Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
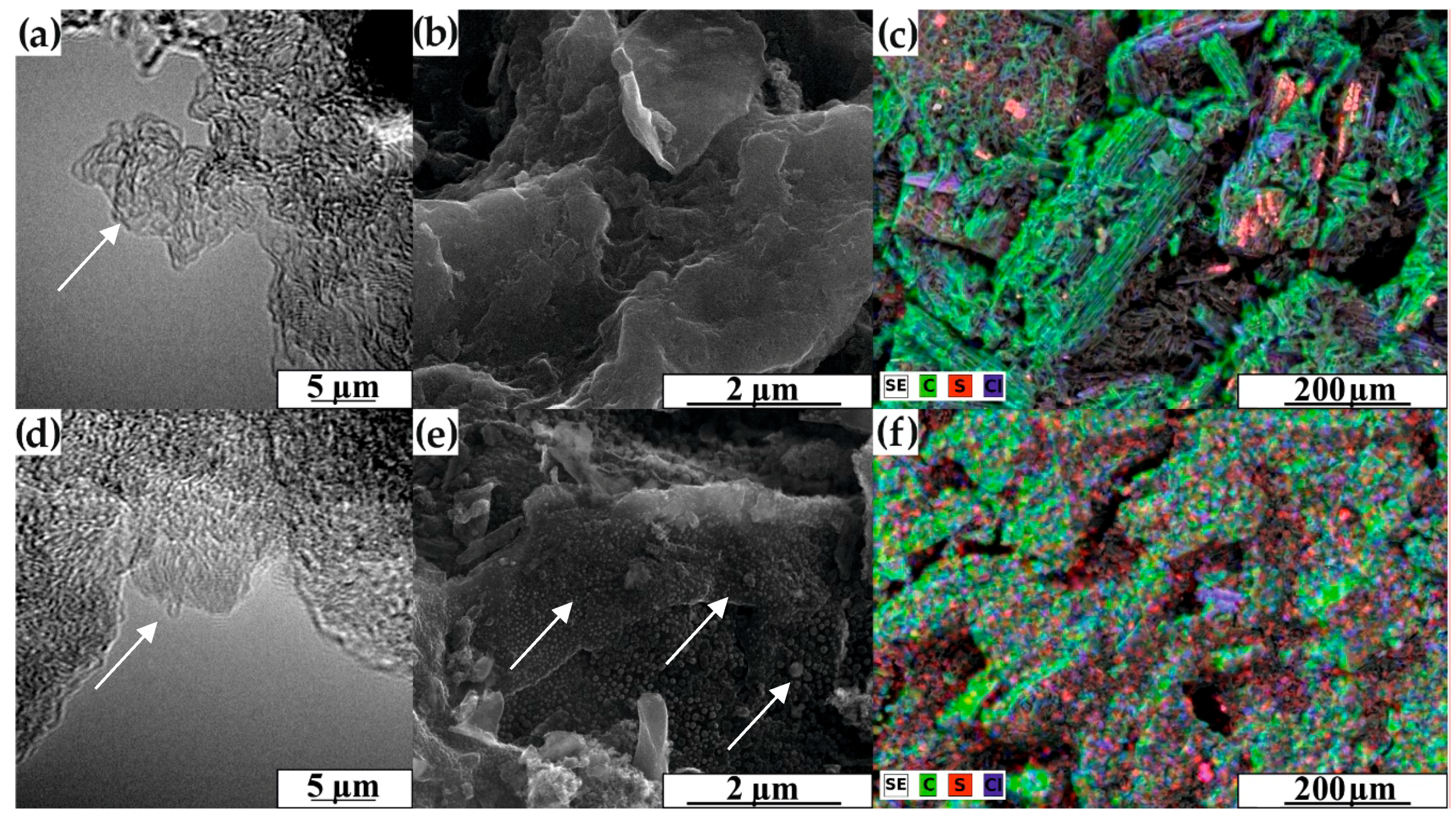
3.1. Morphology and Structural Characterization
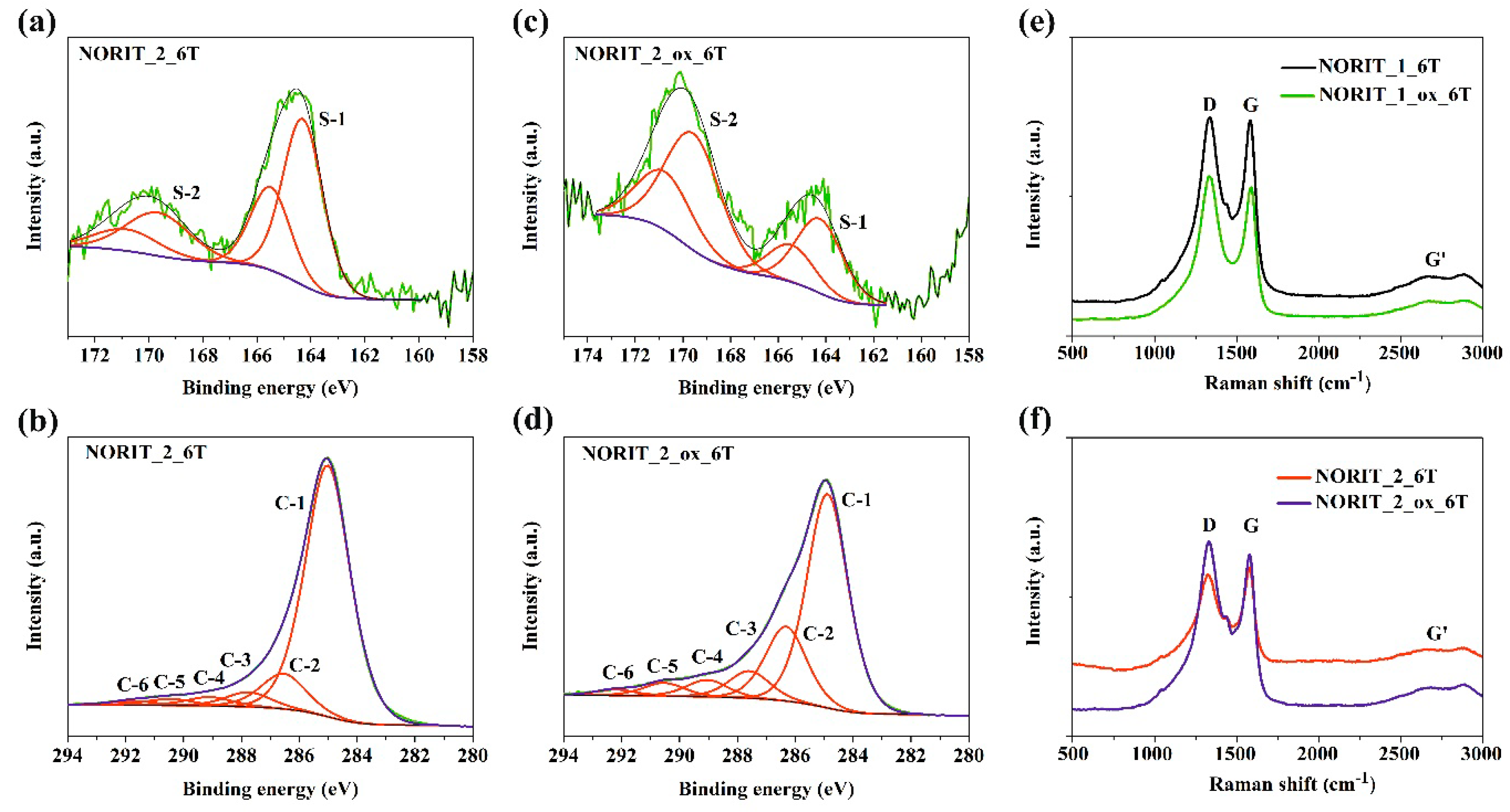
3.2. XPS and Raman Spectroscopy Results
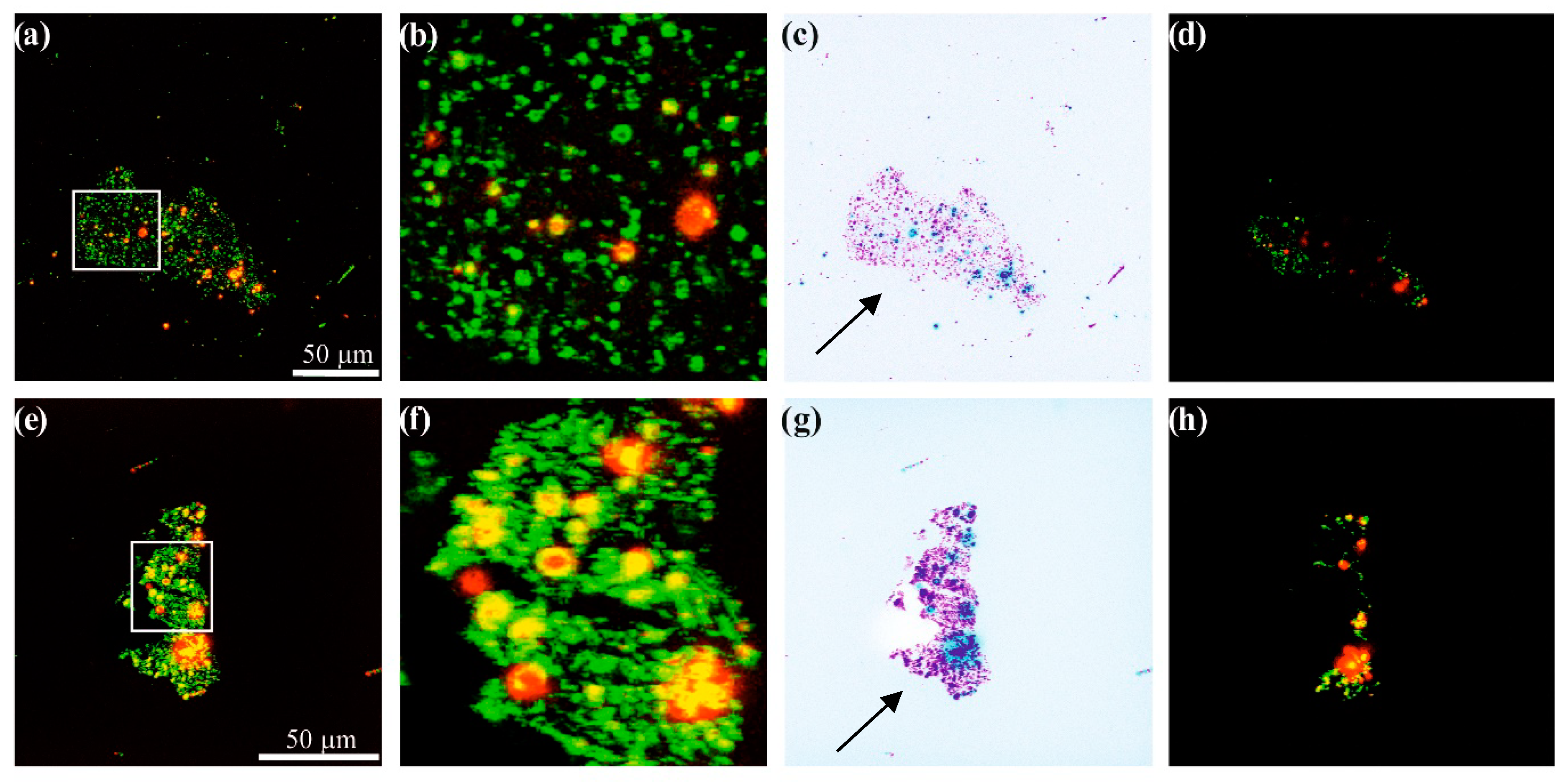
3.3. Confocal Microscopy Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, C.; Chen, X.; Dai, Q.; Wang, M.; Qu, L.; Dai, L. Earth-abundant carbon catalysts for renewable generation of clean energy from sunlight and water. Nano Energy 2017, 41, 367–376. [Google Scholar] [CrossRef]
- Selopal, G.S.; Wu, H.-P.; Lu, J.; Chang, Y.-C.; Wang, M.; Vomiero, A.; Concina, I.; Diau, E.W.-G. Metal-free organic dyes for TiO2 and ZnO dye-sensitized solar cells. Sci. Rep. 2016, 6, 18756. [Google Scholar] [CrossRef] [PubMed]
- Kamedulski, P.; Kaczmarek-Kedziera, A.; Lukaszewicz, J.P. Influence of intermolecular interactions on the properties of carbon nanotubes. Bull. Mater. Sci. 2018, 41, 1–14. [Google Scholar] [CrossRef]
- Loi, M.A.; Gao, J.; Cordella, F.; Blondeau, P.; Menna, E.; Bártová, B.; Hébert, C.; Lazar, S.; Botton, G.A.; Milko, M.; et al. Encapsulation of conjugated oligomers in single-walled carbon nanotubes: Towards nanohybrids for photonic devices. Adv. Mater. 2010, 22, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Sun, S.; Abe, Y.; Krishna, A.; Grimsdale, A.C.; Lam, Y.M. Perovskite-based solar cells: Impact of morphology and device architecture on device performance. J. Mater. Chem. A 2015, 3, 8943–8969. [Google Scholar] [CrossRef]
- Ooyama, Y.; Uenaka, K.; Kamimura, T.; Ozako, S.; Kanda, M.; Koide, T.; Tani, F. Dye-sensitized solar cell based on an inclusion complex of a cyclic porphyrin dimer bearing four 4-pyridyl groups and fullerene C60. RSC Adv. 2016, 6, 16150–16158. [Google Scholar] [CrossRef]
- Janani, M.; Srikrishnarka, P.; Nair, S.V.; Nair, A.S. An in-depth review on the role of carbon nanostructures in dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 17914–17938. [Google Scholar] [CrossRef]
- Melucci, M.; Durso, M.; Zambianchi, M.; Treossi, E.; Xia, Z.-Y.; Manet, I.; Giambastiani, G.; Ortolani, L.; Morandi, V.; De Angelis, F.; et al. Graphene-organic hybrids as processable, tunable platforms for pH-dependent photoemission, obtained by a new modular approach. J. Mater. Chem. 2012, 22, 18237–18243. [Google Scholar] [CrossRef]
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P.; Skorupska, M. Highly effective three-dimensional functionalization of graphite to graphene by wet chemical exfoliation methods. Adsorption 2019, 25, 631–638. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Praveen, V.K.; Das, G.; Ajayaghosh, A. Hybrid materials of 1D and 2D carbon allotropes and synthetic π-systems. NPG Asia Mater. 2018, 10, 107–126. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Li, J.; Pan, M.; Bai, L.; Luoshan, M.; Zhao, X. Graphene quantum dots optimization of dye-sensitized solar cells. Electrochim. Acta 2014, 137, 634–638. [Google Scholar] [CrossRef]
- Scharl, T.; Ferrer-Ruiz, A.; Saura-Sanmartin, A.; Rodríguez-Pérez, L.; Ángeles Herranz, M.; Martin, N.; Guldi, D.M. Charge transfer in graphene quantum dots coupled with tetrathiafulvalenes. Chem. Commun. 2019, 55, 3223–3226. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, F.; Bai, X.; Liu, F.; Kong, W.; Li, M. Tuning the photoluminescence of graphene quantum dots by photochemical doping with nitrogen. Materials 2017, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Lin, Y.; Xie, Y.; Wei, Y. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Jou, J.-H.; Kumar, S.; Agrawal, A.; Li, T.-H.; Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Martin, N. Carbon nanoforms for photovoltaics: Myth or reality? Adv. Energy Mater. 2017, 7, 1601102. [Google Scholar] [CrossRef]
- Ranasinghe, C.S.K.; Jayaweera, E.N.; Kumara, G.R.A.; Rajapakse, R.M.G.; Bandara, H.M.N.; Yoshimura, M. Low-cost dye-sensitized solar cells based on interconnected FTO-activated carbon nanoparticulated counter electrode showing high efficiency. J. Mater. Sci. Eng. A 2015, 5, 361–368. [Google Scholar]
- Joshi, P. Novel counter electrodes of dye-sensitized solar cells based on activated carbon prepared from wood of Choerospondias axillaris seed-stones and Alnus nepalensis plant. Int. J. Eng. Adv. Res. Technol. 2017, 3, 8–11. [Google Scholar]
- Karki, S.; Shakya, S.; Rajbhandari (Nyachhyon), A.; Shrestha, D.; Joshi, P. Low-cost dye-sensitized solar cells with novel counter electrodes based on activated carbon of Rhododendron arboreum plant wood. Int. J. Sci. Eng. Appl. Sci. 2017, 3, 134–138. [Google Scholar]
- Menanteau, T.; Benoit, C.; Breton, T.; Cougnon, C. Enhancing the performance of a diazonium-modified carbon supercapacitor by controlling the grafting process. Electrochem. Commun. 2016, 63, 70–73. [Google Scholar] [CrossRef]
- Biniak, S.; Pakuła, M.; Świątkowski, A.; Bystrzejewski, M.; Błażewicz, S. Influence of high-temperature treatment of granular activated carbon on its structure and electrochemical behavior in aqueous electrolyte solution. J. Mater. Res. 2010, 25, 1617–1628. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Adsorption of dibenzothiophenes on nanoporous carbons: Identification of specific adsorption sites governing capacity and selectivity. Energy Fuels 2010, 24, 3352–3360. [Google Scholar] [CrossRef]
- Yzambart, G.; Zieleniewska, A.; Bauroth, S.; Clark, T.; Bryce, M.R.; Guldi, D.M. Charge-gating dibenzothiophene-S, S-dioxide bridges in electron donor-bridge-acceptor conjugates. J. Phys. Chem. C 2017, 121, 13557–13569. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Si, X.; Zhou, D.; Shi, Y.; Wang, Y.; Jing, X.; Wang, P. Oligothiophene dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 1924–1928. [Google Scholar] [CrossRef]
- Saini, G.; Lucas, N.T.; Jacob, J. Tetrathiophenes with thiophene side chains: Effect of substitution on packing and conjugation. Tetrahedron Lett. 2010, 51, 2956–2958. [Google Scholar] [CrossRef]
- Terzyk, A.P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268, 301–329. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. Simple optimization approach for the characterization of pore size distribution. Langmuir 2000, 16, 1319–1322. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Gauden, P.A.; Kowalczyk, P. What kind of pore size distribution is assumed in the Dubinin-Astakhov adsorption isotherm equation? Carbon 2002, 40, 2879–2886. [Google Scholar] [CrossRef]
- Gauden, P.A.; Kowalczyk, P.; Terzyk, A.P. Toward solving the unstable linear Fredholm equation of the first kind: A new procedure called the adsorption stochastic algorithm (ASA) and its properties. Langmuir 2003, 19, 4253–4268. [Google Scholar] [CrossRef]
- Skrzynski, W. ImageJ—Program do analizy obrazow i jego zastosowania. Inz. I Fiz. Med. 2013, 2, 129–132. [Google Scholar]
- Paternò, G.M.; Robbiano, V.; Fraser, K.J.; Frost, C.; García Sakai, V.; Cacialli, F. Neutron radiation tolerance of two benchmark thiophene-based conjugated polymers: The importance of crystallinity for organic avionics. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamagata, M.; Ishikawa, M. A sulfur-microporous carbon composite positive electrode for lithium/sulfur and silicon/sulfur rechargeble batteries. Prog. Nat. Sci. Mater. Int. 2015, 25, 612–622. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Hodkiewicz, J.; Scientific, T.F. Characterizing carbon materials with Raman spectroscopy. Prog. Mater. Sci 2005, 50, 929–961. [Google Scholar]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Stergiou, A.; Perivoliotis, D.K.; Tagmatarchis, N. (Photo) electrocatalysis of molecular oxygen reduction by S-doped graphene decorated with a star-shaped oligothiophene. Nanoscale 2019, 11, 7335–7346. [Google Scholar] [CrossRef]
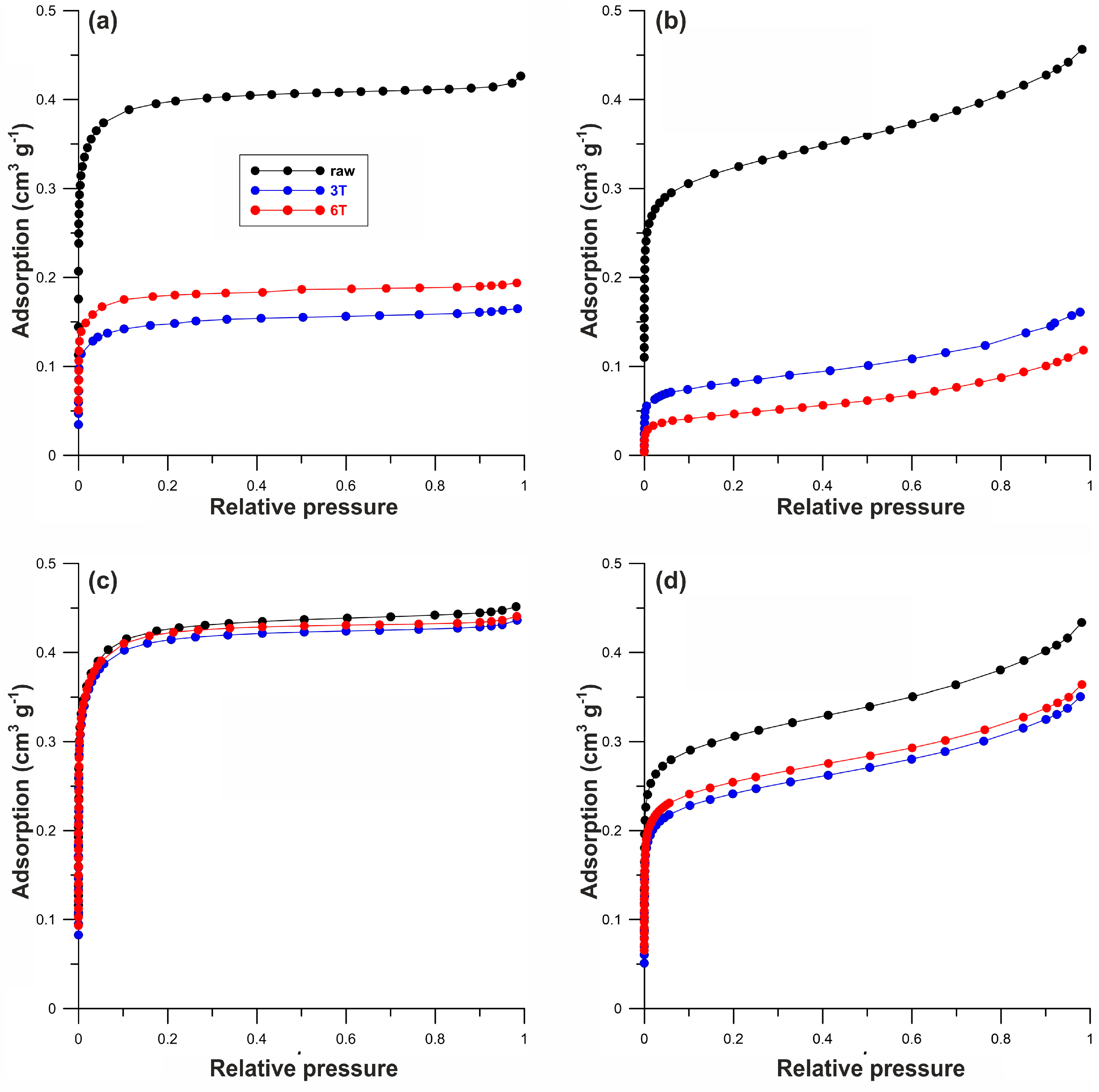
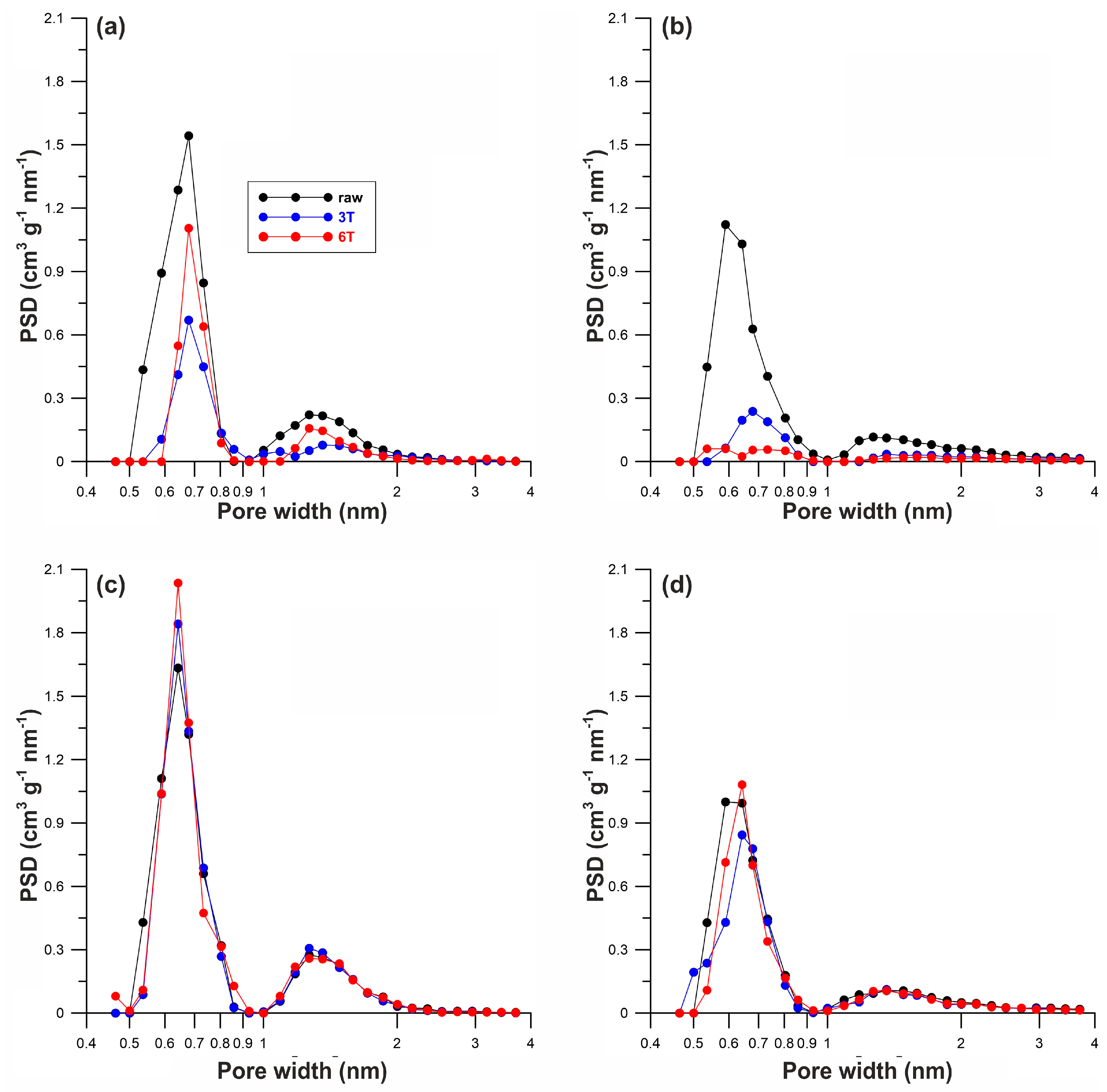
| Sample | Elemental Content (wt%) | SBET | Vmi | Vt | xav | ||
|---|---|---|---|---|---|---|---|
| N | C | H | (m2/g) | (cm3/g) | (cm3/g) | (nm) | |
| Norit_1_raw | 1.0 | 87.6 | 0.9 | 855 | 0.404 | 0.426 | 0.861 |
| Norit_1_3T | 0.4 | 77.1 | 1.5 | 318 | 0.162 | 0.165 | 0.944 |
| Norit_1_6T | 0.4 | 79.4 | 1.7 | 389 | 0.185 | 0.194 | 0.902 |
| Norit_1_ox | 0.5 | 81.2 | 1.9 | 902 | 0.433 | 0.451 | 0.857 |
| Norit_1_ox_3T | 0.5 | 81.2 | 1.1 | 899 | 0.420 | 0.436 | 0.862 |
| Norit_1_ox_6T | 0.5 | 81.3 | 1.1 | 917 | 0.428 | 0.441 | 0.849 |
| Norit_2_raw | 0.8 | 87.0 | 1.0 | 705 | 0.262 | 0.456 | 1.140 |
| Norit_2_3T | 0.5 | 85.2 | 1.7 | 178 | 0.089 | 0.161 | 1.731 |
| Norit_2_6T | 0.4 | 82.3 | 1.7 | 104 | 0.050 | 0.118 | 2.426 |
| Norit_2_ox | 0.7 | 86.6 | 1.3 | 642 | 0.322 | 0.434 | 1.137 |
| Norit_2_ox_3T | 0.4 | 77.2 | 1.0 | 513 | 0.253 | 0.350 | 1.162 |
| Norit_2_ox_6T | 0.5 | 81.2 | 1.1 | 540 | 0.267 | 0.364 | 1.159 |
| Peak | Binding Energy (eV) | Sample | ||
|---|---|---|---|---|
| Norit_2_raw | Norit_2_6T | Norit_2_ox_6T | ||
| Content (at%) | ||||
| C1s (C-1) | 285.01 | 68.73 | 68.62 | 45.37 |
| C1s (C-2) | 286.54 | 8.77 | 9.65 | 15.81 |
| C1s (C-3) | 287.76 | 4.08 | 4.00 | 5.76 |
| C1s (C-4) | 289.10 | 2.47 | 2.42 | 3.59 |
| C1s (C-5) | 290.47 | 2.37 | 1.78 | 2.77 |
| C1s (C-6) | 291.78 | 1.44 | 1.09 | 1.23 |
| Total C | - | 87.86 | 87.56 | 74.53 |
| S2p3 (S-1) | 164.29 | - | 0.78 | 0.66 |
| S2p3 (S-2) | 169.65 | - | 0.62 | 0.79 |
| Total S | - | 0.00 | 1.40 | 1.45 |
| O1s (O-1) | 531.21 | 3.95 | 2.78 | 5.80 |
| O1s (O-2) | 533.29 | 5.23 | 6.58 | 14.13 |
| O1s (O-3) | 535.22 | 0.34 | 0.65 | 1.06 |
| Total O | - | 9.52 | 10.01 | 20.99 |
| Total N | - | 0.46 | 0.44 | 0.64 |
| Total Si | - | 2.16 | 0.58 | 2.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamedulski, P.; Gauden, P.A.; Lukaszewicz, J.P.; Ilnicka, A. Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes. Materials 2019, 12, 3354. https://doi.org/10.3390/ma12203354
Kamedulski P, Gauden PA, Lukaszewicz JP, Ilnicka A. Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes. Materials. 2019; 12(20):3354. https://doi.org/10.3390/ma12203354
Chicago/Turabian StyleKamedulski, Piotr, Piotr A. Gauden, Jerzy P. Lukaszewicz, and Anna Ilnicka. 2019. "Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes" Materials 12, no. 20: 3354. https://doi.org/10.3390/ma12203354
APA StyleKamedulski, P., Gauden, P. A., Lukaszewicz, J. P., & Ilnicka, A. (2019). Effective Synthesis of Carbon Hybrid Materials Containing Oligothiophene Dyes. Materials, 12(20), 3354. https://doi.org/10.3390/ma12203354