Fabrication of Superhydrophobic Magnetic Sawdust as Effective and Recyclable Oil Sorbents
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
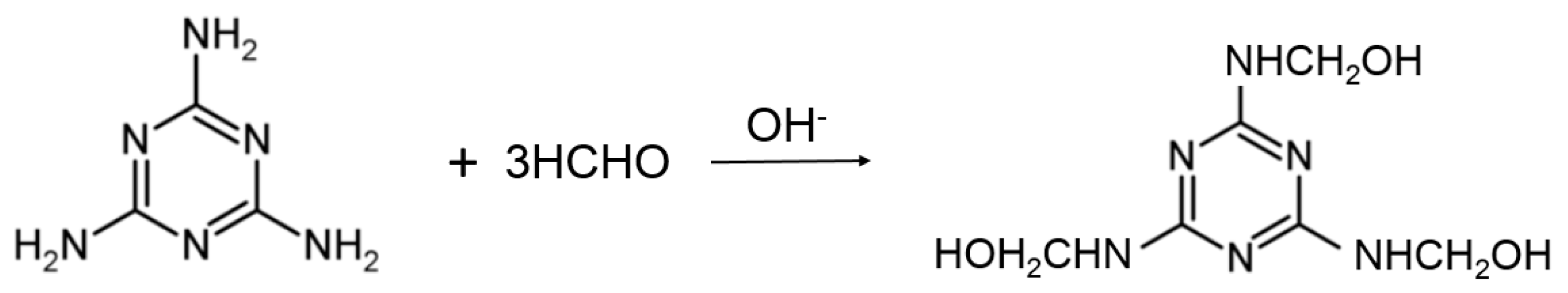
2.2.1. Preparation of Melamine Formaldehyde Resin (MFR)
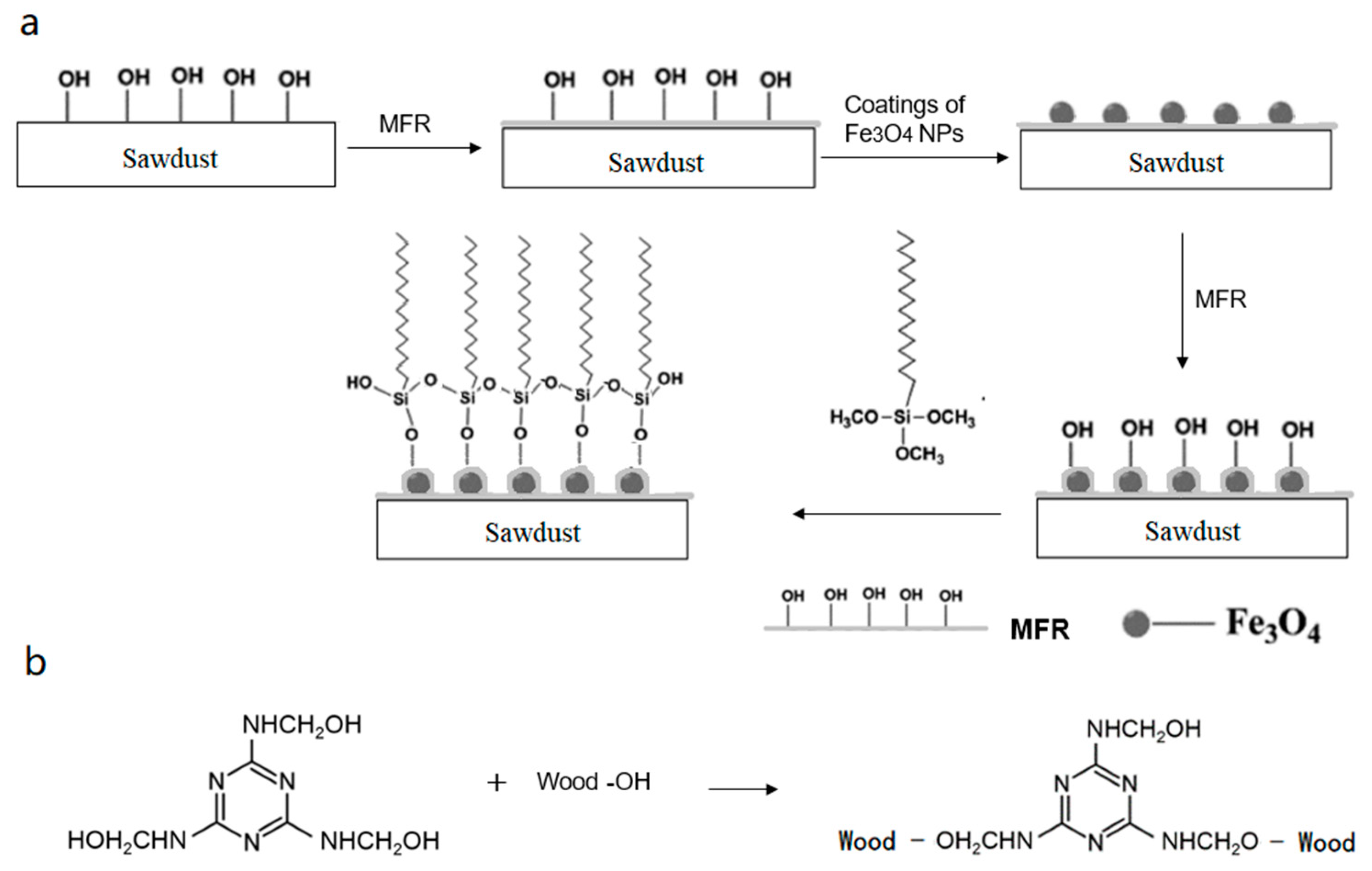
2.2.2. Preparation of Magnetic Sawdust
2.2.3. Modification of Magnetic Sawdust
2.3. Characterization
2.4. Oil Absorption Performance
3. Results and Discussion
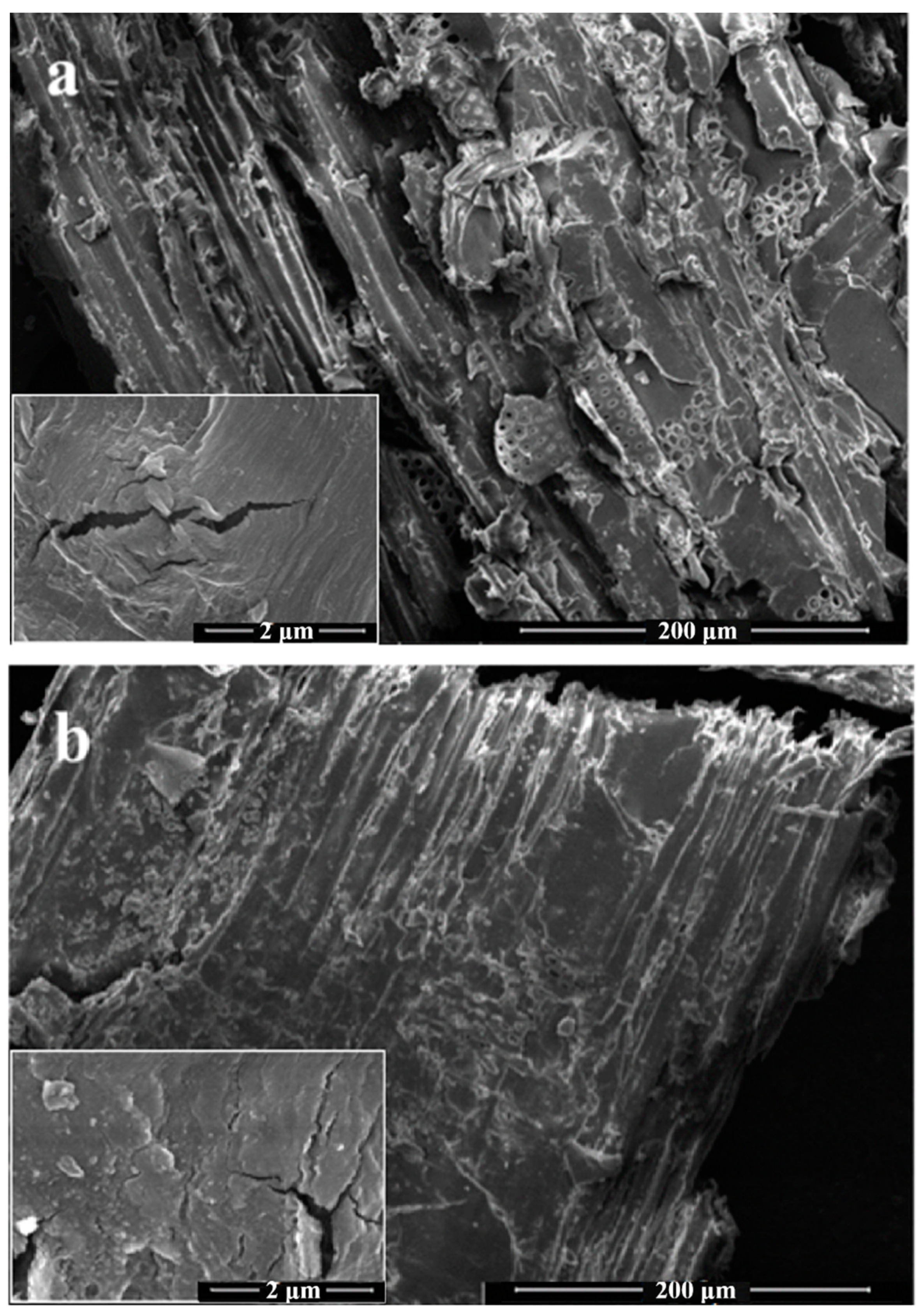
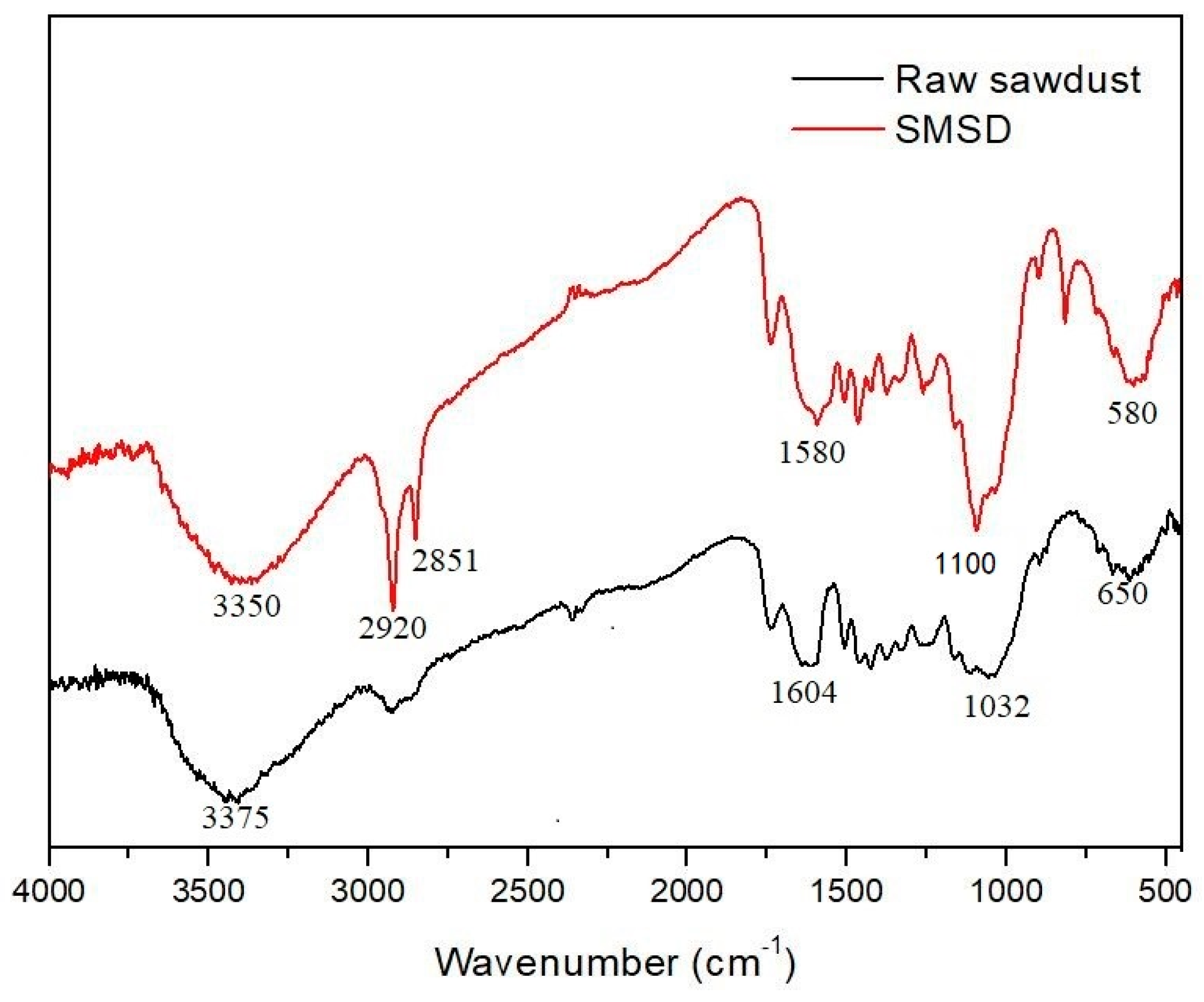
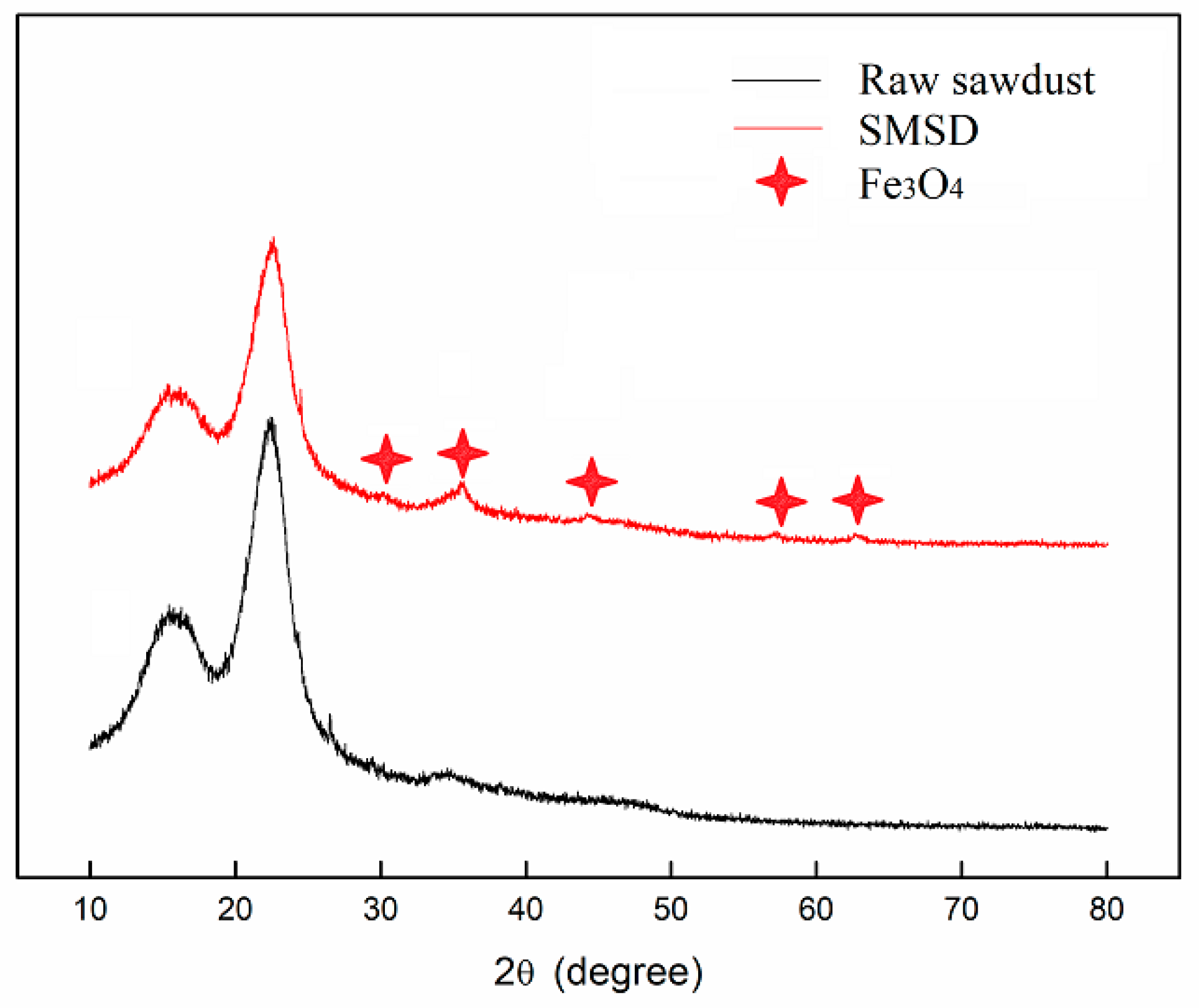
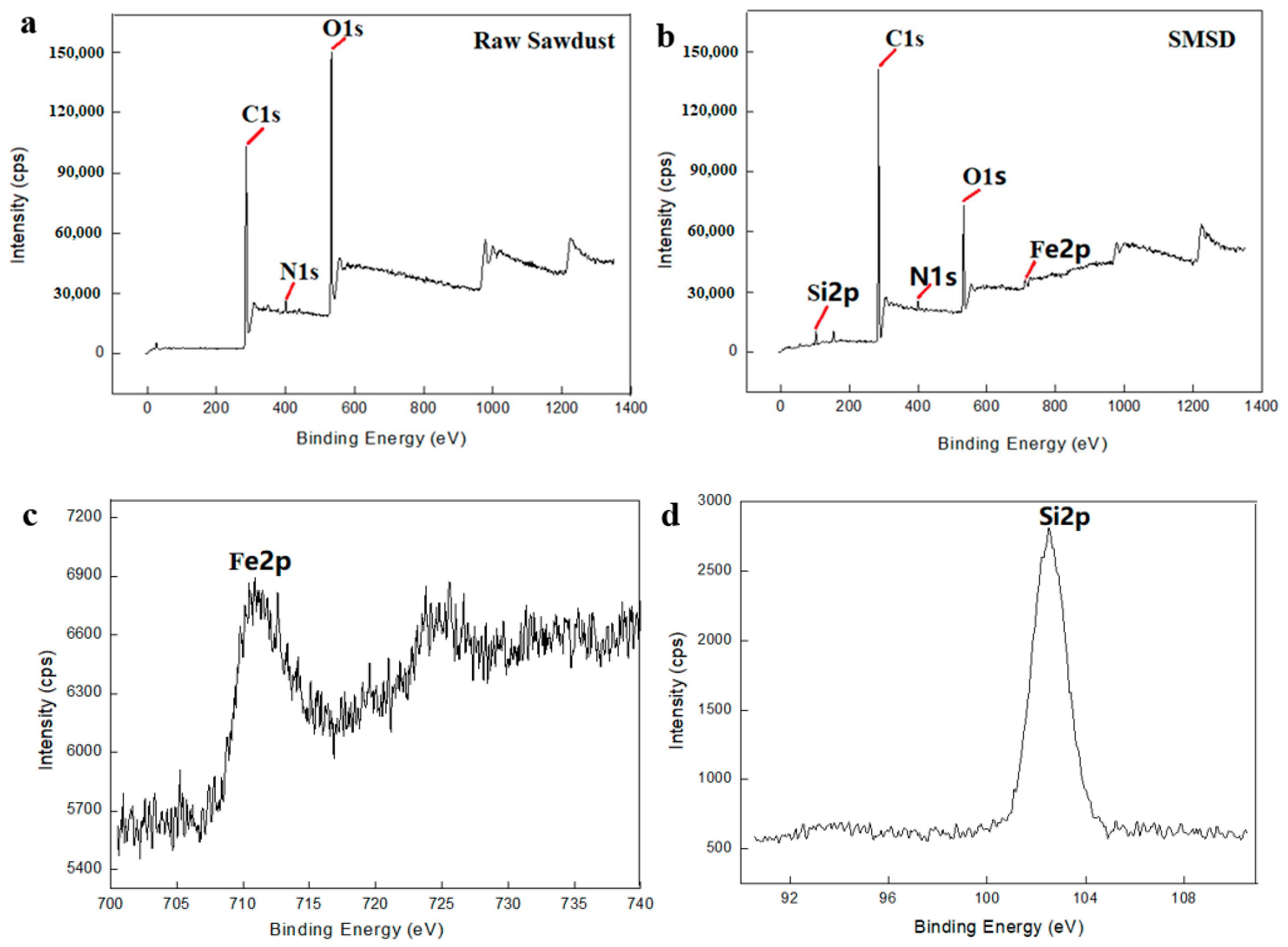
3.1. Composition and Morphology
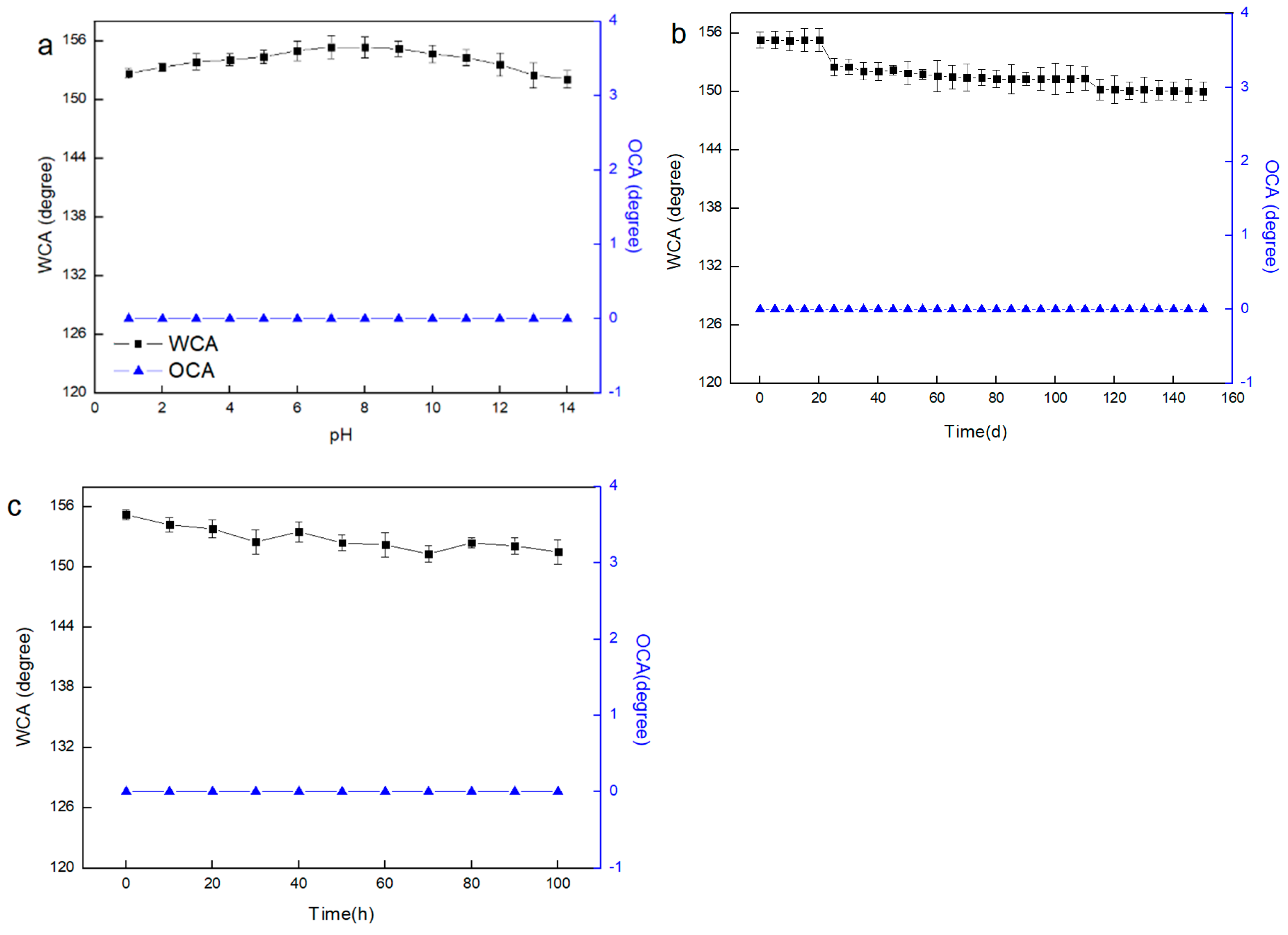
3.2. Surface Wettability
3.3. Application
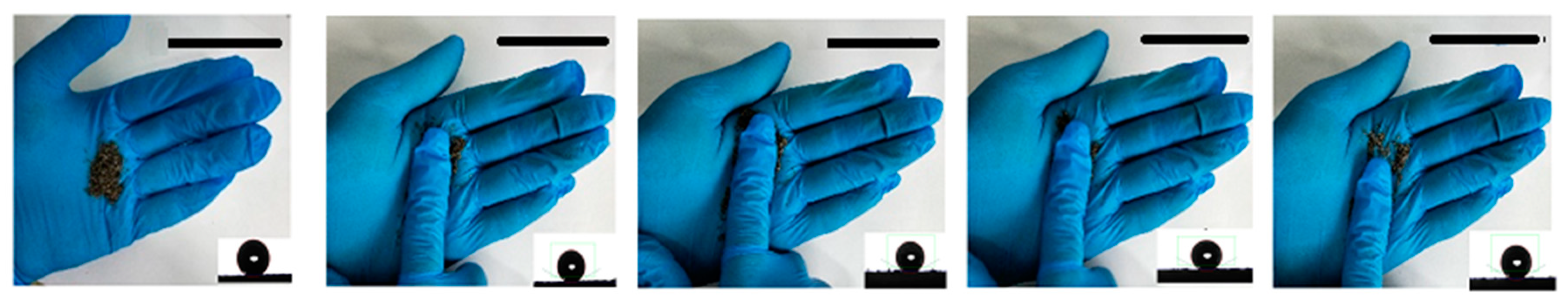
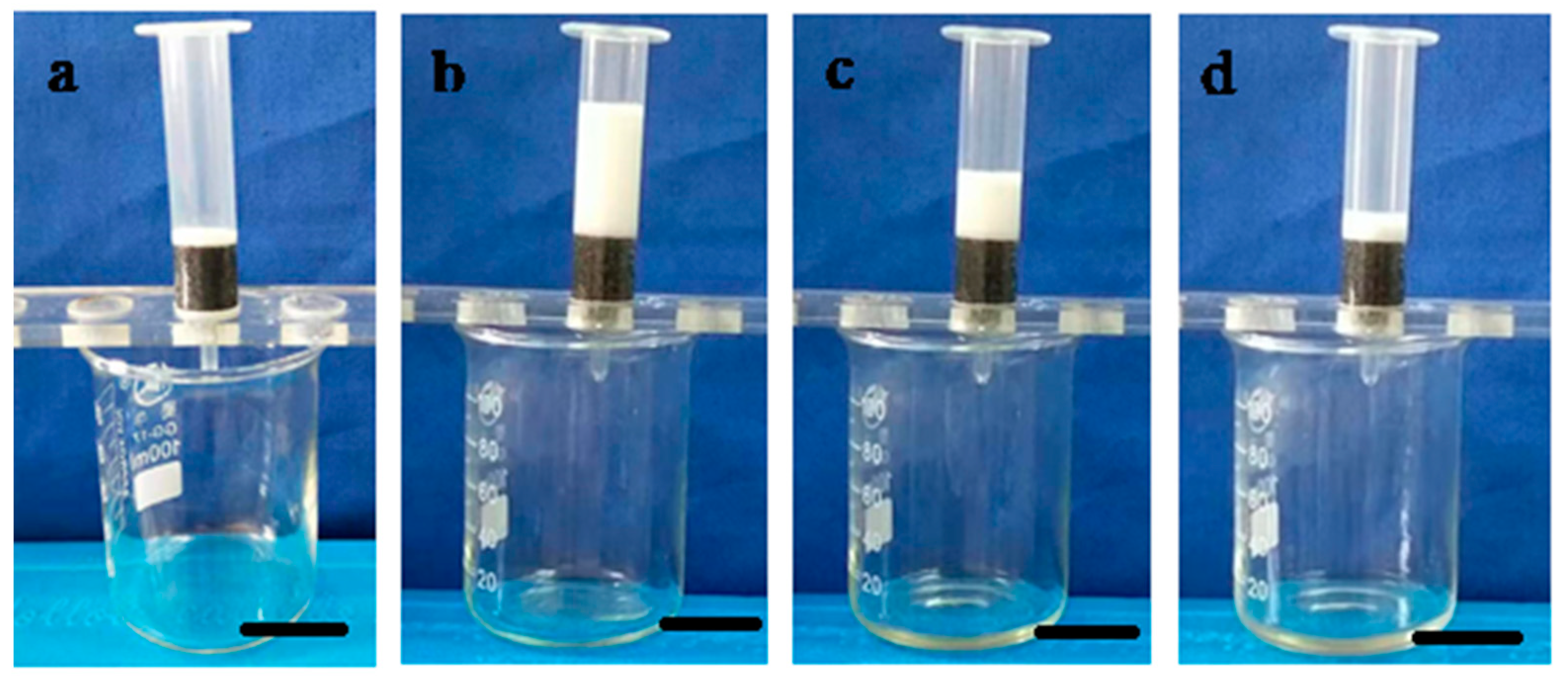
3.3.1. Oil Absorption
3.3.2. Emulsion Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, Z.; Wang, J.; Lai, H.; Du, Y.; Hou, R.; Li, C.; Zhang, N.; Sun, K. pH-controllable on-demand oil/water separation on the switchable superhydrophobic/superhydrophilic and underwater low-adhesive superoleophobic copper mesh film. Langmuir 2015, 31, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic superlyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, H.; Wu, J.; Meng, G.; Wang, Y.; Shi, Y.; Liu, Z.; Guo, X. Preparation of superhydrophobic magnetic cellulose sponge for removing oil from water. Ind. Eng. Chem. Res. 2016, 55, 832–838. [Google Scholar] [CrossRef]
- Duan, C.; Zhu, T.; Guo, J.; Wang, Z.; Liu, X.; Wang, H.; Xu, X.; Jin, Y.; Zhao, N.; Xu, J. Smart enrichment and facile separation of oil from emulsions and mixtures by superhydrophobic/superoleophilic particles. ACS Appl. Mater. Interfaces 2015, 7, 10475–10481. [Google Scholar] [CrossRef]
- Zang, D.; Zhang, M.; Liu, F.; Wang, C. Superhydrophobic/superoleophilic corn straw fibers as effective oil sorbents for the recovery of spilled oil. J. Chem. Tech. Biotech. 2016, 91, 2449–2456. [Google Scholar] [CrossRef]
- Bormashenko, E.Y. Wetting of Real Surfaces; Walter de Gruyter GmbH: Berlin, Germany, 2017. [Google Scholar]
- Gao, L.; Lu, Y.; Zhan, X.; Li, J.; Sun, Q. A robust, anti-acid, and high-temperature—Humidity-resistant superhydrophobic surface of wood based on a modified TiO2 film by fluoroalkyl silane. Surf. Coat. Technol. 2015, 262, 33–39. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Chen, H.; Chen, D. Superhydrophobic behavior achieved from hydrophilic surfaces. Appl. Phys. Lett. 2009, 95, 084104. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.R.; Franssil, S. Robust Superhydrophobic Silicon without a Low Surface-Energy Hydrophobic Coating. Appl. Mater. Interfaces 2015, 7, 941–949. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Tang, J.; Gong, H.; Liu, Y.; Wang, Z.; Shi, P.; Zhang, J.; Chen, X. A novel preparation of polystyrene film with a superhydrophobic surface using a template method. J. Phys. D Appl. Phys. 2007, 40, 3485–3489. [Google Scholar] [CrossRef]
- Xue, C.H.; Li, Y.R.; Zhang, P.; Ma, J.Z.; Jia, S.T. Washable and wear-resistant superhydrophobic surfaces with self-cleaning property by chemical etching of fibers and hydrophobization. ACS Appl. Mater. Interfaces 2014, 6, 10153–10161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, X.; Shen, J.; Yang, G.; Huang, H. Fluorocarbon thin film with superhydrophobic property prepared by pyrolysis of hexafluoropropylene oxide. Appl. Surf. Sci. 2012, 258, 9782–9785. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Gao, P.; Ma, H.; We, Q. Superhydrophobic hybrid films prepared from silica nanoparticles and ionic liquids via layer-by-layer self-assembly. Thin Solid Films 2014, 570, 27–32. [Google Scholar] [CrossRef]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.L.; Guarda, P.A.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon 2013, 59, 150–159. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, L.; Chen, A.; Song, Q.; Chen, C. A rapid one-step process for fabrication of superhydrophobic surface by electrodeposition method. Electrochim. Acta 2012, 59, 168–171. [Google Scholar] [CrossRef]
- Taurino, R.; Fabbri, E.; Pospiech, D.; Synytska, A.; Messori, M. Preparation of scratch resistant superhydrophobic hybrid coatings by sol–gel process. Prog. Org. Coat. 2014, 77, 1635–1641. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability. Chem. Eng. J. 2013, 228, 415–424. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Zhang, M.; Ma, M.; Wang, C.; Li, J. Improvement of mechanical robustness of the superhydrophobic wood surface by coating PVA/SiO2 composite polymer. Appl. Surf. Sci. 2013, 280, 686–692. [Google Scholar] [CrossRef]
- Hurst, S.M.; Farshchian, B.; Choi, J.; Kim, J.; Park, S. A universally applicable method for fabricating superhydrophobic polymer surfaces. Colloids Surf. A 2012, 407, 85–90. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy. Mater. Lett. 2014, 137, 210–213. [Google Scholar] [CrossRef]
- Ji, H.; Chen, G.; Yang, J.; Hu, J.; Song, H.; Zhao, Y. A simple approach to fabricate stable superhydrophobic glass surfaces. Appl. Surf. Sci. 2013, 266, 105–109. [Google Scholar] [CrossRef]
- Park, J.; Lim, H.; Kim, W.; Ko, J.S. Design and fabrication of a superhydrophobic glass surface with micro-network of nanopillars. J. Colloid Interface Sci. 2011, 360, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.; Liu, F.; Zhang, M.; Niu, X.; Gao, Z.; Wang, C. Superhydrophobic coating on fiberglass cloth for selective removal of oil from water. Chem. Eng. J. 2015, 262, 210–216. [Google Scholar] [CrossRef]
- Huang, X.; Wen, X.; Cheng, J.; Yang, Z. Sticky superhydrophobic filter paper developed by dip-coating of fluorinated waterborne epoxy emulsion. Appl. Surf. Sci. 2012, 258, 8739–8746. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of coral-like superhydrophobic coating on filter paper for water–oil separation. Appl. Surf. Sci. 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Zang, D.; Liu, F.; Zhang, M.; Gao, Z.; Wang, C. Novel superhydrophobic and superoleophilic sawdust as a selective oil sorbent for oil spill cleanup. Chem. Eng. Res. Des. 2015, 102, 34–41. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Li, J.; Cao, J. Self-Assembly of a Superhydrophobic ZnO Nanorod Arrays Film on Wood Surface Using a Hydrothermal Method. Key Eng. Mater. 2014, 609, 468–471. [Google Scholar] [CrossRef]




| Oils | Liquid Paraffin | Ethyl Acetate | Benzene | Soya-Bean Oil | Diesel Oil | Engine Oil |
|---|---|---|---|---|---|---|
| Surface tension (mN·m−1) | 26.2 (60 °C) | 26.3 (25 °C) | 28.2 (25 °C) | 34.2 (25 °C) | 26.8 (25 °C) | 40.5 (25 °C) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Pei, S.; Shen, T.; Xu, G.; Li, Y.; Fan, W. Fabrication of Superhydrophobic Magnetic Sawdust as Effective and Recyclable Oil Sorbents. Materials 2019, 12, 3432. https://doi.org/10.3390/ma12203432
Fan S, Pei S, Shen T, Xu G, Li Y, Fan W. Fabrication of Superhydrophobic Magnetic Sawdust as Effective and Recyclable Oil Sorbents. Materials. 2019; 12(20):3432. https://doi.org/10.3390/ma12203432
Chicago/Turabian StyleFan, Shumin, Shuai Pei, Tianyu Shen, Guangri Xu, Yuanchao Li, and Wenxiu Fan. 2019. "Fabrication of Superhydrophobic Magnetic Sawdust as Effective and Recyclable Oil Sorbents" Materials 12, no. 20: 3432. https://doi.org/10.3390/ma12203432
APA StyleFan, S., Pei, S., Shen, T., Xu, G., Li, Y., & Fan, W. (2019). Fabrication of Superhydrophobic Magnetic Sawdust as Effective and Recyclable Oil Sorbents. Materials, 12(20), 3432. https://doi.org/10.3390/ma12203432





