Size and Shape-Dependent Solubility of CuO Nanostructures
Abstract
:1. Introduction
2. Thermodynamic Description of CuO Dissolution
2.1. Dissolution of Bulk CuO
2.2. Dissolution of CuO Nanostructures
3. Estimation of the Interfacial Energy CuO/Aqueous Solution
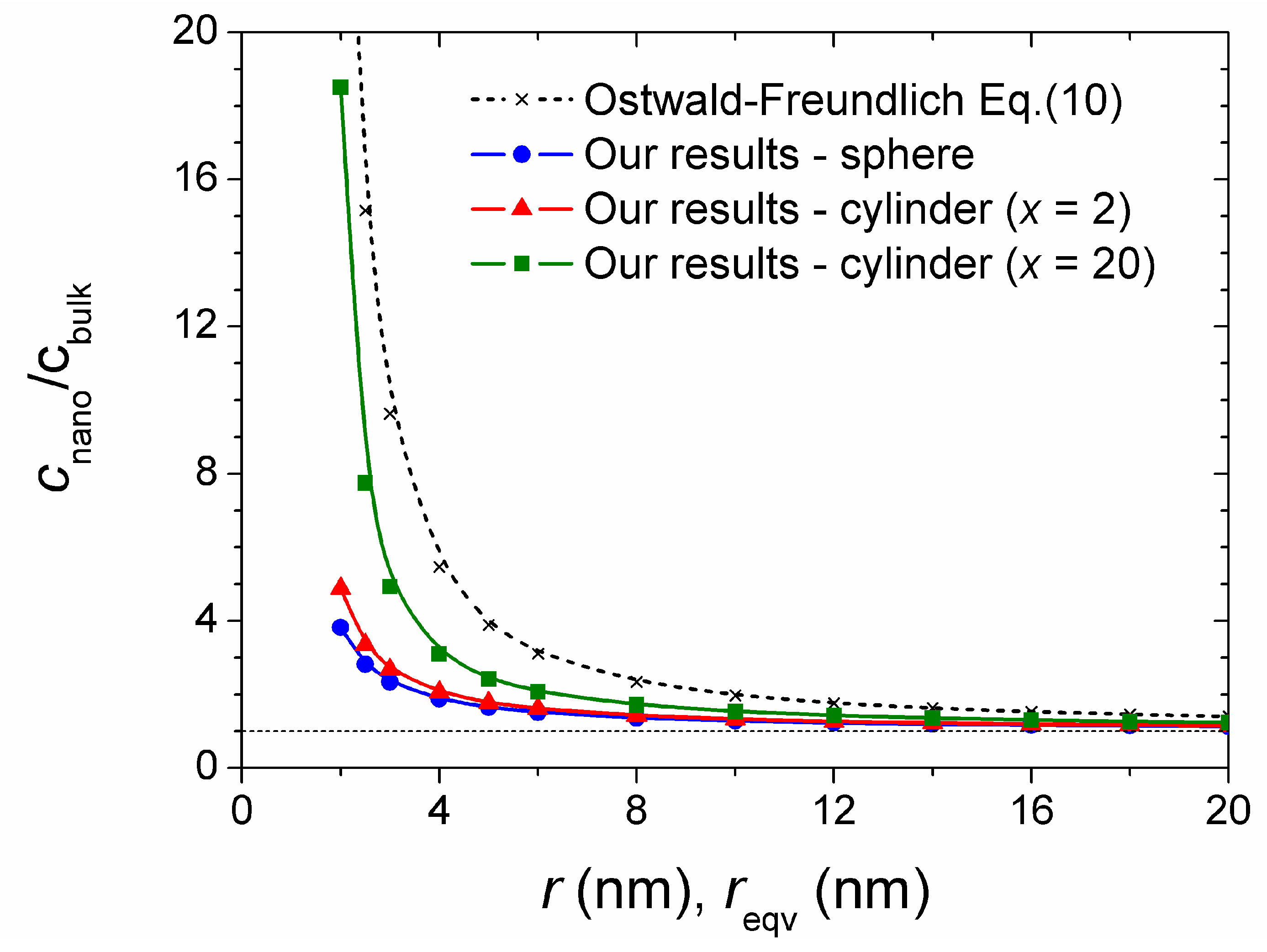
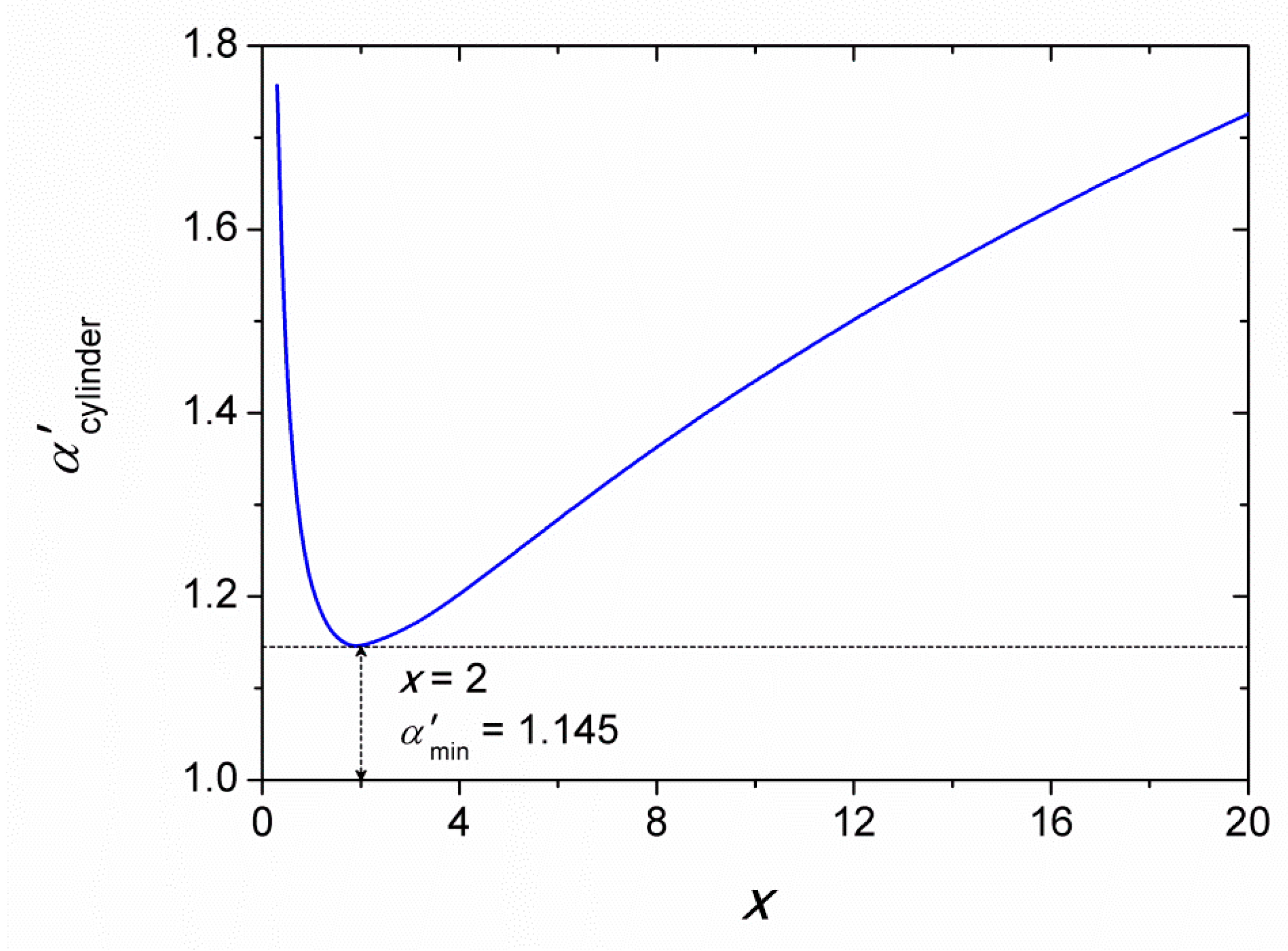
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Djerdj, I.; Arčon, D.; Jagličić, Z.; Niederberger, M. Nonaqueous synthesis of metal oxide nanoparticles: Short review and doped titanium dioxide as case study for the preparation of transition metal-doped oxide nanoparticles. J. Solid State Chem. 2008, 181, 1571–1581. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef] [PubMed]
- Bartůněk, V.; Huber, Š.; Sedmidubský, D.; Sofer, Z.; Šimek, P.; Jankovský, O. CoO and Co3O4 nanoparticles with a tunable particle size. Ceram. Int. 2014, 40, 12591–12595. [Google Scholar] [CrossRef]
- Jankovský, O.; Sedmidubský, D.; Šimek, P.; Sofer, Z.; Ulbrich, P.; Bartůněk, V. Synthesis of MnO, Mn2O3 and Mn3O4 nanocrystal clusters by thermal decomposition of manganese glycerolate. Ceram. Int. 2015, 41, 595–601. [Google Scholar] [CrossRef]
- Lamastra, F.; Grilli, M.; Leahu, G.; Belardini, A.; Voti, R.L.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Photoacoustic spectroscopy investigation of zinc oxide/diatom frustules hybrid powders. Int. J. Thermophys. 2018, 39, 110. [Google Scholar] [CrossRef]
- Lamastra, F.; Grilli, M.; Leahu, G.; Belardini, A.; Voti, R.L.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Diatom frustules decorated with zinc oxide nanoparticles for enhanced optical properties. Nanotechnology 2017, 28, 375704. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Henson, T.E.; Navratilova, J.; Tennant, A.H.; Bradham, K.D.; Rogers, K.R.; Hughes, M.F. In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology 2019, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thit, A.; Selck, H.; Bjerregaard, H.F. Toxic mechanisms of copper oxide nanoparticles in epithelial kidney cells. Toxicol. Vitr. 2015, 29, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, B.; Xia, B.; Han, Q.; Zhu, L.; Qu, K. Are CuO nanoparticles effects on hemocytes of the marine scallop (Chlamys farreri) caused by particles and/or corresponding released ions? Ecotoxicol. Environ. Saf. 2017, 139, 65–72. [Google Scholar] [CrossRef]
- Kaptay, G. On the size and shape dependence of the solubility of nano-particles in solutions. Int. J. Pharm. 2012, 430, 253–257. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Du, J.; Zhao, R.; Xue, Y. Effects of sizes of nano-copper oxide on the equilibrium constant and thermodynamic properties for the reaction in nanosystem. J. Chem. Thermodyn. 2012, 45, 48–52. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic origin of copper (II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef]
- Misra, S.K.; Nuseibeh, S.; Dybowska, A.; Berhanu, D.; Tetley, T.D.; Valsami-Jones, E. Comparative study using spheres, rods and spindle-shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials. Nanotoxicology 2014, 8, 422–432. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.-M.; Grassian, V.H. Dissolution of ZnO nanoparticles at circumneutral pH: A study of size effects in the presence and absence of citric acid. Langmuir 2011, 28, 396–403. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A. Solubility of nanocrystalline cerium dioxide: Experimental data and thermodynamic modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef]
- Leitner, J.; Sedmidubský, D.; Jankovský, O. Effect of ZnO nanosizing on its solubility in aqueous media. Micro Nano Lett. 2018, 13, 1585–1589. [Google Scholar] [CrossRef]
- Laha, D.; Pramanik, A.; Laskar, A.; Jana, M.; Pramanik, P.; Karmakar, P. Shape-dependent bactericidal activity of copper oxide nanoparticle mediated by DNA and membrane damage. Mater. Res. Bull. 2014, 59, 185–191. [Google Scholar] [CrossRef]
- Palmer, D.A. The solubility of crystalline cupric oxide in aqueous solution from 25 °C to 400 °C. J. Chem. Thermodyn. 2017, 114, 122–134. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for copper at 25 to 300 °C. J. Electrochem. Soc. 1997, 144, 3476–3483. [Google Scholar] [CrossRef]
- Preis, W.; Gamsjäger, H. Solid–solute phase equilibria in aqueous solution XVI. Thermodynamic properties of malachite and azurite—Predominance diagrams for the system Cu2+–H2O–CO2. J. Chem. Thermodyn. 2002, 34, 631–650. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands Part 2: The Cu2+, OH−, Cl−, CO32−, SO42−, and PO43− systems (IUPAC Technical Report). Pure Appl. Chem. 2007, 79, 895–950. [Google Scholar] [CrossRef]
- Voňka, P.; Leitner, J. Calculation of chemical equilibria in heterogeneous multicomponent systems. Calphad 1995, 19, 25–36. [Google Scholar] [CrossRef]
- Barry, T.I. Chemical Thermodynamics in Industry: Models and Computation; Blackwell Science Incorporated: Boston, MA, USA, 1985. [Google Scholar]
- Puigdomenech, I.; Taxén, C. Thermodynamic Data for Copper. Implications for the Corrosion of Copper under Repository Conditions; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2000. [Google Scholar]
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L. The NBS tables of chemical thermodynamic properties: Selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys. Chem. Ref. Data 1982, 18, 1807–1812. [Google Scholar]
- Leitner, J.; Sedmidubský, D. Thermodynamic Equilibria in Systems with Nanoparticles. In Thermal Physics and Thermal Analysis; Springer International Publishing: Cham, Switzerland, 2017; pp. 385–402. [Google Scholar]
- Forsyth, J.; Hull, S. The effect of hydrostatic pressure on the ambient temperature structure of CuO. J. Phys. Condens. Matter. 1991, 3, 5257. [Google Scholar] [CrossRef]
- Leitner, J.; Sedmidubský, D. Thermodynamic Modeling of Oxidation of Tin Nanoparticles. J. Ph. Equilib. Diffus. 2019, 40, 10–20. [Google Scholar] [CrossRef]
- Vargaftik, N.; Volkov, B.; Voljak, L. International tables of the surface tension of water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Hu, J.; Li, D.; Lu, J.G.; Wu, R. Effects on electronic properties of molecule adsorption on CuO surfaces and nanowires. J. Phys. Chem. C 2010, 114, 17120–17126. [Google Scholar] [CrossRef]
- Maimaiti, Y.; Nolan, M.; Elliott, S.D. Reduction mechanisms of the CuO (111) surface through surface oxygen vacancy formation and hydrogen adsorption. PCCP 2014, 16, 3036–3046. [Google Scholar] [CrossRef]
- Mishra, A.K.; Roldan, A.; de Leeuw, N.H. CuO surfaces and CO2 activation: A dispersion-corrected DFT+ U study. J. Phys. Chem. C 2016, 120, 2198–2214. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, F.; Gilbert, B.; Banfield, J.F. Molecular dynamics simulations, thermodynamic analysis, and experimental study of phase stability of zinc sulfide nanoparticles. J. Phys. Chem. B 2003, 107, 13051–13060. [Google Scholar] [CrossRef]
- Navrotsky, A. Nanoscale effects on thermodynamics and phase equilibria in oxide systems. Chem. Phys. Chem. 2011, 12, 2207–2215. [Google Scholar] [CrossRef]
- Ogwu, A.; Bouquerel, E.; Ademosu, O.; Moh, S.; Crossan, E.; Placido, F. An investigation of the surface energy and optical transmittance of copper oxide thin films prepared by reactive magnetron sputtering. Acta. Mater. 2005, 53, 5151–5159. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Z.; Li, J.; Zhang, Z.; Ren, L. Super-hydrophobic property of nano-sized cupric oxide films. Surf. Coat. Technol. 2010, 204, 3200–3204. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Ye, Y.; Zhou, H.; Chen, J. Fabrication of superhydrophobic CuO surfaces with tunable water adhesion. J. Phys. Chem. C 2011, 115, 4726–4729. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sudhagar, P.; Ravidhas, C.; Christy, A.J.; Kirubakaran, D.D.; Venkatesh, R.; Devadoss, A.; Terashima, C.; Nakata, K.; Fujishima, A. Self-cleaning and superhydrophobic CuO coating by jet-nebulizer spray pyrolysis technique. Cryst. Eng. Comm. 2015, 17, 2624–2628. [Google Scholar] [CrossRef]
| Substance | ∆fGo (kJ/mol) | Substance | ∆fGo (kJ/mol) |
|---|---|---|---|
| H2O(l) | −237.129 | Cu2(OH)22+(aq) | −285.1 |
| H+(aq) | 0 | Cu3(OH)42+(aq) | −633.0 |
| OH−(aq) | −157.244 | CuCO3(aq) | −501.5 |
| O2(aq) | −16.4 | Cu(CO3)22−(aq) | −1048.98 |
| CO2(aq) | −385.98 | CuHCO3+(aq) | −532.08 |
| CO32−(aq) | −527.81 | N2(g) | 0 |
| HCO3−(aq) | −586.77 | O2(g) | 0 |
| Cu+(aq) | 48.87 | CO2(g) | −394.359 |
| CuOH(aq) | −122.32 | Cu2O(s) | −147.90 |
| Cu(OH)2−(aq) | −333.05 | CuO(s) | −128.29 |
| Cu2+ (aq) | 65.04 | Cu(OH)2(s) | −359.92 |
| CuOH+(aq) | −126.66 | CuCO3(s) | −528.20 |
| Cu(OH)2(aq) | −316.54 | Cu2(OH)2CO3(s) | −902.35 |
| Cu(OH)3−(aq) | −493.98 | Cu3(OH)2(CO3)2(s) | −1431.43 |
| Cu(OH)42−(aq) | −657.48 |
| (hkl) | γ(hkl) (mJ m−2) | ||
|---|---|---|---|
| Ref. [36] | Ref. [37] | Ref. [38] | |
| (111) | 740 | 720 | 750 |
| (−111) | 860 | 890 | |
| (011) | 930 | 910 | 940 |
| (101) | 1160 | 1170 | |
| (110) | 1290 | 1180 | 1185 |
| (010) | 1370 | 1680 | 1485 |
| (100) | 2280 | 2240 | 1755 |
| Average-Equation (9) | 1094.6 | 1142.4 | 1083.2 |
| p(CO2)/po | m(Cu)tot (mol kg−1) | pH | Im (mol kg−1) | Dominant Aqueous Cu Apecies # |
|---|---|---|---|---|
| 4 × 10−4 | 7.74 × 10−6 | 6.41 | 2.29 × 10−5 | Cu2+(95.8), Cu(OH)+(2.6), CuCO3 (1.0) |
| 0 | 1.10 × 10−7 | 7.37 | 3.21 × 10−7 | Cu2+(77.3), Cu(OH)+(19.9), Cu(OH)2 (2.5) |
| r or rekv (nm) | m(Cu)tot (mol kg−1) | ||
|---|---|---|---|
| Sphere | Cylinder (x = 2) | Cylinder (x = 20) | |
| 2 | 2.959 × 10−5 | 3.779 × 10−5 | 1.433 × 10−4 |
| 3 | 1.811 × 10−5 | 2.072 × 10−5 | 3.818 × 10−5 |
| 4 | 1.450 × 10−5 | 1.595 × 10−5 | 2.395 × 10−5 |
| 5 | 1.275 × 10−5 | 1.373 × 10−5 | 1.871 × 10−5 |
| 6 | 1.171 × 10−5 | 1.245 × 10−5 | 1.601 × 10−5 |
| 8 | 1.054 × 10−5 | 1.104 × 10−5 | 1.327 × 10−5 |
| 10 | 9.911 × 10−6 | 1.028 × 10−5 | 1.189 × 10−5 |
| 12 | 9.509 × 10−6 | 9.798 × 10−6 | 1.106 × 10−5 |
| 14 | 9.232 × 10−6 | 9.473 × 10−6 | 1.050 × 10−5 |
| 16 | 9.031 × 10−6 | 9.236 × 10−6 | 1.011 × 10−5 |
| 18 | 8.877 × 10−6 | 9.056 × 10−6 | 9.893 × 10−6 |
| 20 | 8.755 × 10−6 | 8.914 × 10−6 | 9.579 × 10−6 |
| bulk | 7.741 × 10−6 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitner, J.; Sedmidubský, D.; Jankovský, O. Size and Shape-Dependent Solubility of CuO Nanostructures. Materials 2019, 12, 3355. https://doi.org/10.3390/ma12203355
Leitner J, Sedmidubský D, Jankovský O. Size and Shape-Dependent Solubility of CuO Nanostructures. Materials. 2019; 12(20):3355. https://doi.org/10.3390/ma12203355
Chicago/Turabian StyleLeitner, Jindřich, David Sedmidubský, and Ondřej Jankovský. 2019. "Size and Shape-Dependent Solubility of CuO Nanostructures" Materials 12, no. 20: 3355. https://doi.org/10.3390/ma12203355
APA StyleLeitner, J., Sedmidubský, D., & Jankovský, O. (2019). Size and Shape-Dependent Solubility of CuO Nanostructures. Materials, 12(20), 3355. https://doi.org/10.3390/ma12203355







