A Kinetic Study of the Pozzolanic Reaction of Fly Ash, CaO, and Na2O in the Preparation of Fly Ash Belite Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Specimens
2.3. Conversion of CaO
3. Results
3.1. Analysis of Kinetic Characteristics
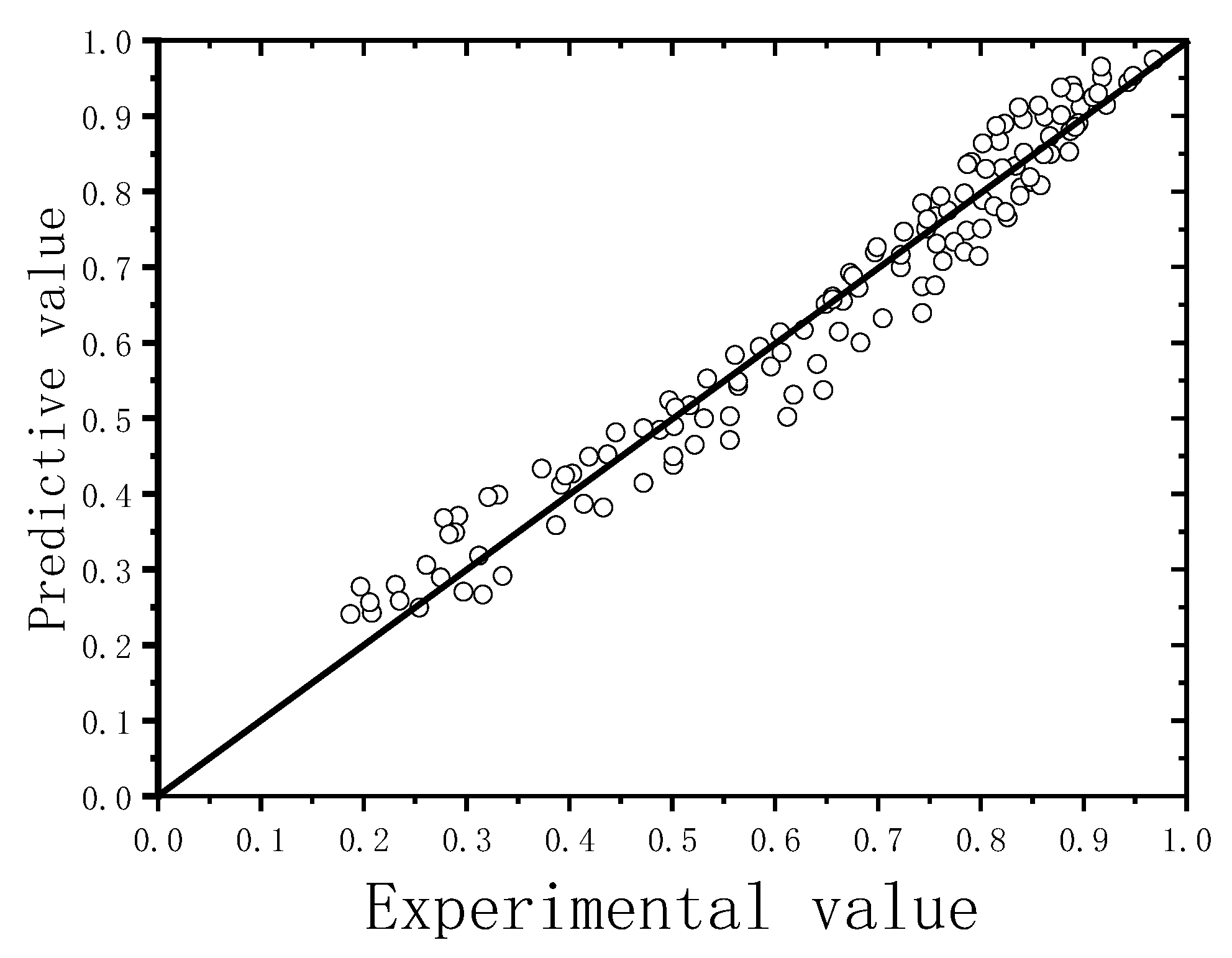
3.2. Kinetic Expression of a Hydrothermal Synthesis Reaction
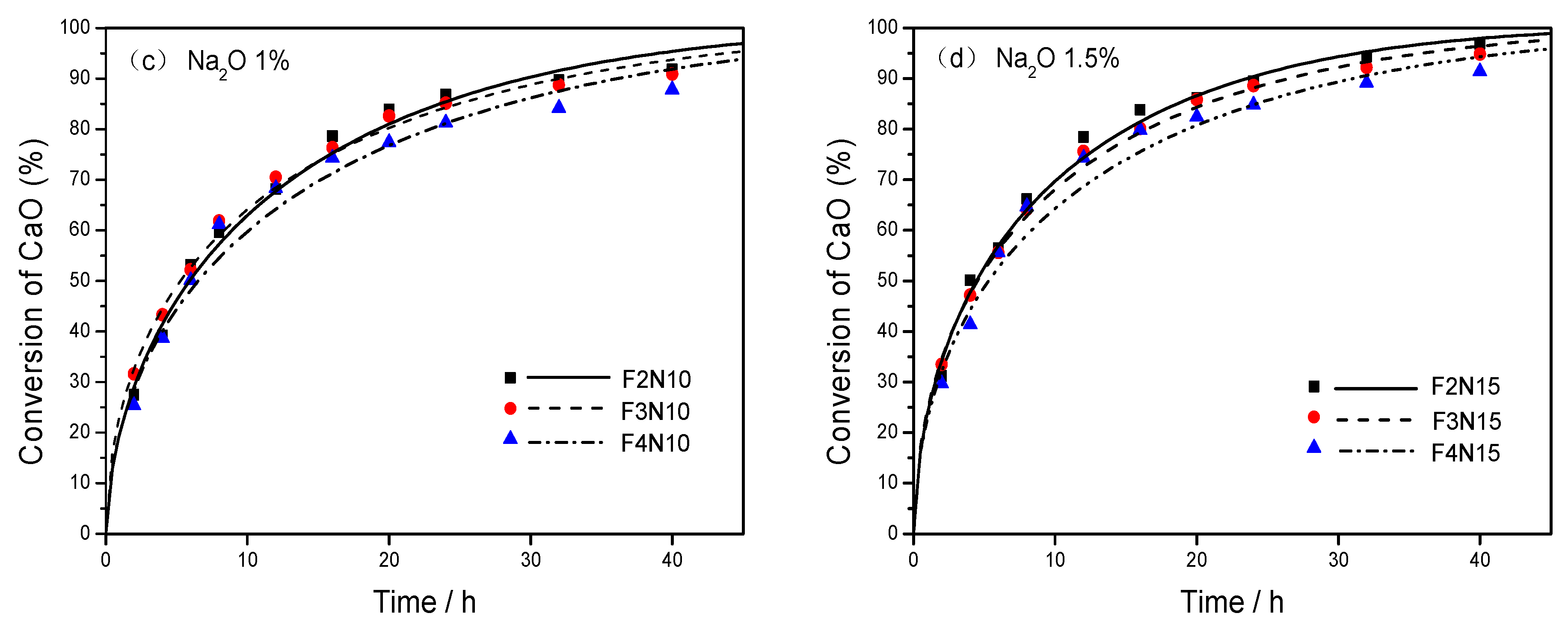
3.3. Kinetic Expression in the Presence of Na2O
4. Discussion
4.1. Effect of Alkali Activation
4.2. Effect of Higher Collision Frequency
5. Conclusions
- (1)
- Na2O, as a network modifier for the structure, incorporated the Ca2+ ions to generate more precursors and increase the reactivity. When the dosage of Na2O was ranging from 0% to 1.5%, the acceleration by alkali was more remarkable with the increase of dosage of Na2O.
- (2)
- The kinetic analyses based on the conversion of CaO indicated that the reaction process was controlled by the Kondo’s modified Jander equation. The trend of reaction in the presence of Na2O was very similar. The activation energy Ea in the process was determined to be 67.76 kJ/mol. It was inferred that the conversion of CaO values increased with the increase in the FA/CA.
- (3)
- A kinetic model for the hydrothermal reaction of lime–fly ash in the presence of Na2O was proposed, which could accurately predict the hydrothermal reaction degree as characterized by the absorption rate of CaO. NaOH could accelerate the dissolution of the amorphous phase in hydrothermal synthesis hydrates, and the dosage of Na2O was set as the correction of the parameter FA/CA. It was possible to increase the pre-exponential factor A in the presence of Na2O, which resulted in an increase in collision frequency between Ca2+ ions and silicon-oxygen ions.
Author Contributions
Funding
Conflicts of Interest
References
- Mccarthy, M.J.; Zheng, L.; Dhir, R.K.; Tella, G. Dry-processing of long-term wet-stored fly ash for use as an addition in concrete. Cem. Concr. Compos. 2017, 92, 205–215. [Google Scholar] [CrossRef]
- Ma, B.; Luo, Z.; Li, X.; Zhang, M.; Wang, Y. High activated mineral admixture slurry made by wet-discharged fly-ash promoted by matrix bonding component. J. Wuhan Univ. Technol. Sci. Ed. 2009, 24, 826–829. [Google Scholar] [CrossRef]
- Rungchet, A.; Poon, C.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Improving the 3D printability of high volume fly ash mixtures via the use of nano attapulgite clay. Compos. Part B Eng. 2019, 165, 75–83. [Google Scholar] [CrossRef]
- Mazouzi, W.; Kacimi, L.; Cyr, M.; Clastres, P. Properties of low temperature belite cements made from aluminosilicate wastes by hydrothermal method. Cem. Concr. Compos. 2014, 53, 170–177. [Google Scholar] [CrossRef]
- Ávalos-Rendón, T.L.; Chelala, E.A.P.; Escobedo, C.J.M.; Figueroa, I.A.; Lara, V.H.; Palacios-Romero, L.M. Synthesis of belite cements at low temperature from silica fume and natural commercial zeolite. Mater. Sci. Eng. B 2018, 229, 79–85. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Chen, X.; Zhang, W.; Yang, D. Synthesis and calorimetric study of hydration behavior of sulfate-rich belite sulfoaluminate cements with different phase compositions. J. Therm. Anal. Calorim. 2018, 133, 1281–1289. [Google Scholar] [CrossRef]
- Sinyoung, S.; Kunchariyakun, K.; Asavapisit, S.; MacKenzie, K.J. Synthesis of belite cement from nano-silica extracted from two rice husk ashes. J. Environ. Manag. 2017, 190, 53–60. [Google Scholar] [CrossRef]
- Kacimi, L.; Cyr, M.; Clastres, P. Synthesis of α′L-C2S cement from fly-ash using the hydrothermal method at low temperature and atmospheric pressure. J. Hazard. Mater. 2010, 181, 593–601. [Google Scholar] [CrossRef]
- Guo, W.; Wang, C.; Yu, P.S.; Jiang, J.H.; Wen, Z.J. Mineral formation mechanism of calcium sulphoaluminate cement clinker in hydrothermal-low temperature sintering process. J. Chin. Ceram. Soc. 2018, 46, 892–896. [Google Scholar]
- Goñi, S.; Guerrero, A.; Luxán, M.; Macías, A. Activation of the fly ash pozzolanic reaction by hydrothermal conditions. Cem. Concr. Res. 2003, 33, 1399–1405. [Google Scholar] [CrossRef]
- Gong, Y.F.; Fang, Y.H. Preparation of belite cement from stockpiled high-carbon fly ash using granule-hydrothermal synthesis method. Constr. Build. Mater. 2016, 111, 175–181. [Google Scholar] [CrossRef]
- Du, C.; Liu, F.; Fang, Y.H.; Wan, Y.; Gong, Y. Preparation of Fly Ash Belite Cement from High-carbon Low-quality Fly Ash and Its Properties. Mater. Rev. 2017, 31, 30–34. [Google Scholar]
- Wang, S.Z. Quantitative kinetics of pozzolanic reactions in coal/cofired biomass fly ashes and calcium hydroxide (CH) mortars. Constr. Build. Mater. 2014, 51, 364–371. [Google Scholar] [CrossRef]
- Zhu, B.R.; Yang, Q.B. Pozzolanic reactivity and reaction kinetics of fly ash. J. Chin. Ceram. Soc. 2004, 32, 892–896. [Google Scholar]
- Fang, Y.H.; Cheng, L.Q.; Gong, Y.F.; Yang, W. Development and future of fly ash belite cement. J. Chin. Ceram. Soc. 2015, 2, 165–173. [Google Scholar]
- Shi, C.; Day, R.L. Pozzolanic reaction in the presence of chemical activators: Part I. Reaction kinetics. Cem. Concr. Res. 2000, 30, 51–58. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Pozzolanic reaction in the presence of chemical activators: Part II—Reaction products and mechanism. Cem. Concr. Res. 2000, 30, 607–613. [Google Scholar] [CrossRef]
- Renedo, M.; Fernández, J. Kinetic modelling of the hydrothermal reaction of fly ash, Ca(OH)2 and CaSO4 in the preparation of desulfurant sorbents. Fuel 2004, 83, 525–532. [Google Scholar] [CrossRef]
- Fernandez, J.; Renedo, M.J.; Pesquera, A.; Irabien, J.A. Kinetic study of the hydrothermal reaction of fly ash with Ca(OH)2 in the preparation of desulfurant sorbents. Chem. Eng. Commun. 2002, 189, 310–321. [Google Scholar] [CrossRef]
- Han, F.; Wang, D.; Yan, P. Hydration kinetics of composite binder containing different content of slag or fly ash. J. Chin. Ceram. Soc. 2014, 42, 613–620. [Google Scholar]
- Nath, S.K.; Mukherjee, S.; Maitra, S.; Kumar, S. Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry. J. Therm. Anal. Calorim. 2017, 127, 1953–1961. [Google Scholar] [CrossRef]
- Guerrero, A.; Goñi, S.; Dolado, J.S. Belite Cements: Modifications of Calcium Silicate Hydrate (C-S-H) Gel by Alkaline Hydrothermal Activation. ACI Mater. J. 2009, 106, 138–143. [Google Scholar]
- Guerrero, A.; Goñi, S.; Allegro, V. Effect of temperature on the durability of class C fly ash belite cement in simulated radioactive liquid waste: Synergy of chloride and sulphate ions. J. Hazard. Mater. 2009, 165, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Herrero, M.J.; Fernandez-Jimenez, A.; Palomo, A. Alkaline Hydration of C2S and C3S. J. Am. Ceram. Soc. 2016, 99, 604–611. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A.; Sánchez-Herrero, M.J.; Fernández-Jiménez, A. C3S and C2S hydration in the presence of Na2CO3 and Na2SO4. J. Am. Ceram. Soc. 2017, 100, 3188–3198. [Google Scholar] [CrossRef]
- Gong, Y.F.; Fang, Y.H.; Yan, Y.R.; Chen, L.Q. Investigation on alkali activated recycled cement mortar powder cementitious material. Mater. Res. Innov. 2014, 18, 784–787. [Google Scholar] [CrossRef]
- Guerrero, A.; Goñi, S.; Campillo, I.; Moragues, A. Belite cement clinker from coal fly ash of high Ca content. Optimization of synthesis parameters. Environ. Sci. Technol. 2004, 38, 3209–3213. [Google Scholar] [CrossRef]
- Goñi, S.; Guerrero, A. Study of alkaline hydrothermal activation of belite cements by thermal analysis. J. Therm. Anal. Calorim. 2009, 99, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Nocuò-Wczelik, W. Effect of Na and Al on the phase composition and morphology of autoclaved calcium silicate hydrates. Cem. Concr. Res. 1999, 29, 1759–1767. [Google Scholar] [CrossRef]
- Viallis-Terrisse, H.; Nonat, A.; Petit, J.-C. Zeta-Potential Study of Calcium Silicate Hydrates Interacting with Alkaline Cations. J. Colloid Interface Sci. 2001, 244, 58–65. [Google Scholar] [CrossRef]

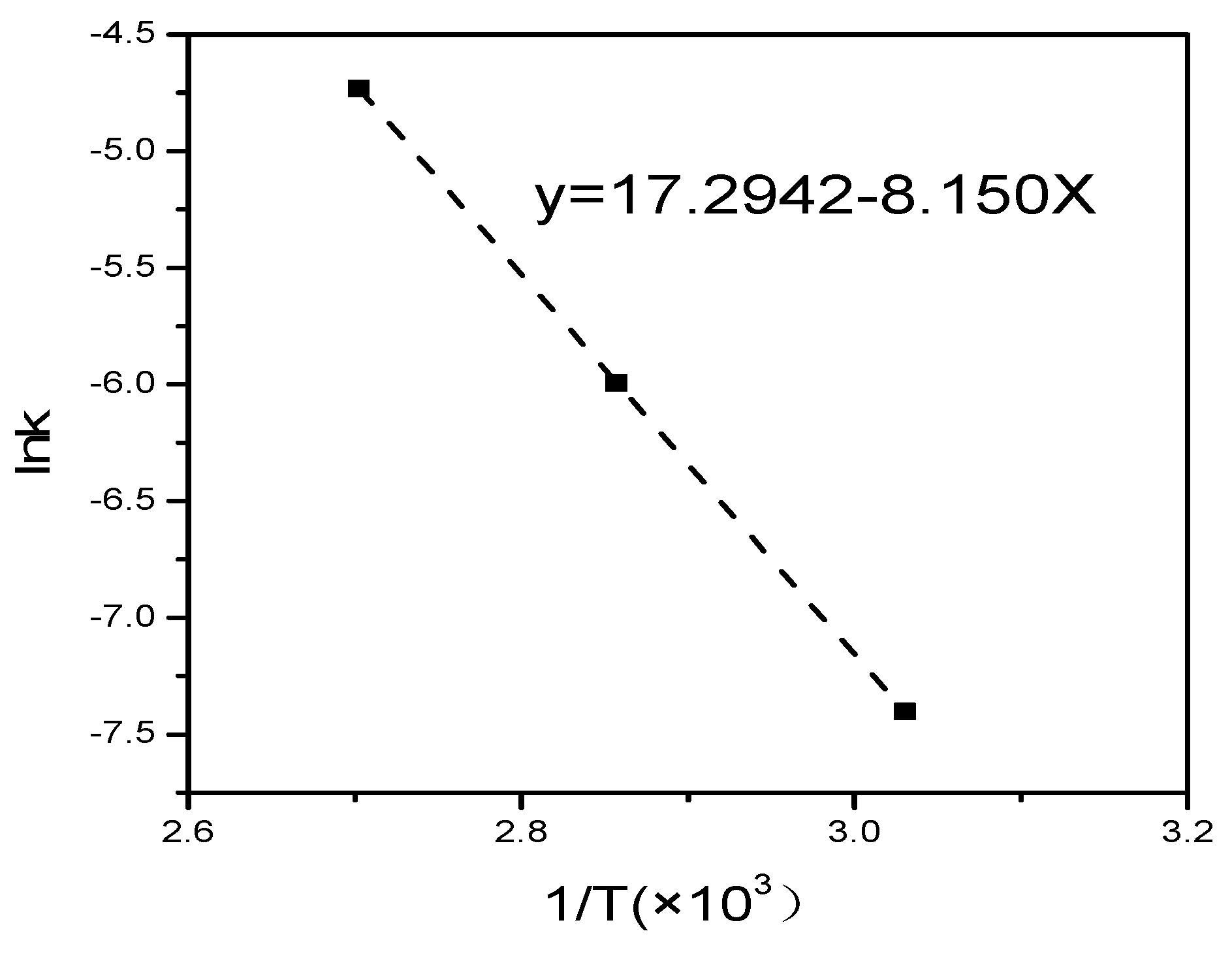

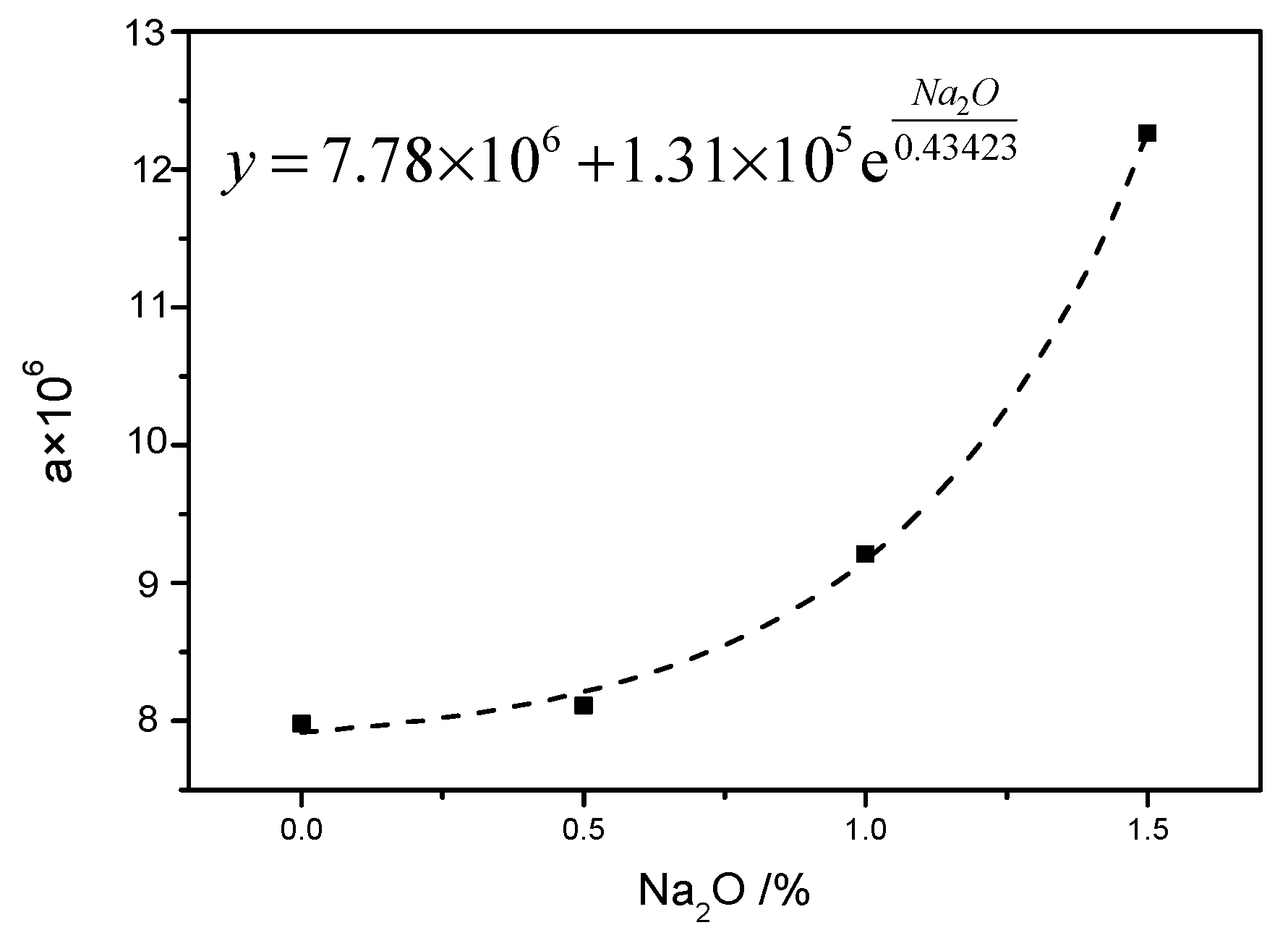
| Chemical Composition | Fly Ash |
|---|---|
| SiO2 | 49.21 |
| Al2O3 | 29.46 |
| Fe2O3 | 4.36 |
| CaO | 2.26 |
| MgO | 0.80 |
| TiO2 | 1.21 |
| Na2O | 0.39 |
| K2O | 0.28 |
| SO3 | 0.18 |
| Loss on ignition | 10.08 |
| No. | FA/CA/Na2O * | Absorption Rate of CaO (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 12 h | 16 h | 20 h | 24 h | 32 h | 40 h | ||
| F1 | 80/20/0 | 26.1 | 37.3 | 49.7 | 58.5 | 72.3 | 76.8 | 82.1 | 86.7 | 89.1 | 91.7 |
| F2 | 75/25/0 | 19.7 | 32.1 | 44.5 | 56.4 | 64.9 | 69.9 | 74.3 | 80.5 | 84.1 | 87.8 |
| F3 | 70/30/0 | 20.6 | 27.8 | 41.9 | 50.3 | 60.5 | 67.6 | 72.5 | 76.1 | 80.2 | 83.7 |
| F4 | 65/35/0 | 18.7 | 28.3 | 39.6 | 47.2 | 56.1 | 65.6 | 72.2 | 74.8 | 78.7 | 81.5 |
| F2N5 | 75/25/0.5 | 23.1 | 33.1 | 48.8 | 53.4 | 66.6 | 75.7 | 80.2 | 83.4 | 86.2 | 88.9 |
| F2N10 | 75/25/1.0 | 27.5 | 39.2 | 53.1 | 59.6 | 68.1 | 78.6 | 83.9 | 86.8 | 89.7 | 91.8 |
| F2N15 | 75/25/1.5 | 31.2 | 50.1 | 56.4 | 66.2 | 78.4 | 83.8 | 86.1 | 89.5 | 94.3 | 96.2 |
| F3N5 | 70/30/0.5 | 23.5 | 29.2 | 43.7 | 51.7 | 62.8 | 67.3 | 74.7 | 78.4 | 81.8 | 85.6 |
| F3N10 | 70/30/1.0 | 31.6 | 43.3 | 52.2 | 61.8 | 70.5 | 76.3 | 82.6 | 85.1 | 88.7 | 90.9 |
| F3N15 | 70/30/1.5 | 33.5 | 47.2 | 55.6 | 64.1 | 75.6 | 80.1 | 85.8 | 88.6 | 92.2 | 94.8 |
| F4N5 | 65/35/0.5 | 20.8 | 28.9 | 40.3 | 50.2 | 60.6 | 65.6 | 69.7 | 75.6 | 79.1 | 82.3 |
| F4N10 | 65/35/1.0 | 25.4 | 38.7 | 50.1 | 61.2 | 68.3 | 74.3 | 77.4 | 81.3 | 84.2 | 87.7 |
| F4N15 | 65/35/1.5 | 29.7 | 41.4 | 55.6 | 64.7 | 74.3 | 79.8 | 82.4 | 84.8 | 89.2 | 91.4 |
| F3 a | 70/30/0 | 16.6 | 26.8 | 33.9 | 40.3 | 45.7 | 49.6 | 52.5 | 56.3 | 60.3 | 62.7 |
| F3 b | 70/30/0 | N/A | 6.4 | N/A | 10.1 | 13.6 | 16.2 | 18.7 | 20.1 | N/A | N/A |
| No. | Mixed Control | α = 1 − (1 − e(lnt − lnk)/N)3 | ||||
|---|---|---|---|---|---|---|
| 1/N | (ln k)/N | k × 102 (h−1) | N | Standard Deviation (%) | ||
| F1 | 0.61 | −2.71 | 1.29 | 1.63 | 2.68 | α1 = 1 − (1 − e(lnt − 4.350)/1.63)3 |
| F2 | 0.65 | −2.95 | 1.07 | 1.54 | 3.59 | α2 = 1 − (1 − e(lnt − 4.537)/1.54)3 |
| F3 | 0.63 | −2.99 | 0.88 | 1.58 | 3.29 | α3 = 1 − (1 − e(lnt − 4.733)/1.58)3 |
| F4 | 0.64 | −3.07 | 0.85 | 1.55 | 3.04 | α4 = 1 − (1 − e(lnt − 4.676)/1.55)3 |
| F2N5 | 0.63 | −2.84 | 1.13 | 1.58 | 3.30 | α5 = 1 − (1 − e(lnt − 4.483)/1.58)3 |
| F2N10 | 0.59 | −2.63 | 1.18 | 1.69 | 2.43 | α6 = 1 − (1 − e(lnt − 4.439)/1.69)3 |
| F2N15 | 0.57 | −2.43 | 1.43 | 1.75 | 2.14 | α7 = 1 − (1 − e(lnt − 4.247)/1.75)3 |
| F3N5 | 0.61 | −2.88 | 0.88 | 1.64 | 2.97 | α8 = 1 − (1 − e(lnt − 4.733)/1.64)3 |
| F3N10 | 0.53 | −2.46 | 0.96 | 1.89 | 1.79 | α9 = 1 − (1 − e(lnt − 4.646)/1.89)3 |
| F3N15 | 0.54 | −2.40 | 1.19 | 1.85 | 1.41 | α10 = 1 − (1 − e(lnt − 4.431)/1.85)3 |
| F4N5 | 0.61 | −2.94 | 0.83 | 1.63 | 3.08 | α11 = 1 − (1 − e(lnt − 4.791)/1.63)3 |
| F4N10 | 0.56 | −2.62 | 0.90 | 1.80 | 3.39 | α12 = 1 − (1 − e(lnt − 4.710)/1.80)3 |
| F4N15 | 0.54 | −2.48 | 1.02 | 1.85 | 3.43 | α13 = 1 − (1 − e(lnt − 4.585)/1.85)3 |
| F3 a | 0.50 | −3.02 | 0.249 | 0.50 | 2.59 | α14 = 1 − (1 − e(lnt − 5.995)/1.98)3 |
| F3 b | 0.62 | −4.59 | 0.061 | 0.62 | 11.88 | α15 = 1 − (1 − e(lnt − 7.402)/1.61)3 |
| No. | k × 102 (h−1) | Standard Deviation (%) | |
|---|---|---|---|
| Predictive Value | Experimental Value | ||
| F1 | 1.28 | 1.22 | 3.00 |
| F2 | 1.06 | 1.07 | 0.50 |
| F3 | 0.92 | 0.88 | 2.00 |
| F4 | 0.82 | 0.85 | 1.50 |
| F3 a | 0.261 | 0.249 | 0.60 |
| F3 b | 0.064 | 0.061 | 0.15 |
| No. | k (10−2)/h−1 | a (106) | Average of a (106) |
|---|---|---|---|
| F2N5 | 1.13 | 8.78 | 8.11 |
| F3N5 | 0.88 | 7.34 | |
| F4N5 | 0.83 | 8.21 | |
| F2N10 | 1.18 | 9.39 | 9.21 |
| F3N10 | 0.96 | 8.60 | |
| F4N10 | 0.90 | 9.64 | |
| F2N10 | 1.43 | 12.57 | 12.26 |
| F3N10 | 1.19 | 12.23 | |
| F4N10 | 1.02 | 11.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Yu, B.; Fang, Y.; Yang, D.; Wu, S.-a.; Yan, Y. A Kinetic Study of the Pozzolanic Reaction of Fly Ash, CaO, and Na2O in the Preparation of Fly Ash Belite Cement. Materials 2019, 12, 3303. https://doi.org/10.3390/ma12203303
Gong Y, Yu B, Fang Y, Yang D, Wu S-a, Yan Y. A Kinetic Study of the Pozzolanic Reaction of Fly Ash, CaO, and Na2O in the Preparation of Fly Ash Belite Cement. Materials. 2019; 12(20):3303. https://doi.org/10.3390/ma12203303
Chicago/Turabian StyleGong, Yongfan, Ben Yu, Yonghao Fang, Dingyi Yang, Shu-an Wu, and Yurong Yan. 2019. "A Kinetic Study of the Pozzolanic Reaction of Fly Ash, CaO, and Na2O in the Preparation of Fly Ash Belite Cement" Materials 12, no. 20: 3303. https://doi.org/10.3390/ma12203303
APA StyleGong, Y., Yu, B., Fang, Y., Yang, D., Wu, S.-a., & Yan, Y. (2019). A Kinetic Study of the Pozzolanic Reaction of Fly Ash, CaO, and Na2O in the Preparation of Fly Ash Belite Cement. Materials, 12(20), 3303. https://doi.org/10.3390/ma12203303






