Symposium, Dresden, Germany, 7–10 October 2012; pp. 523–526. [Google Scholar]
- Liu, Q.H. Large-scale simulations of electromagnetic and acoustic measurements using the pseudospectral time-domain (PSTD) algorithm. IEEE Trans. Geosci. Electron. 1999, 37, 917–926. [Google Scholar]
- Caputo, M.; Carcione, J.M.; Cavallini, F. Wave simulation in biologic media based on the Kelvin-voigt fractional-derivative stress-strain relation. Ultrasound Med. Biol. 2011, 37, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Meza-Fajardo, K.C.; Papageorgiou, A.S. On the stability of a non-convolutional perfectly matched layer for isotropic elastic media. Soil Dyn. Earthq. Eng. 2010, 30, 68–81. [Google Scholar] [CrossRef]
- Robertson, J.; Martin, E.; Cox, B.; Treeby, B.E. Sensitivity of simulated transcranial ultrasound fields to acoustic medium property maps. Phys. Med. Biol. 2017, 62, 2559–2580. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Jung, Y.; Song, I.U.; Chung, Y.A.; Yoo, S.S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep. 2015, 5, 8743. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.B.; Hoffmann, H.; Grandchamp, P.-A. Ultrasound Axicon: A Device for Focussing Over Large Depth. J. Opt. Soc. Am. 1973, 54, 1628–1630. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Fujieda, K.; Tamura, T.; Kawamoto, S.; Takagi, T.; Okubo, K. BodyParts3D: 3D structure database for anatomical concepts. Nucleic Acids Res. 2009, 37, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Watanabe, S.; Sakurai, K.; Kunieda, E.; Watanabe, S.; Taki, M.; Yamanaka, Y. Development of realistic high-resolution whole-body voxel models of Japanese adult males and females of average height and weight, and application of models to radio-frequency electromagnetic-field dosimetry. Phys. Med. Biol. 2004, 49, 1. [Google Scholar] [CrossRef] [PubMed]


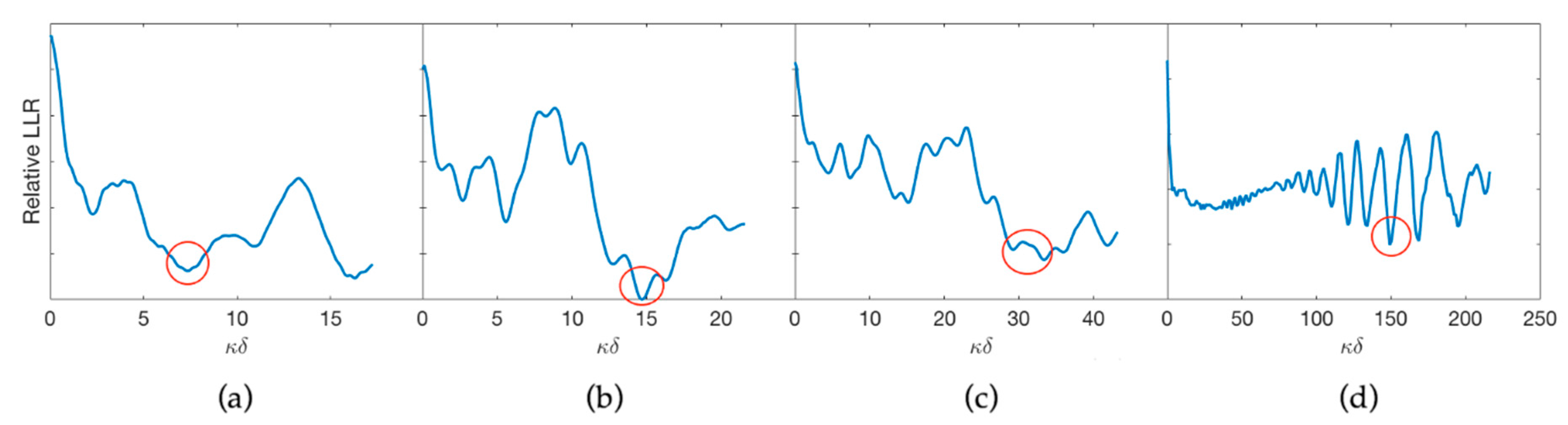
| Comp. Speed (ms−1) | Shear Speed (ms−1) | Absorption (dB cm−1) | Density (kg m−3) |
|---|---|---|---|
| 2495 ± 8 | 1081 ± 31 | 3.70 ± 0.1 | 1180 |
| Parameter | Value |
|---|---|
| Transducer frequency (f) | 0.445 MHz |
| Transducer diameter (D) | 28 mm |
| Transducer near field in water (N) | 64.75 mm (−6 dB) |
| Ratio of F/N (F/N) | 0.16 |
| Axicon lens angle (φ) | 144° |
| Focal length (F) | 10.5 mm |
| Depth of focus (DOF) | 11 mm (−3 dB)/22 mm (−6 dB) |
| Focus diameter (dF) | 2.5 mm (−3 dB)/3.5 mm (−6 dB) |
| Insertion loss (IL) | 8.4 dB |
| Stand-off (δ) | 30 mm |
| Encapsulation Echo Reduction (ER) | −12 dB |
| Thickness Skull (ts) | 0.75 mm ts < λ/4 | 1.25 mm ts < λ/4 | 1.5 mm λ/4 < ts < λ/2 | 2.5 mm λ/4 < ts < λ/2 | 3 mm λ/2 < ts < λ | 5 mm λ/2 < ts < λ | 6 mm ts > λ |
|---|---|---|---|---|---|---|---|
| F (mm) | 10.75 | 11 | 11 | 9.75 | 8.25 | 9.5 | 10.5 |
| dF (mm) | 3.5 | 3.5 | 3.5 | 5 | 4 | 4 | 4 |
| DOF (mm) | 17 | 18 | 18 | 17.5 | 17 | 17.5 | 17 |
| SLL (dB) | −8.2 | −10.3 | −11.5 | −8.4 | −8.7 | −9.9 | −9.5 |
| Beam Properties | Simulated | Scanned |
|---|---|---|
| dF (mm) | 4 | 5 |
| DOF (mm) | 17.5 | 18 |
| SLL (dB) | −9.9 | −14.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquaticci, F.; Lew, S.E.; Gwirc, S.N. Ultrasound Axicon: Systematic Approach to Optimize Focusing Resolution through Human Skull Bone. Materials 2019, 12, 3433. https://doi.org/10.3390/ma12203433
Acquaticci F, Lew SE, Gwirc SN. Ultrasound Axicon: Systematic Approach to Optimize Focusing Resolution through Human Skull Bone. Materials. 2019; 12(20):3433. https://doi.org/10.3390/ma12203433
Chicago/Turabian StyleAcquaticci, Fabián, Sergio E. Lew, and Sergio N. Gwirc. 2019. "Ultrasound Axicon: Systematic Approach to Optimize Focusing Resolution through Human Skull Bone" Materials 12, no. 20: 3433. https://doi.org/10.3390/ma12203433
APA StyleAcquaticci, F., Lew, S. E., & Gwirc, S. N. (2019). Ultrasound Axicon: Systematic Approach to Optimize Focusing Resolution through Human Skull Bone. Materials, 12(20), 3433. https://doi.org/10.3390/ma12203433




