Amorphous Tin Oxide Applied to Solution Processed Thin-Film Transistors
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursor Solutions
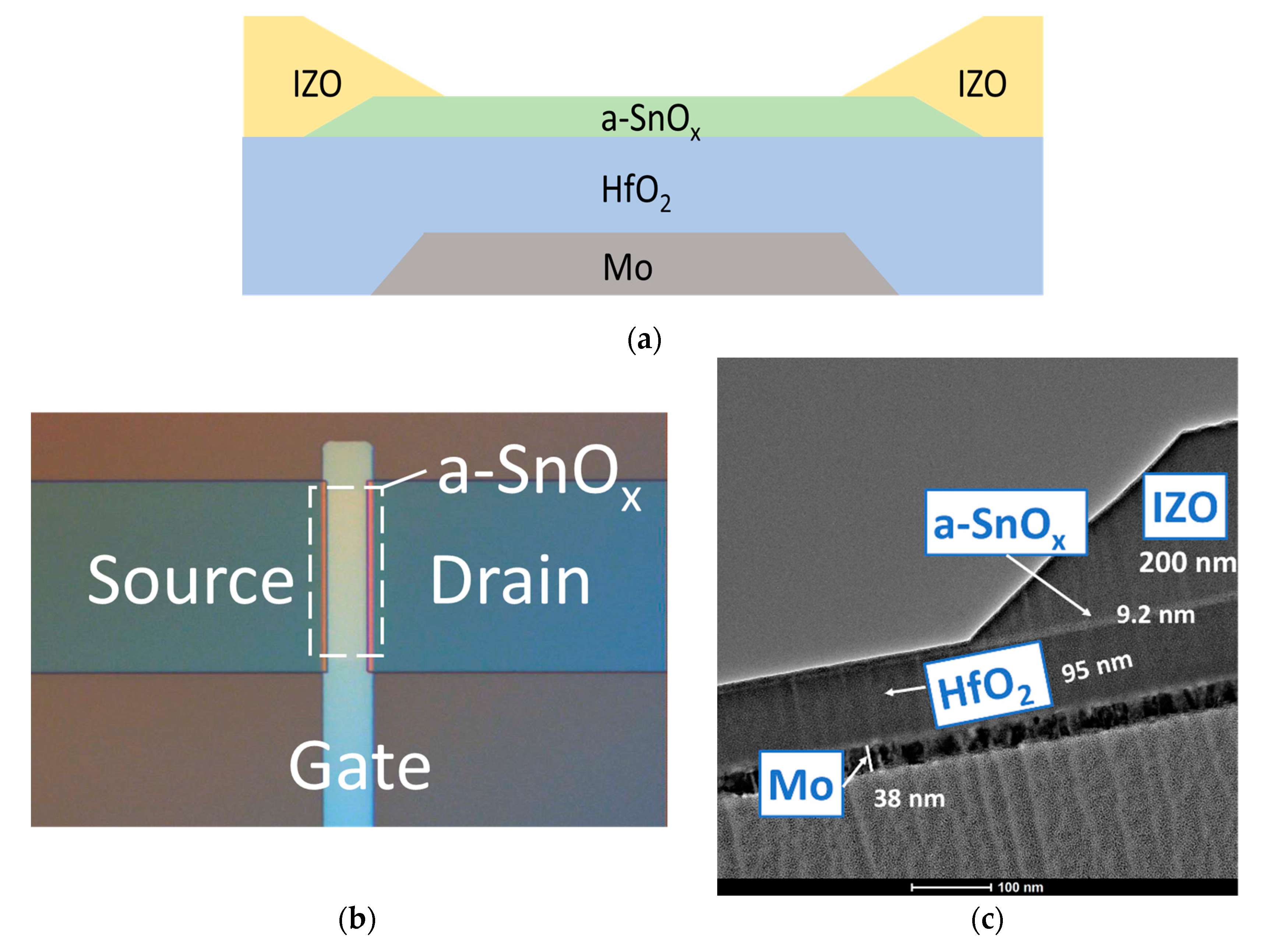
2.2. Thin-Film Transistor Fabrication and Characterization
2.3. Thin Film Characterization
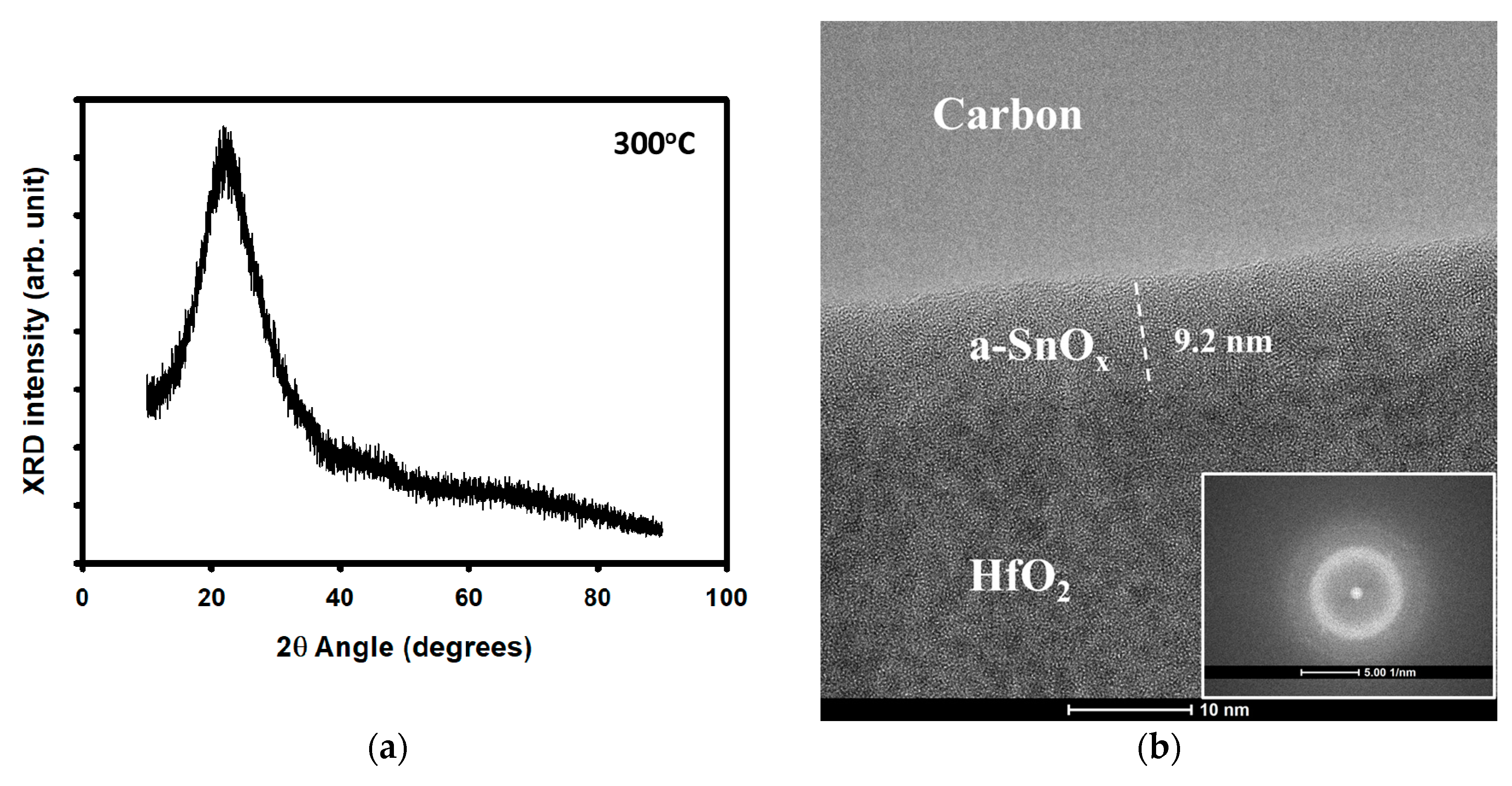
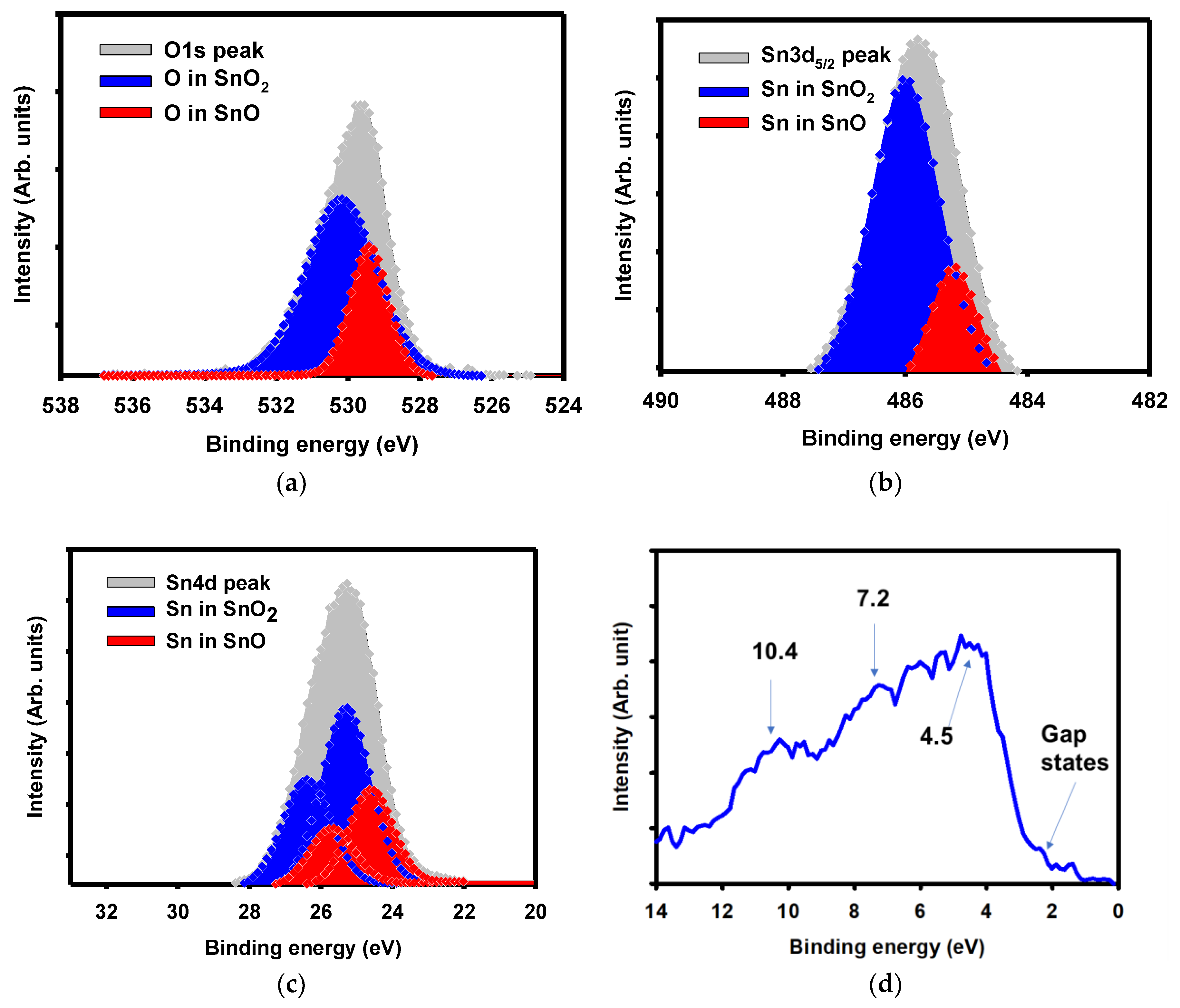
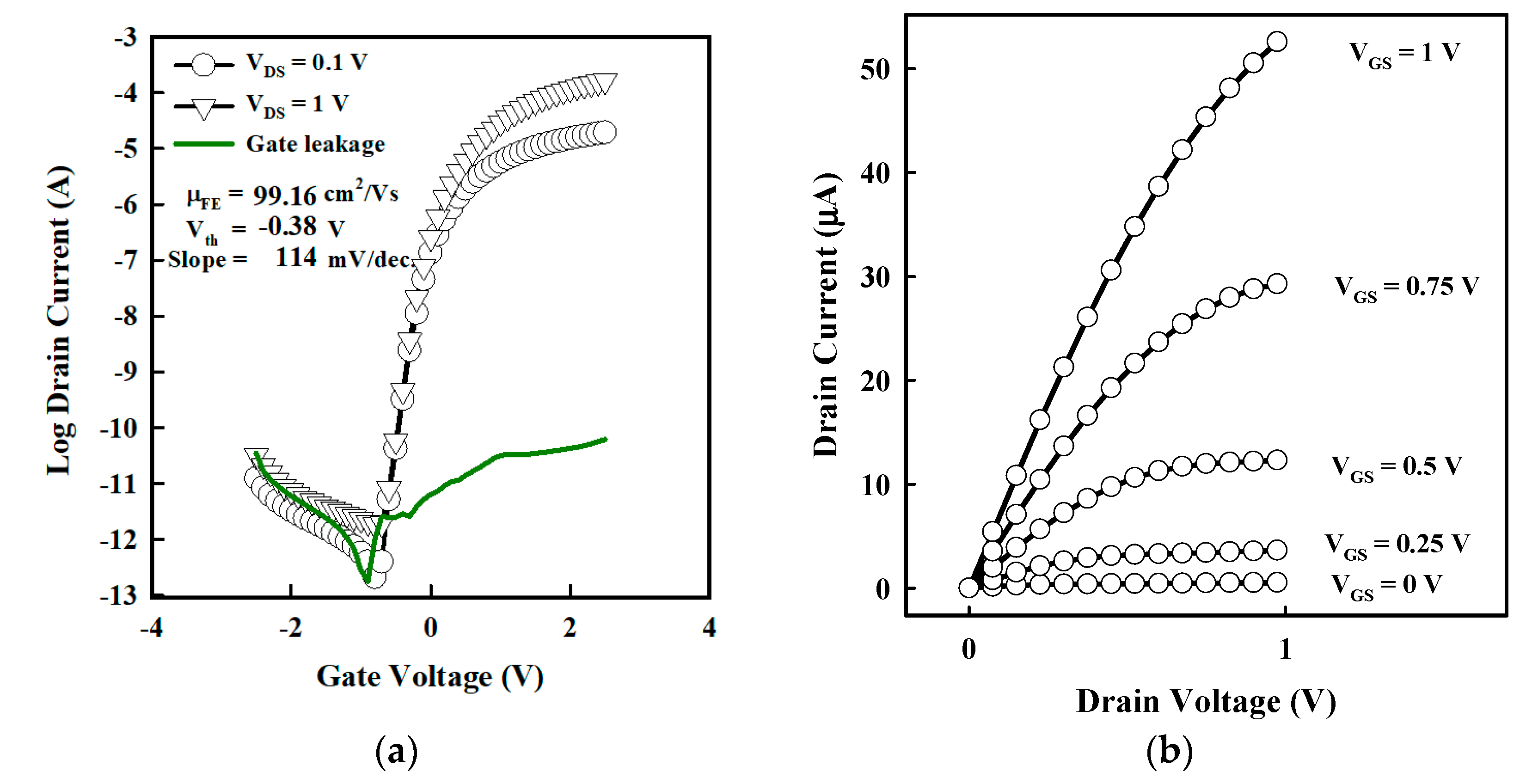
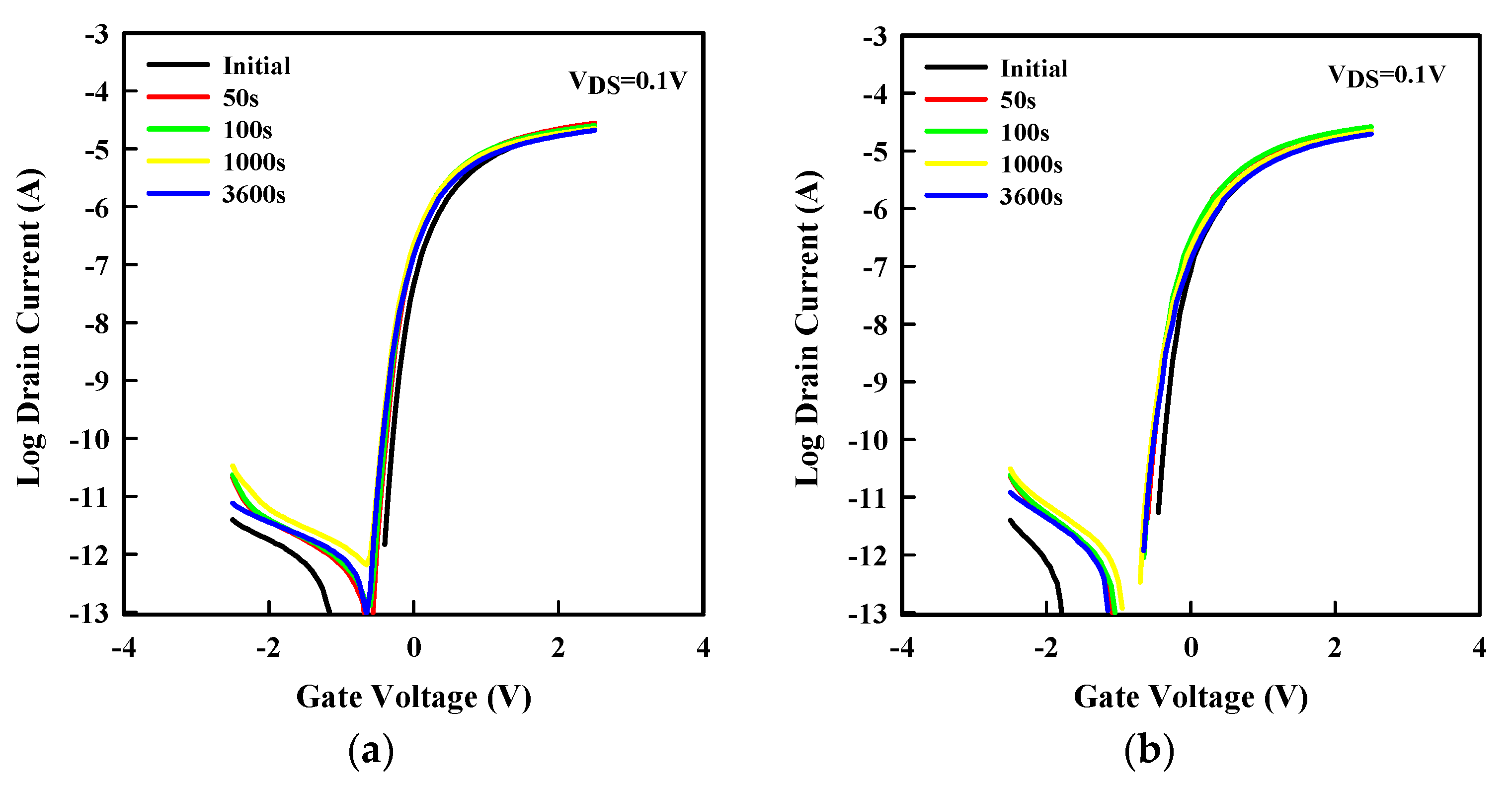
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Jeong, H.; Han, K.; Hong, T.; Park, J. Review of recent advances in flexible oxide semiconductor thin-film transistors. J. Inf. Disp. 2017, 18, 159–172. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Avis, C.; Hwang, H.R.; Jang, J. Effect of Channel Layer Thickness on the Performance of Indium Zinc Tin Oxide Thin Film Transistors Manufactured by Inkjet Printing. ACS Appl. Mater. Interfaces 2014, 6, 10941–10945. [Google Scholar] [CrossRef] [PubMed]
- Banger, K.K.; Yamashita, Y.; Mori, K.; Peterson, R.; Leedham, T.; Rickard, J.; Sirringhaus, H. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol–gel on chip’ process. Nat. Mater. 2011, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Marks, T.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Ye, L.; Xu, J. The role of solution-processed high-k gate dielectrics in electrical performance of oxide thin-film transistors. Mater. Chem. C 2014, 2, 5389–5396. [Google Scholar] [CrossRef]
- Pal, B.N.; Mukund Dhar, B.; See, K.C.; Katz, H.E. Solution-deposited sodium beta-alumina gate dielectrics for low-voltage and transparent field-effect transistors. Nat. Mater. 2009, 8, 898–903. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Sun, H.; Xu, Y.; Noh, Y.-Y. Solution Processed Metal Oxide High-κ Dielectrics for Emerging Transistors and Circuits. Adv. Mater. 2018, 30, 1–39. [Google Scholar] [CrossRef]
- Avis, C.; Jang, J. High-performance solution processed oxide TFT with aluminum oxide gate dielectric fabricated by a sol–gel method. J. Mater. Chem. 2011, 21, 10649–10652. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Ueda, K.; Kamiya, T.; Hirano, M.; Hosono, H. Thin-Film Transistor Fabricated in Single-Crystalline Transparent Oxide Semiconductor. Science 2003, 300, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Kim, T.; Avis, C.; Lee, S.H.; Jang, J. Stable and High-Performance Indium Oxide Thin-Film Transistor by Ga Doping. IEEE Trans. Electron. Dev. 2016, 63, 1078–1084. [Google Scholar] [CrossRef]
- Klasens, H.A.; Koelmans, H. A tin oxide field-effect transistor. Solid State Electron. 1964, 7, 701–702. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Hosono, H. Ambipolar Oxide Thin-Film Transistor. Adv. Mater. 2011, 23, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Sajiz, K.J.; Reena Mary, A.P. Tin Oxide Based P and N-Type Thin Film Transistors Developed by RF Sputtering. ECS J. Solid State Sci. Technol. 2015, 4, Q101–Q104. [Google Scholar] [CrossRef]
- Presley, R.E.; Munsee, C.L.; Park, C.-H.; Hong, D.; Wager, J.F.; Keszler, D.A. Tin Oxide Transparent Thin-Film Transistors. J. Phys. D Appl. Phys. 2004, 37, 2810–2813. [Google Scholar] [CrossRef]
- Huang, G.; Duan, L.; Dong, G.; Zhang, D.; Qiu, Y. High-Mobility Solution-Processed Tin Oxide Thin-Film Transistors with High κ Alumina Dielectric Working in Enhancement Mode. ACS Appl. Mater. Interfaces 2014, 6, 20786–20794. [Google Scholar] [CrossRef]
- Jang, J.; Kitsomboonloha, R.; Swisher, S.L.; Park, E.S.; Kang, H.; Subramanian, V. Transparent High-Performance Thin Film Transistors from Solution-Processed SnO2/ZrO2 Gel-like Precursors. Adv. Mater. 2013, 25, 1042–1047. [Google Scholar] [CrossRef]
- Priyadarshini, D.M.; Ramanjaneyulu, M.; Rao, M.S.; Das Gupta, N. Effect of annealing ambient on SnO2 thin film transistors. Appl. Surf. Sci. 2017, 418, 414–417. [Google Scholar] [CrossRef]
- Shih, C.; Chin, A.; Lu, C.F.; Su, W.F. Remarkably high mobility ultrathin-film metal-oxide transistor with strongly overlapped orbitals. Sci. Rep. 2016, 6, 19023. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mat. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaics. J. Mater. Chem. C 2016, 7, 1467–1475. [Google Scholar] [CrossRef]
- Kang, Y.; Song, H.; Nahm, H.; Jeon, S.; Cho, Y.; Han, S. Intrinsic nature of visible-light absorption in amorphous semiconducting oxides. Appl. Mater. 2014, 2, 032108. [Google Scholar] [CrossRef]
- Themlin, J.M.; Chtaib, M.; Henrard, L.; Lambin, P.; Darville, J.; Gilles, J.M. Characterization of tin oxides by X-ray-photoemission spectroscopy. Phys. Rev. B 1992, 46, 2460–2466. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Czempika, G.; Szuber, J. XPS study of the surface chemistry of L-CVD SnO2 thin films after oxidation. Thin Solid Films 2005, 490, 36–42. [Google Scholar] [CrossRef]
- Akgul, F.; Gumus, C.; Er, A.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and electronic properties of SnO2. J. Alloy. Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Robertson, J.; Wallace, R.M. High-K materials and metal gates for CMOS applications. Mater. Sci. Eng. R Rep. 2015, 88, 1–41. [Google Scholar] [CrossRef]
- Bukke, R.; Avis, C.; Chowdhury, M.D.H.; Kim, T.; Lin, T.; Jang, J. Improvement in performance of solution processed indium–zinc–tin oxide thin-film transistors by UV/O3 treatment on zirconium oxide gate insulator. J. Jpn. J. Appl. Phys. 2016, 55, 03CC02. [Google Scholar] [CrossRef]
- Fan, C.; Tseng, F.; Tseng, C. Electrical Performance and Reliability Improvement of Amorphous-Indium-Gallium-Zinc-Oxide Thin-Film Transistors with HfO2 Gate Dielectrics by CF4 Plasma Treatment. Materials 2018, 11, 824. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avis, C.; Kim, Y.; Jang, J. Amorphous Tin Oxide Applied to Solution Processed Thin-Film Transistors. Materials 2019, 12, 3341. https://doi.org/10.3390/ma12203341
Avis C, Kim Y, Jang J. Amorphous Tin Oxide Applied to Solution Processed Thin-Film Transistors. Materials. 2019; 12(20):3341. https://doi.org/10.3390/ma12203341
Chicago/Turabian StyleAvis, Christophe, YounGoo Kim, and Jin Jang. 2019. "Amorphous Tin Oxide Applied to Solution Processed Thin-Film Transistors" Materials 12, no. 20: 3341. https://doi.org/10.3390/ma12203341
APA StyleAvis, C., Kim, Y., & Jang, J. (2019). Amorphous Tin Oxide Applied to Solution Processed Thin-Film Transistors. Materials, 12(20), 3341. https://doi.org/10.3390/ma12203341




