Influence of the Presence of Different Alkali Cations and the Amount of Fe(CN)6 Vacancies on CO2 Adsorption on Copper Hexacyanoferrates
Abstract
:1. Introduction
2. Materials and Methods
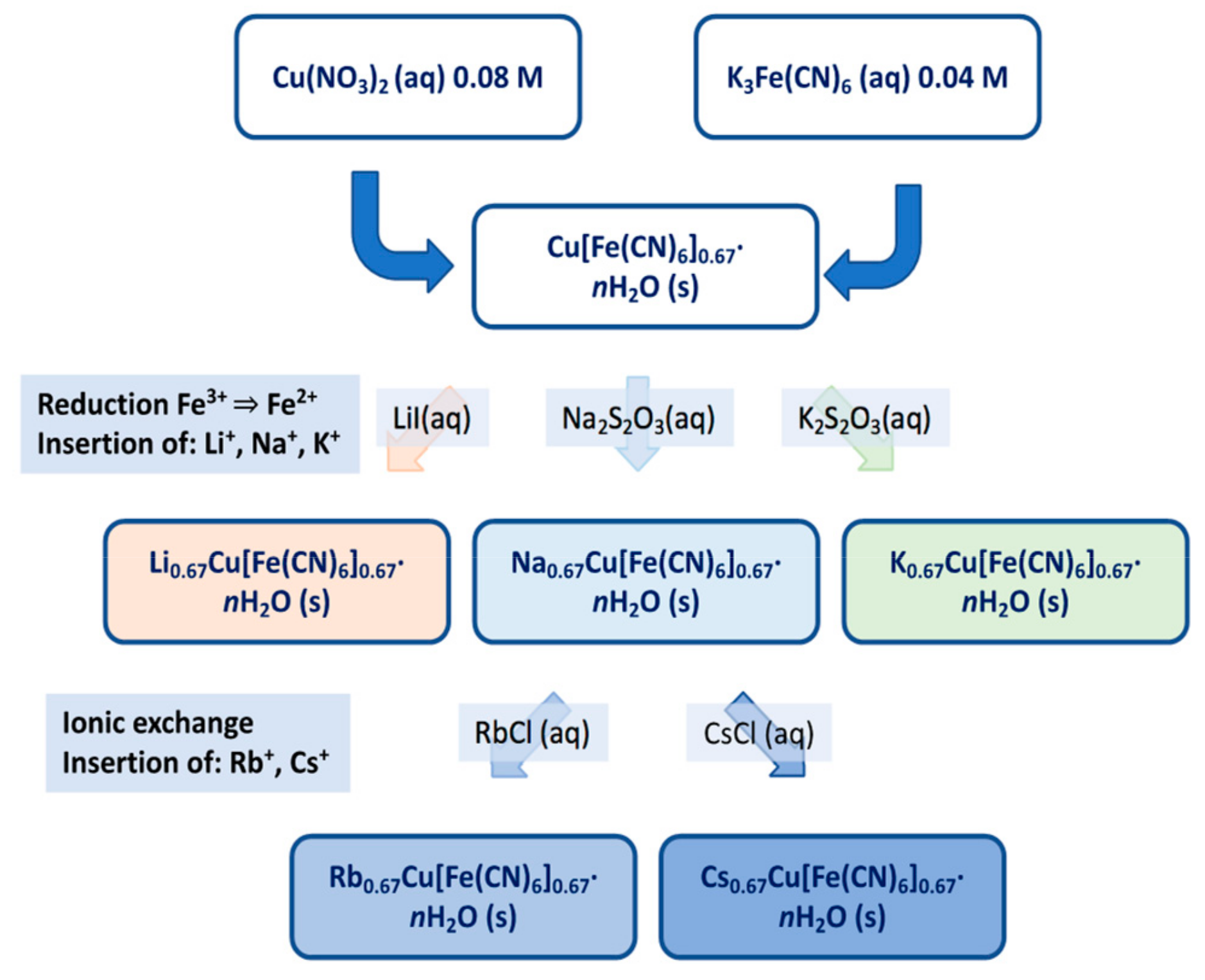
2.1. Synthesis
2.2. Validation of Compositions and Structures
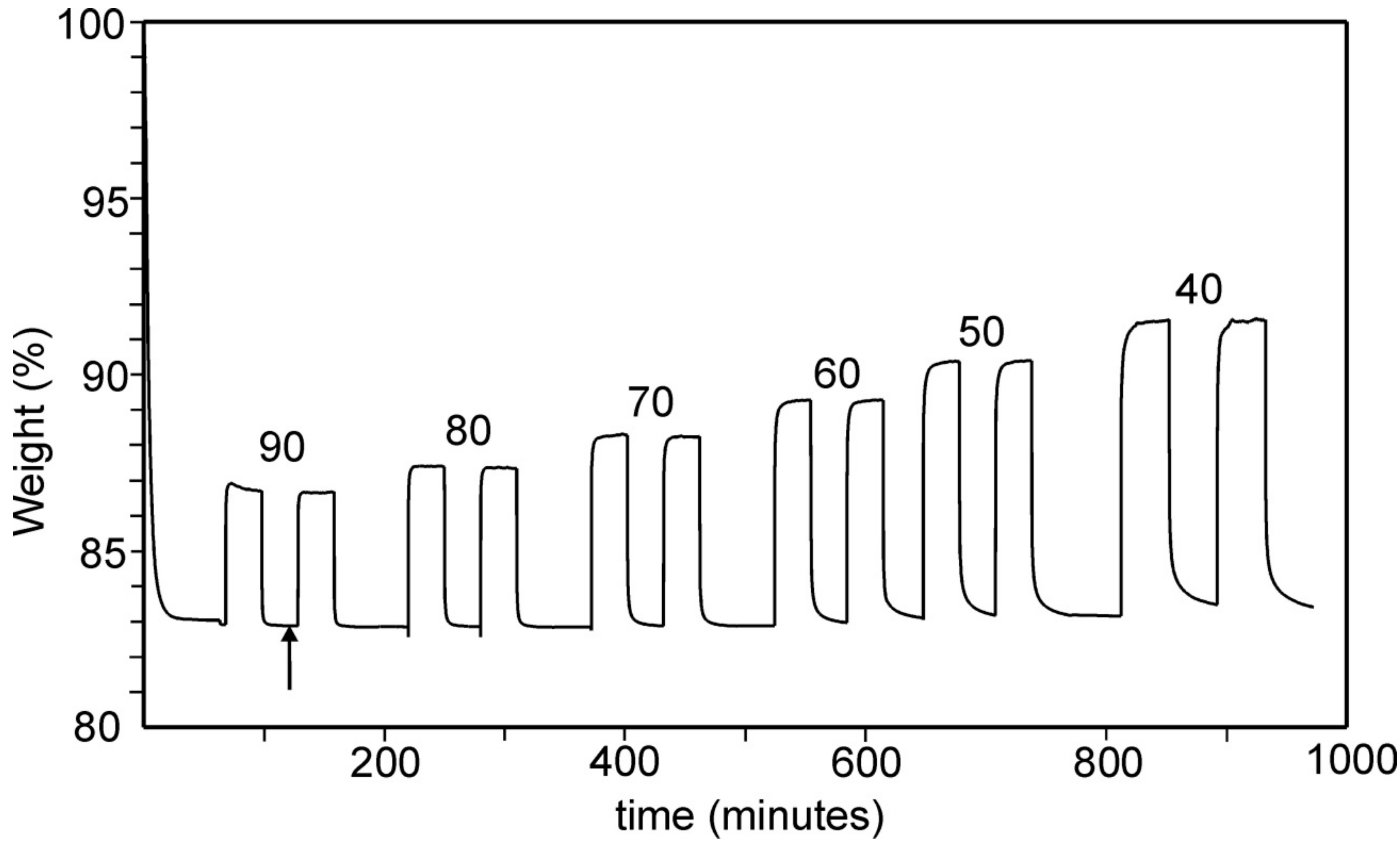
2.3. CO2 Adsorption by Thermogravimetric Analysis
3. Results
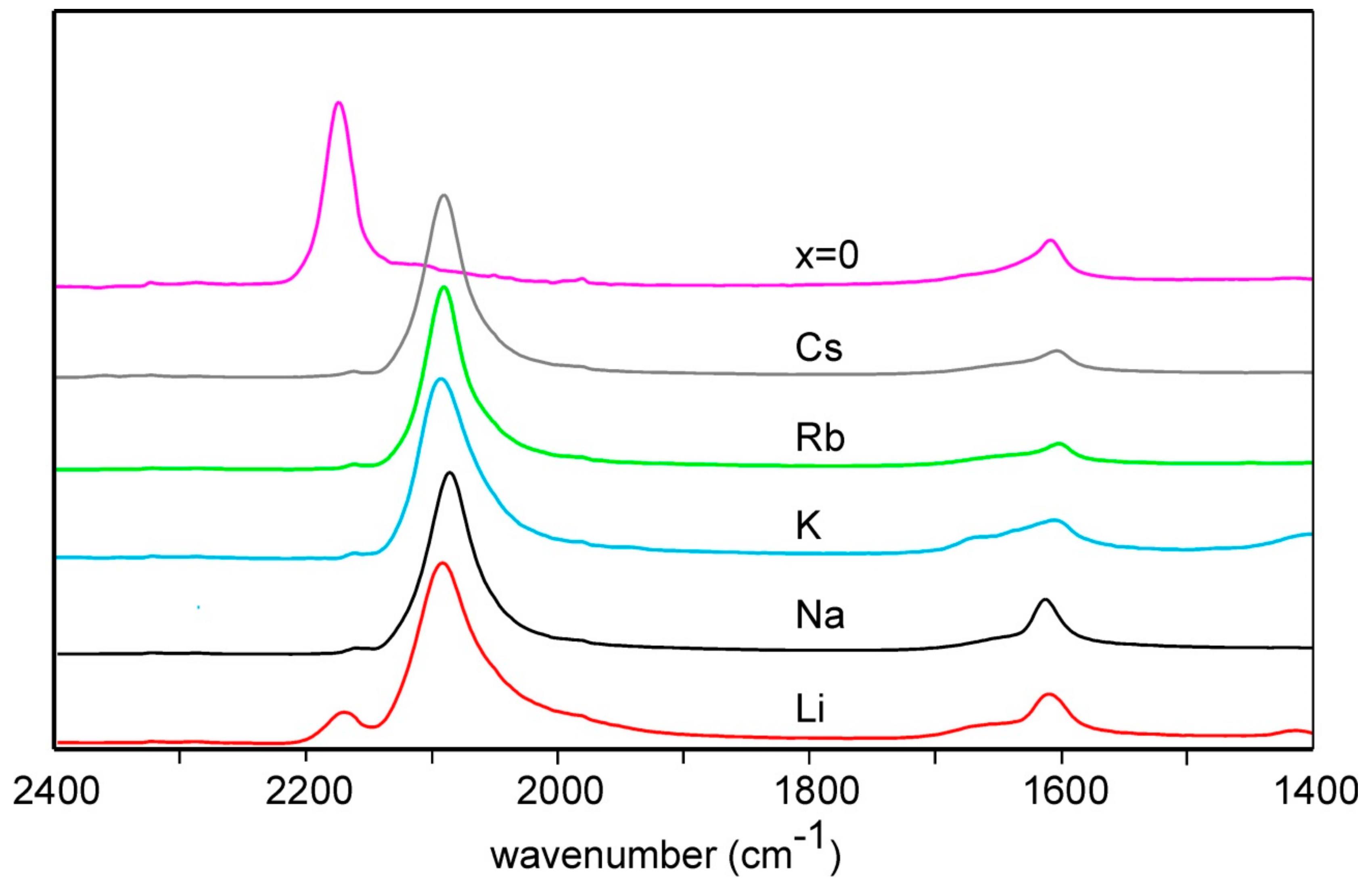
3.1. Scanning Electron Microscopy, Energy Dispersive Spectroscopy and Infra-Red Spectroscopy
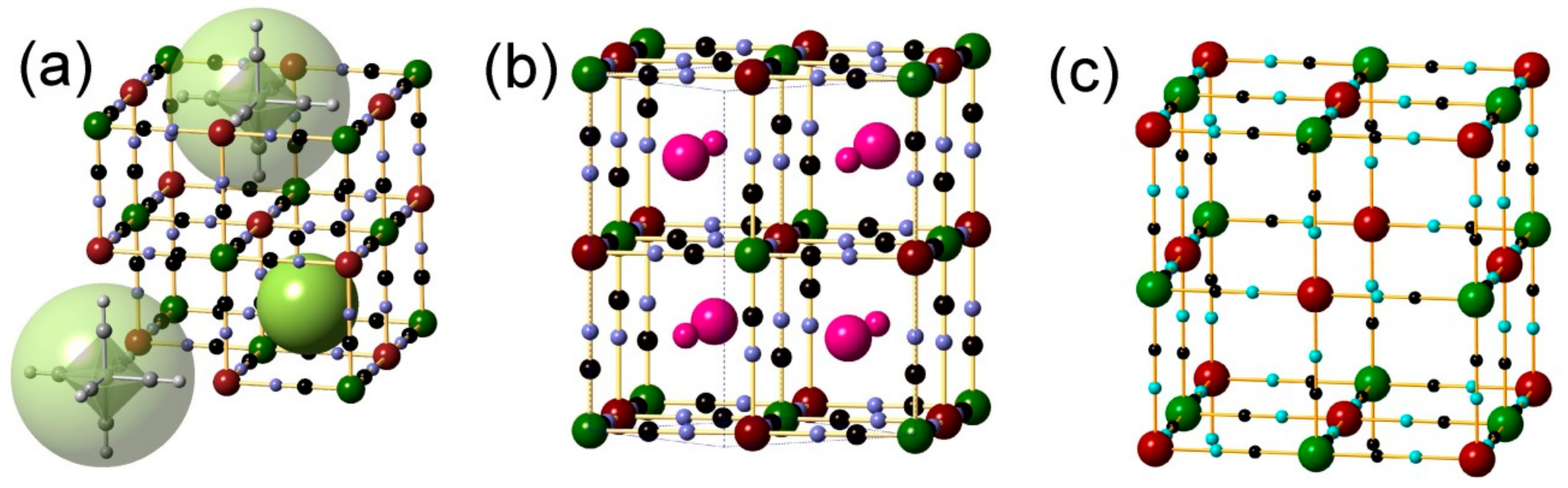
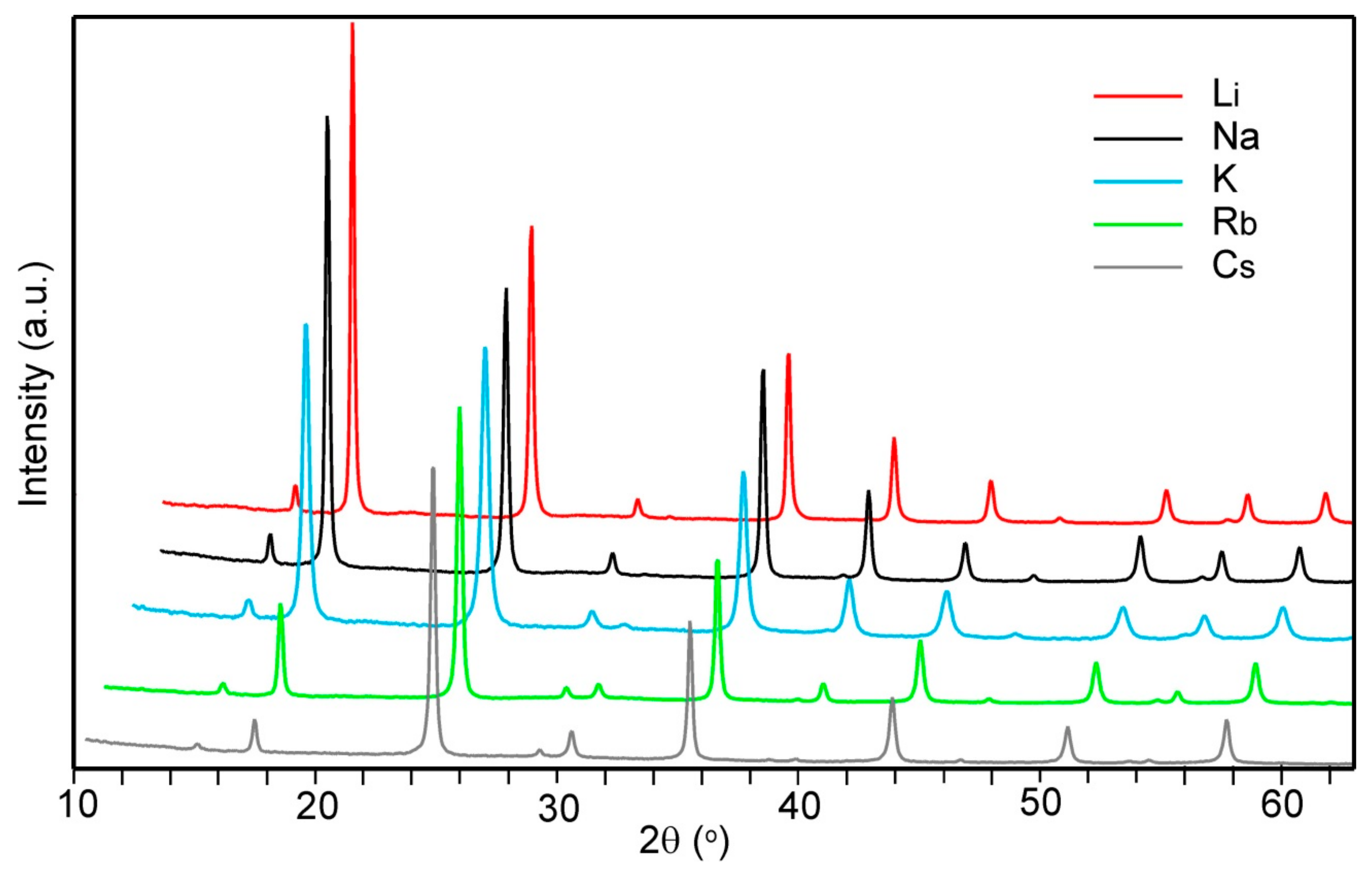
3.2. Powder X-ray Diffraction
3.3. Thermogravimetric Analysis
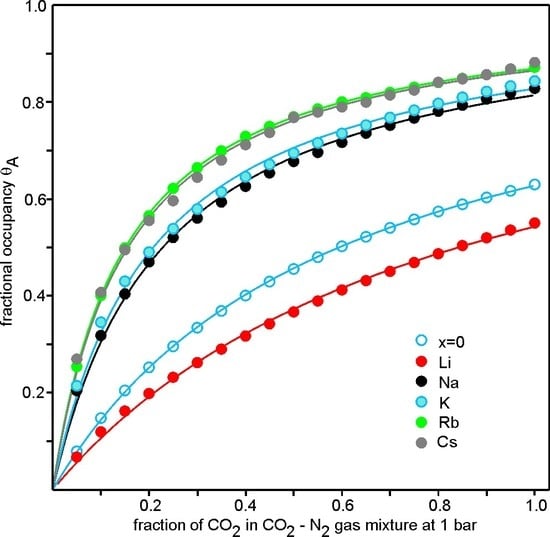
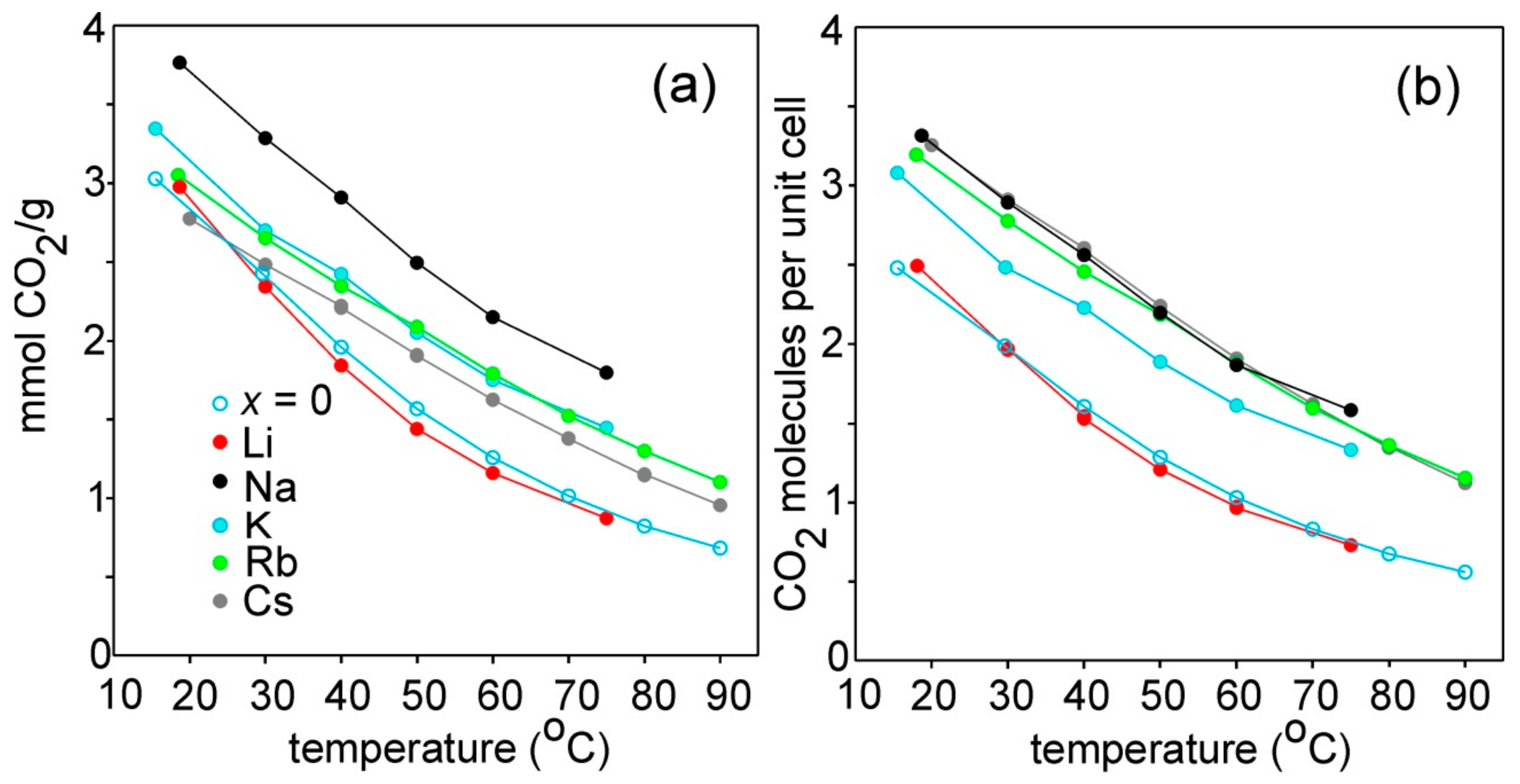
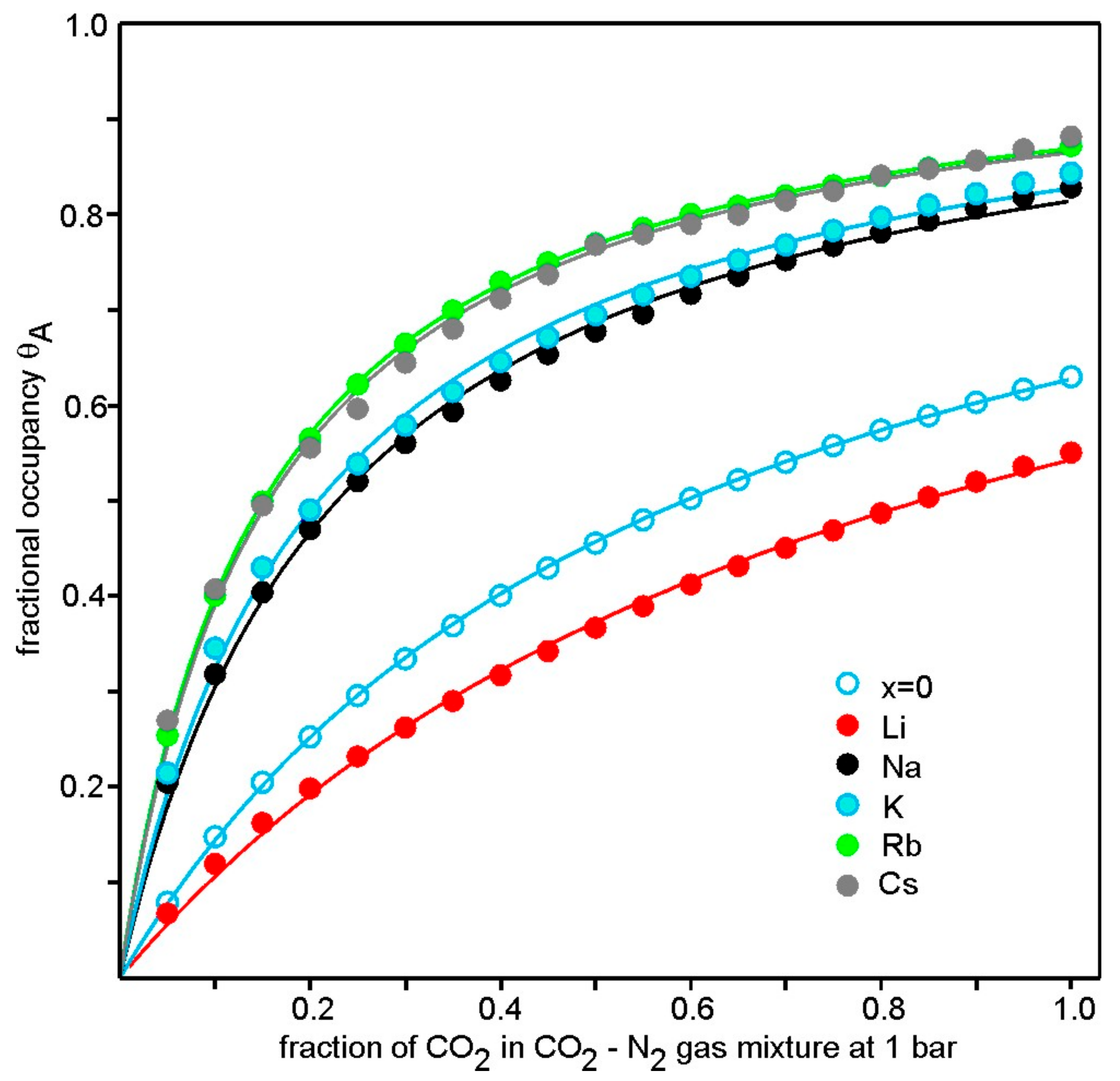
3.4. CO2 Adsorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karadas, F.; El-Faki, H.; Deniz, E.; Yavuz, C.T.; Aparicio, S.; Atilhan, M. CO2 adsorption studies on Prussian blue analogues. Microporous Mesoporous Mater. 2012, 162, 91–97. [Google Scholar] [CrossRef]
- Ogilvie, S.H.; Duyker, S.G.; Southon, P.D.; Peterson, V.K.; Kepert, C.J. Identification of bridged CO2 binding in a Prussian blue analogue using neutron powder diffraction. Chem. Commun. 2013, 49, 9404–9406. [Google Scholar] [CrossRef] [PubMed]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Ojwang, D.; Grins, J.; Svensson, G. The adsorption kinetics of CO2 on copper hexacyanoferrate studied by thermogravimetric analysis. Microporous Mesoporous Mater. 2018, 272, 70–78. [Google Scholar] [CrossRef]
- Verdaguer, M.; Girolami, G.S. Magnetic Prussian Blue Analogs. In Magnetism Molecules to Materials; Miller, J.S., Drillon, M., Eds.; Wiley-VCH: New York, NY, USA, 2005; Volume 5, pp. 283–346. [Google Scholar]
- Buser, H.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3·xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Ojwang, D.O.; Grins, J.; Wardecki, D.; Valvo, M.; Renman, V.; Häggström, L.; Ericsson, T.; Gustafsson, T.; Mahmoud, A.; Hermann, R.P.; et al. Structure characterization and properties of K-containing copper hexacyanoferrate. Inorg. Chem. 2016, 55, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- Wardecki, D.; Ojwang, D.O.; Grins, J.; Svensson, G. Neutron diffraction and EXAFS studies of K2x/3Cu[Fe(CN)6]2/3·nH2O. Cryst. Growth Des. 2017, 17, 1285–1292. [Google Scholar] [CrossRef]
- Åkerblom, I.E.; Ojwang, D.O.; Grins, J.; Svensson, G. A thermogravimetric study of thermal dehydration of copper hexacyanoferrate by means of model-free kinetic analysis. J. Therm. Anal. Calorim. 2017. [Google Scholar] [CrossRef]
- Ayrault, S.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.J.; Loos-Neskovic, C. Sorption mechanism of Caesium on Cu2[Fe(CN)6] and Cu3[Fe(CN)6]2: Hexacyanoferrates and their relation to the crystalline structure. J. Solid State Chem. 1998, 141, 475–485. [Google Scholar] [CrossRef]
- Avila, M.; Reguera, L.; Rodríguez-Hernández, J.; Balmaseda, J.; Reguera, E. Porous framework of T2[Fe(CN)6] xH2O with T = Co, Ni, Cu, Zn, and H2 storage. J. Solid State Chem. 2008, 181, 2899–2907. [Google Scholar] [CrossRef]
- Ayrault, S.; Loos-Neskovic, C.; Fedoroff, M.; Garnier, E. Copper hexacyanoferrates: Preparation, composition and structure. Talanta 1994, 41, 1435–1452. [Google Scholar] [CrossRef]
- Matsuda, T.; Kim, J.; Moritomo, Y. Control of the alkali cation alignment in Prussian Blue framework. Dalton Trans. 2012, 41, 7620–7623. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carjaval, J. Fullprof: A Program for Rietveld Refinement and Pattern Matching analysis. In Proceedings of the Abstract of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Tolouse, France, 16–19 July 1990; p. 127. [Google Scholar]
- Sato, O.; Einaga, Y.; Fujishima, A.; Hashimoto, K. Photoinduced longe-range magnetic ordering of a cobalt-iron cyanide. Inorg. Chem. 1999, 38, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nature Commun. 2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, M.; Bian, X.; Wu, H.; Xu, M. Heat-induced changes in microstructure and magnetic properties of Co0.75Ni0.75[Fe(CN)6]·xH2O. J. Coord. Chem. 2010, 63, 3907–3913. [Google Scholar] [CrossRef]
- Ng, C.W.; Ding, J.; Shi, Y.; Gan, L.M. Structure and magnetic properties of copper(II) hexacyanoferrate(III) compound. J. Phys. Chem. Solids 2001, 62, 767–775. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.K.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review. Int. J. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef]


| Nominal Composition | Fe | Li/Na/K/Rb/Cs | K | Fraction of Fe2+ |
|---|---|---|---|---|
| Cu[Fe(CN)6]2/3 (x = 0) * | 0.67(3) | - | 0.06(3) | 0.0 |
| Li2/3Cu[Fe(CN)6]2/3 | 0.6(1) | - | 0.03(1) | 0.83(2) |
| Na2/3Cu[Fe(CN)6]2/3 | 0.69(7) | 0.68(6) | 0.02(1) | 0.95(2) |
| K2/3Cu[Fe(CN)6]2/3* | 0.64(1) | 0.61(3) | 1.0 | |
| Rb2/3Cu[Fe(CN)6]2/3 | 0.8(1) | 0.70(7) | 0.01(1) | 0.96(2) |
| Cs2/3Cu[Fe(CN)6]2/3 | 0.69(6) | 0.63(9) | 0.02(1) | 0.96(2) |
| Cu[Fe(CN)6]1/2 | 0.56(6) | - | - | 1.0 |
| KCu[Fe(CN)6]3/4 | 0.75(9) | 1.1(1) | - | 1.0 |
| CsCu[Fe(CN)6] | 1.0(1) | 0.9(1) | - | 1.0 |
| A | χ2 | RF | B(Fe/Cu) | B(coor) | B(A/zeol) | sof(Fe) | n(zeol) | n(coor) | n(tot) |
|---|---|---|---|---|---|---|---|---|---|
| x = 0 * | 2.1 | 3.6 | 1.80(2) | 3 | 2 | 0.70(1) | 5.3(4) | 2.2(2) | 7.5(4) |
| Li | 4.9 | 8.8 | 3.5(1) | 5.5(6) | 12(1) | 0.74(2) | 6.7(5) | 2.0(8) | 8.7(9) |
| Na | 5.1 | 7.0 | 3.3(1) | 4.0(4) | 12.7(7) | 0.69(1) | 5.6(3) | 5.2(5) | 10.8(6) |
| K | 2.6 | 4.9 | 2.7(1) | 5.6(5) | 11.0(6) | 0.70(1) | 5.5(3) | 4.4(6) | 10.4(7) |
| Rb | 4.9 | 5.0 | 1.7(1) | 3.9(4) | 6.9(2) | 0.74(2) | 6.5(3) | 2.7(5) | 9.2(6) |
| Cs | 3.7 | 6.8 | 2.4(2) | 3.7(6) | 4.6(3) | 0.75(2) | 6.2(5) | 3.6(8) | 9.8(9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svensson, G.; Grins, J.; Eklöf, D.; Eriksson, L.; Wardecki, D.; Thoral, C.; Bodoignet, L. Influence of the Presence of Different Alkali Cations and the Amount of Fe(CN)6 Vacancies on CO2 Adsorption on Copper Hexacyanoferrates. Materials 2019, 12, 3371. https://doi.org/10.3390/ma12203371
Svensson G, Grins J, Eklöf D, Eriksson L, Wardecki D, Thoral C, Bodoignet L. Influence of the Presence of Different Alkali Cations and the Amount of Fe(CN)6 Vacancies on CO2 Adsorption on Copper Hexacyanoferrates. Materials. 2019; 12(20):3371. https://doi.org/10.3390/ma12203371
Chicago/Turabian StyleSvensson, Gunnar, Jekabs Grins, Daniel Eklöf, Lars Eriksson, Darius Wardecki, Clara Thoral, and Loic Bodoignet. 2019. "Influence of the Presence of Different Alkali Cations and the Amount of Fe(CN)6 Vacancies on CO2 Adsorption on Copper Hexacyanoferrates" Materials 12, no. 20: 3371. https://doi.org/10.3390/ma12203371
APA StyleSvensson, G., Grins, J., Eklöf, D., Eriksson, L., Wardecki, D., Thoral, C., & Bodoignet, L. (2019). Influence of the Presence of Different Alkali Cations and the Amount of Fe(CN)6 Vacancies on CO2 Adsorption on Copper Hexacyanoferrates. Materials, 12(20), 3371. https://doi.org/10.3390/ma12203371