Experimental Study on the Energy-Release Characteristics of Fine-Grained Fe/Al Energetic Jets under Impact Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Samples
- (1)
- The mixed powders were loaded into a custom mold and sintered in a vacuum hot-pressing sintering furnace (R-C-ZKQY-07, Chenrong Electric Furnace Co., Ltd. Shanghai, China), and high-purity nitrogen was used as a protective gas.
- (2)
- After 15 min, the power supply for the heater was turned on, and the water-cooling system was turned on when the temperature reached 150 °C. The heating rate was set to 1 °C/min. The sintering pressure was maintained at 10 MPa.
- (3)
- After the temperature reached the highest sintering temperature (500, 520, 550, or 600 °C), the temperature was maintained for 4 h; then, the mould was cooled to 300 °C at a cooling rate of 30 °C/h. This temperature was maintained for 1 h.
- (4)
- As the temperature was gradually lowered, most of the pressure was released. After the temperature reached room temperature, the mold was removed. The preparation process for the Fe/Al composites is shown in Figure 1. SEM images of the Al and Fe powders are shown in Figure 2 and Figure 3, respectively.
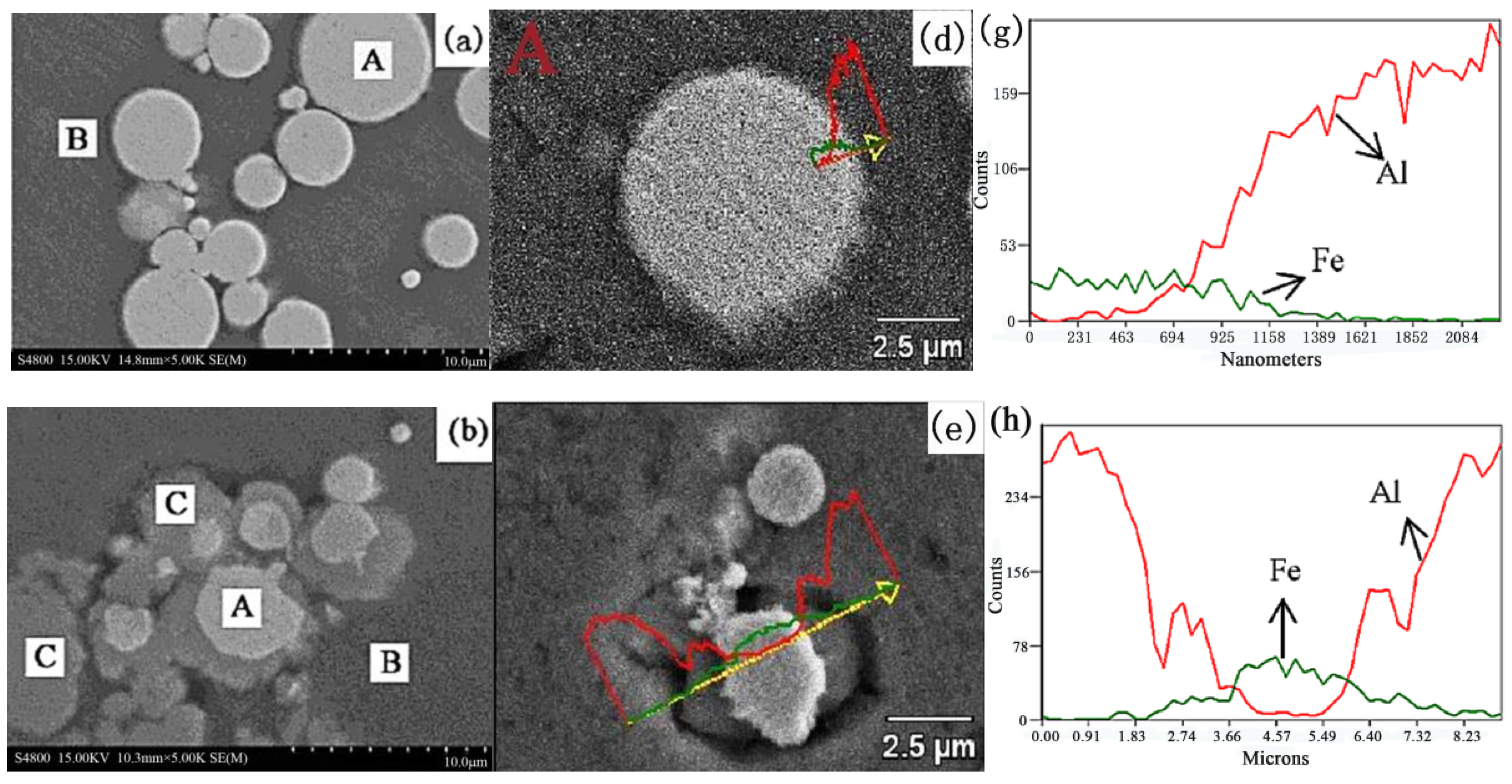
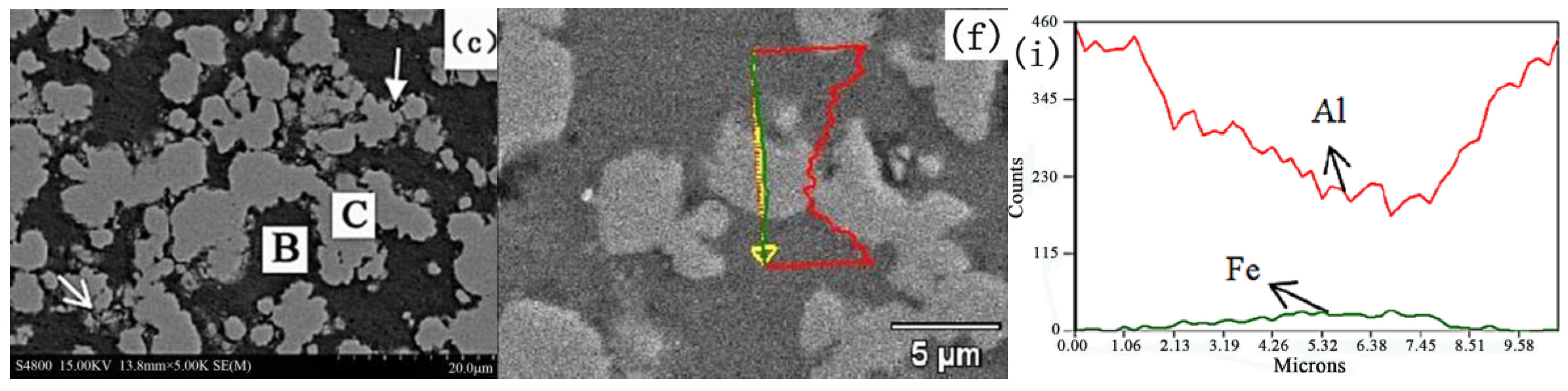
2.2. Microstructure Analysis of the Composites
3. Experimental Design and Scheme
3.1. Dynamic Compression Properties Experiment
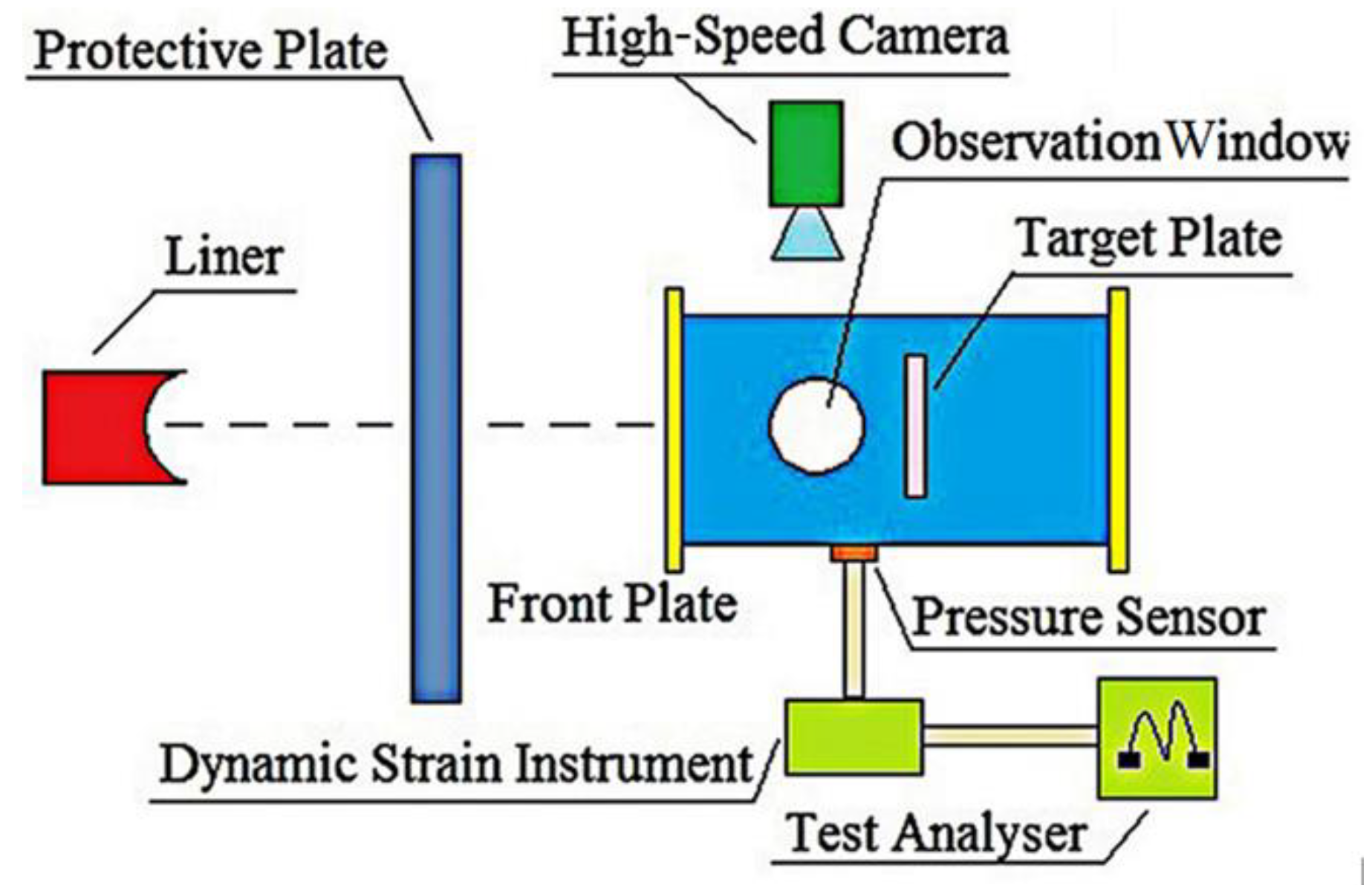
3.2. Experiment on the Energy-Release Characteristics under Impact Loading
4. Results and Discussion
4.1. Dynamic Compression Properties
4.2. Impact-Induced Reaction Characteristics
5. Conclusions
- (1)
- When the sintering temperature is 550 °C, a solid-state diffusion reaction between Al and Fe begins to take place at a pressure of 10 MPa, forming Fe2Al5. When the temperature exceeds 600 °C, the diffusion reaction occurs.
- (2)
- Fe/Al materials prepared at different maximum sintering temperatures show the characteristics of brittle materials with poor ductility. The fracture surface reflects intergranular brittle fracturing. When the maximum sintering temperature is 550 °C, the compressive strength of the fine-grained Fe/Al composites is the highest among the different composites. To maintain the activity and strength of the Fe/Al energetic material, the maximum sintering temperature should be 550 °C.
- (3)
- The reaction behavior of the fine-grained Fe/Al energetic jet under impact is related to the impact energy. With increasing impact energy, the energy release of the fine-grained Fe/Al energetic jet increases. The reaction mechanism is as follows: The aluminum oxidation reaction and the aluminum–iron intercalation reaction occur mainly under low-impact-energy conditions, and a great quantity of oxidative reactions of energetic substances is induced by high-impact energy. There is an impact-energy threshold at 121.1 J/mm2, at which the chemical reaction of the energetic material in the jet is saturated, and the highest reaction rate is 95.3%.
- (4)
- The Fe/Al energetic jet with fine particles and proper hot-pressing sintering has a higher energy efficiency than those created with coarse-grained and traditional mechanical processing under the same impact conditions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, E.L.; Daniels, A.S.; Ng, K.W. Barnie: A Unitary Demolition Warhead. In Proceedings of the 19th International Symposium of Ballistics, Interlaken, Switzerland, 7–11 May 2001. [Google Scholar]
- Zhang, X.F.; Zhao, X.N. Review on multifunctional energetic structural materials. Chin. J. Energy Mater. 2009, 17, 731–739. [Google Scholar] [CrossRef]
- Peng, F.; Yu, D.Q.; Yang, S.Q.; Jiang, J.P.; Lou, J.; Wang, W.M. Damage effects of energetic fragment warhead. Chin. J. Energy Mater. 2011, 19, 450–453. [Google Scholar]
- Liu, C.T.; George, E.P.; Maziasz, P.J.; Schneibel, J.H. Recent advances in B2 iron aluminide alloys: Deformation, fracture and alloy design. Mater. Sci. Eng. 1998, 258, 84–98. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Chi, Y.; Ge, Y.; Tan, P.; Xu, Z. Research on the preoxidation performance of Fe3Al porous materials. Mater. Sci. Eng. 2012, 41, 822. [Google Scholar]
- Sheasby, J.S. Powder metallurgy of iron-aluminium. Int. J. Powder Metall. 1979, 15, 301–305. [Google Scholar]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.H.; Väyrynen, I.J.; Kokko, K.; Ropo, M.; Punkkinen, M.; Pitkänen, H.; Alatalo, M.; Kollár, J.; et al. High temperature oxidation of Fe–Al and Fe–Cr–Al alloys: The role of Cr as a chemically active element. Corros. Sci. 2010, 52, 3394–3404. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Zhang, X.F.; Cheng, Z.P.; An, C.W.; Guo, X.D.; Li, F.S. Preparation and characterization of composite with micron Al coating in nanometer Fe particles. J. Funct. Mater. 2004, 11, 1900–1902. [Google Scholar]
- Wang, X.Y.; Wang, Y.W.; Wang, Z.C.; Jiang, C.L.; Li, Q. Effect of composition and sintering temperature on reaction heat of Fe-al reactive material. Mater. Sci. Eng. 2017, 46, 3043–3047. [Google Scholar]
- Gedevanishvili, S.; Deevi, S. Processing of iron aluminides by pressureless sintering through Fe+Al elemental route. Mater. Sci. Eng. 2002, 325, 163–176. [Google Scholar] [CrossRef]
- Wang, X.Q.; Sui, Y.J.; Lv, H.B. Fe and Al atoms diffusion in intermetallic formation. J. Shanghai Univ. 1998, 4, 661–667. [Google Scholar]
- Zheng, C.; Fan, J.; Gong, X.; Yang, C.; Liu, T.; State Key Laboratory for Powder Metallurgy; Central South University. Dynamic constitutive relationship of fine-grained 93W-4.9Ni-2.1Fe alloys. Mater. Sci. Eng. 2013, 42, 2043–2047. [Google Scholar]
- Zhang, X.F.; Zhao, X.N.; Qiao, L. Theoretical analysis of impact reactive metal. Explos. Shock Wave 2010, 30, 145–151. [Google Scholar]
- Walker, F.E.; Wasley, R.J. Critical energy for shock initiation of heterogeneous explosives. Explosivestoffe 1969, 17, 9–13. [Google Scholar]
- Ames, R.G. Vented chamber calorimetry for impact-initiated energetic materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005; pp. 10–13. [Google Scholar]

















| No. | Highest Sintering Temperature (°C) | Density (g/cm3) | Mass (g) |
|---|---|---|---|
| 1 | 500 | 3.59 | 3.38 |
| 2 | 520 | 3.65 | 3.43 |
| 3 | 550 | 3.74 | 3.52 |
| 4 | 600 | 3.88 | 3.65 |
| No. | Mass Ratio (Fe:Al) | Target Thick-Ness (mm) | Mass (g) |
|---|---|---|---|
| 1 | 4:6 | 2 | 3.84 |
| 2 | 4:6 | 2.5 | 3.84 |
| 3 | 4:6 | 3 | 3.84 |
| 4 | 4:6 | 3.5 | 3.84 |
| 5 | 4:6 | 4 | 3.84 |
| 6 | 4:6 | 4.5 | 3.84 |
| 7 | 4:6 | 5 | 3.84 |
| 8 | Cu | 4 | 3.84 |
| 9 | Fe/Al Mix-ture a | 4.5 | 3.88 |
| 10 | Fe/Al Mix-ture b | 4.5 | 3.84 |
| ρ1a kg/m3 | ρ2b kg/m3 | C1c m/s | C2d m/s | S1e | S2f | Vg m/s | Up m/s | Us2h m/s |
|---|---|---|---|---|---|---|---|---|
| 3660 | 7850 | 4624 | 4569 | 1.57 | 1.49 | 3555 | 1309 | 6520 |
| Product | Fe | Fe/Al | FeA12 | FeA13 | Fe2A15 | Al | Al2O3 | Fe2O3 | Fe3O4 |
|---|---|---|---|---|---|---|---|---|---|
| (kJ/mol) | / | 50.3 | 79.2 | 112.3 | 187.7 | / | 1669.68 | 824.2 | 1118.4 |
| No. | Impact Energy (J/mm2) | (MPa) | Platform Pressure (MPa) | Platform Time (ms) | Q1 (kJ) | Q2 (kJ) | QT (kJ) | Energy Efficiency Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 56.1 | 0.97 | 0.88 | 0.15 | 35.6 | 3.5 | 27.7 | 34.0 |
| 2 | 67.3 | 1.17 | 1.09 | 1.02 | 42.9 | 8.4 | 39.9 | 49.0 |
| 3 | 79.1 | 1.33 | 1.21 | 0.94 | 48.8 | 11.1 | 48.5 | 59.5 |
| 4 | 94.2 | 1.52 | 1.39 | 1.66 | 55.7 | 13.6 | 57.9 | 71.1 |
| 5 | 103.5 | 1.77 | 1.58 | 2.53 | 64.9 | 18.2 | 71.7 | 88.0 |
| 6 | 121.1 | 1.8 | 1.68 | 4.61 | 66.0 | 23 | 77.6 | 95.3 |
| 7 | 134.5 | 1.78 | 1.70 | 3.65 | 65.3 | 22.8 | 76.7 | 94.1 |
| 8 | --- | 0.31 | --- | --- | 11.4 | --- | 11.4 a | --- |
| 9 | --- | 0.82 | --- | --- | 30.1 | --- | 18.7 | 22.9 |
| 10 | --- | 0.53 | --- | --- | 19.4 | --- | 8.0 | 9.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Du, Y. Experimental Study on the Energy-Release Characteristics of Fine-Grained Fe/Al Energetic Jets under Impact Loading. Materials 2019, 12, 3317. https://doi.org/10.3390/ma12203317
Li Q, Du Y. Experimental Study on the Energy-Release Characteristics of Fine-Grained Fe/Al Energetic Jets under Impact Loading. Materials. 2019; 12(20):3317. https://doi.org/10.3390/ma12203317
Chicago/Turabian StyleLi, Qiang, and Ye Du. 2019. "Experimental Study on the Energy-Release Characteristics of Fine-Grained Fe/Al Energetic Jets under Impact Loading" Materials 12, no. 20: 3317. https://doi.org/10.3390/ma12203317
APA StyleLi, Q., & Du, Y. (2019). Experimental Study on the Energy-Release Characteristics of Fine-Grained Fe/Al Energetic Jets under Impact Loading. Materials, 12(20), 3317. https://doi.org/10.3390/ma12203317




