Microstructure Evolution of the Semi-Macro Segregation Induced Banded Structure in High Strength Oil Tubes During Quenching and Tempering Treatments
Abstract
1. Introduction
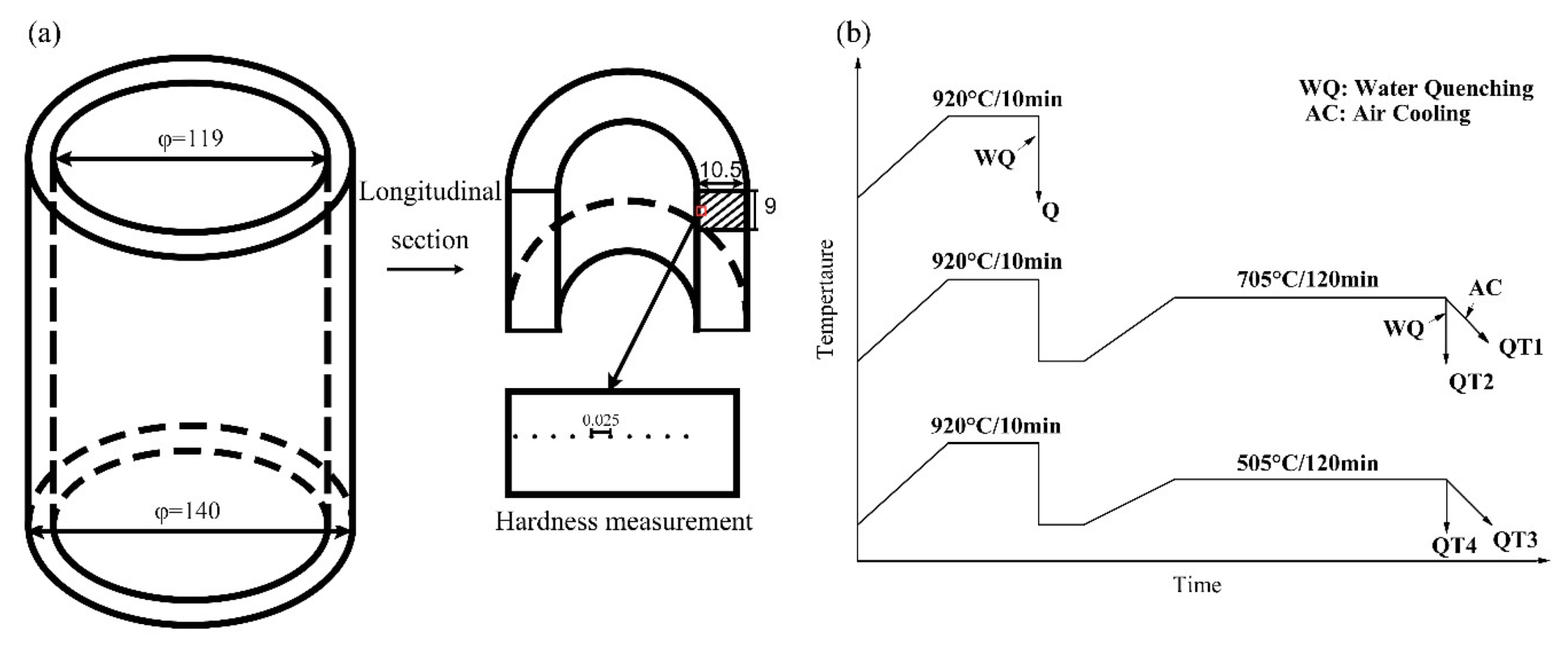
2. Materials and Methods
3. Results
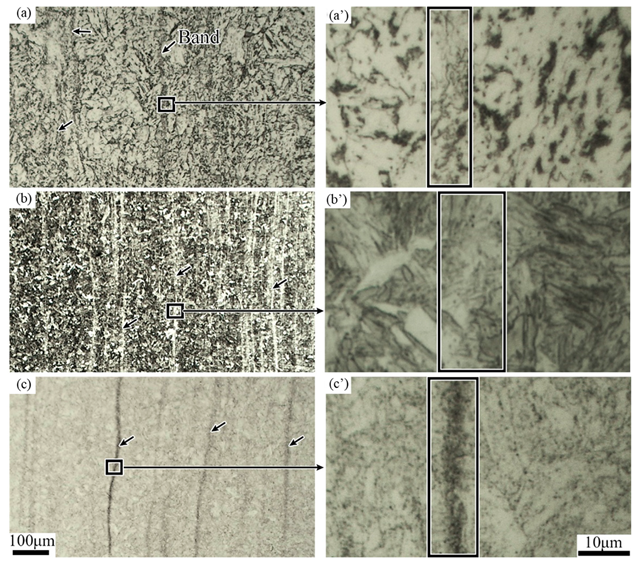
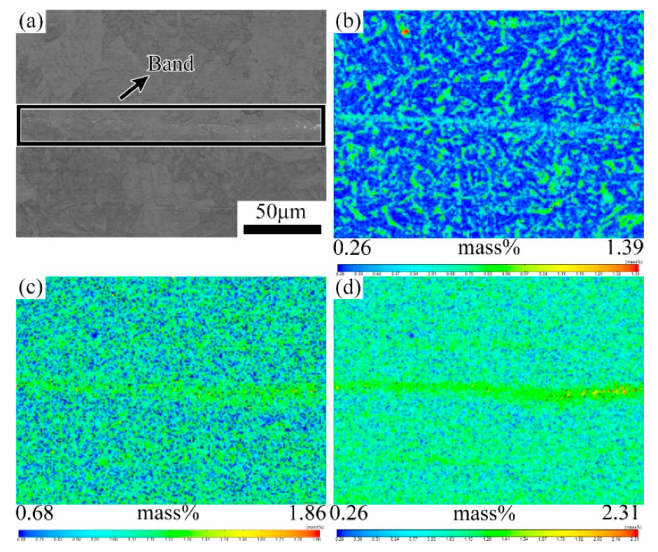
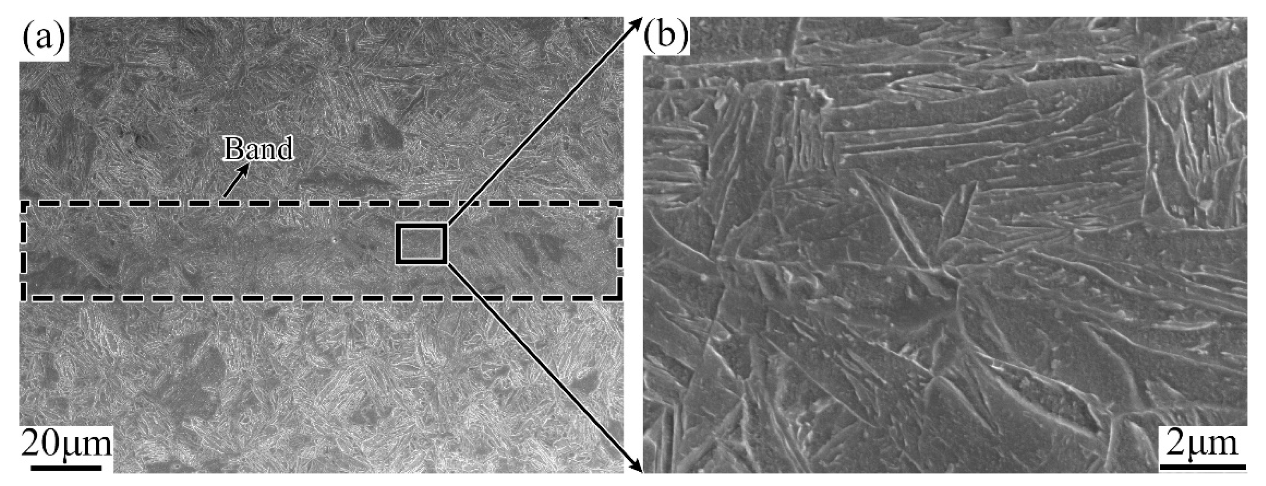
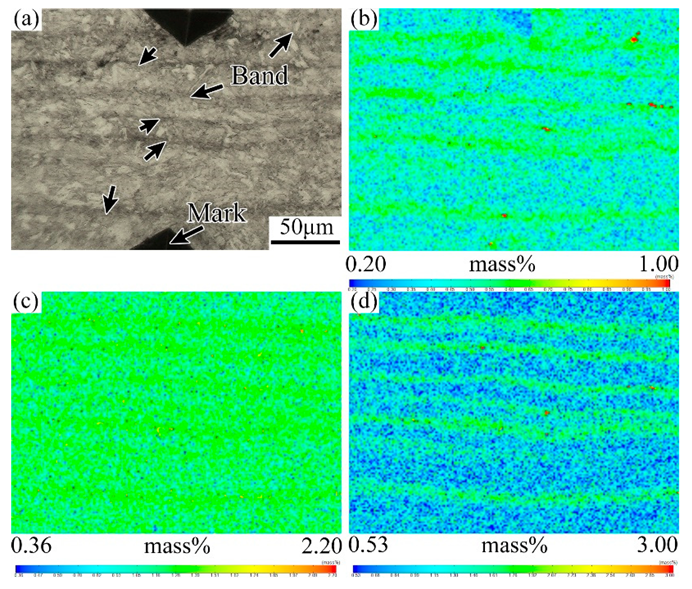
3.1. Evolution of the Banded during the QT Hot Treatment
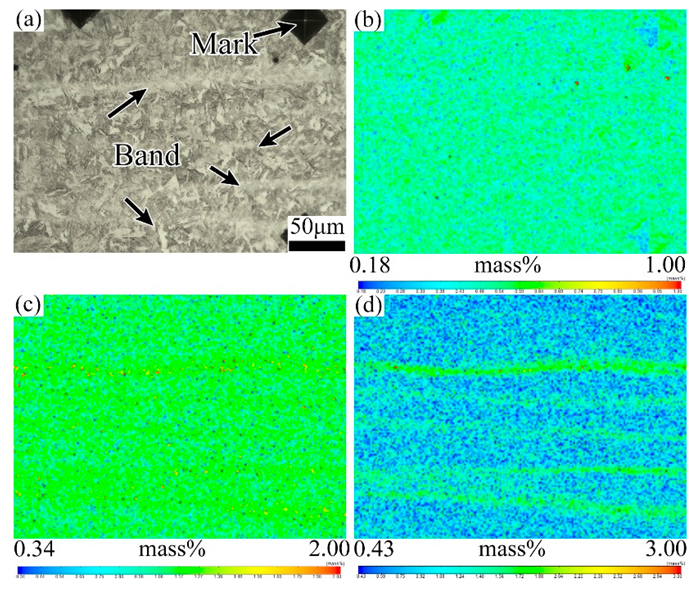
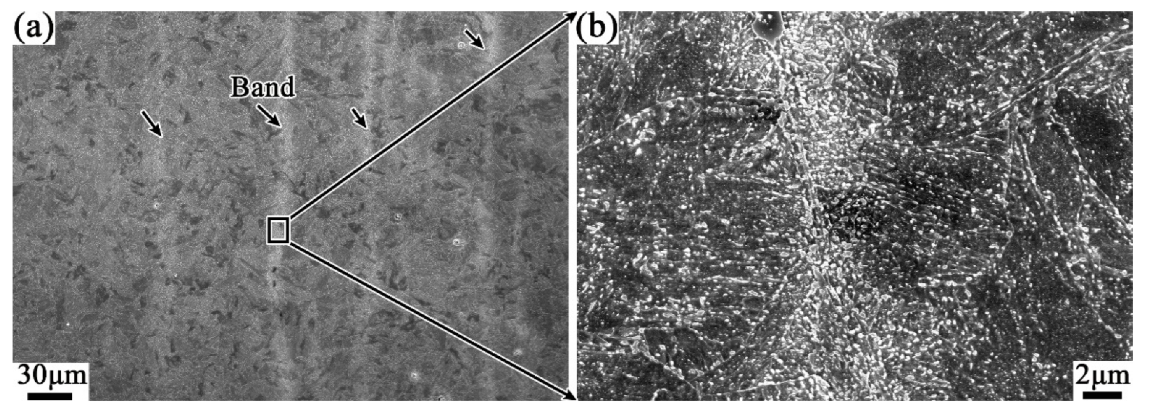
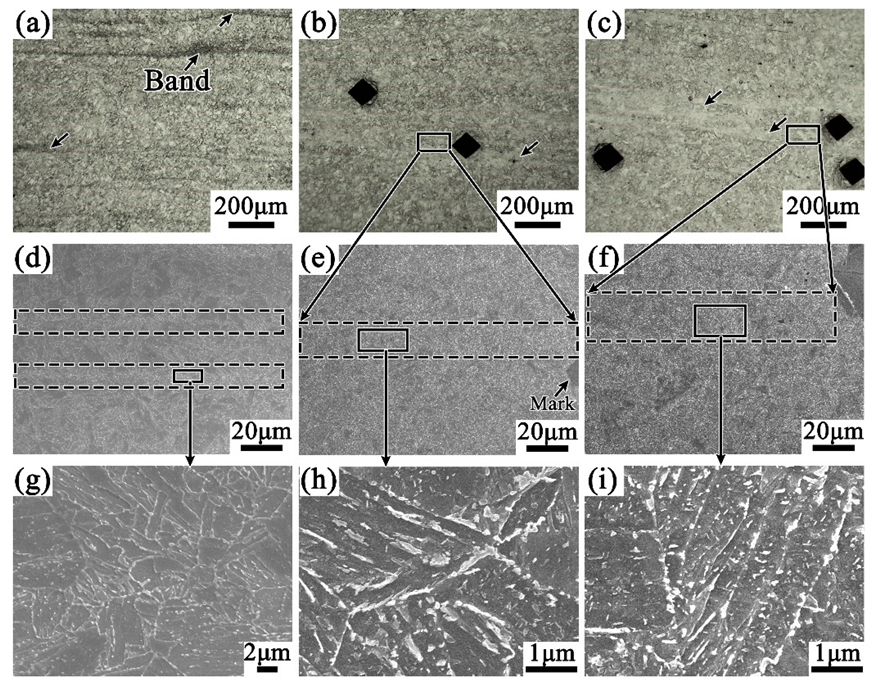
3.2. Effect of Tempering Temperature and Cooling Rate on Carbide Banding
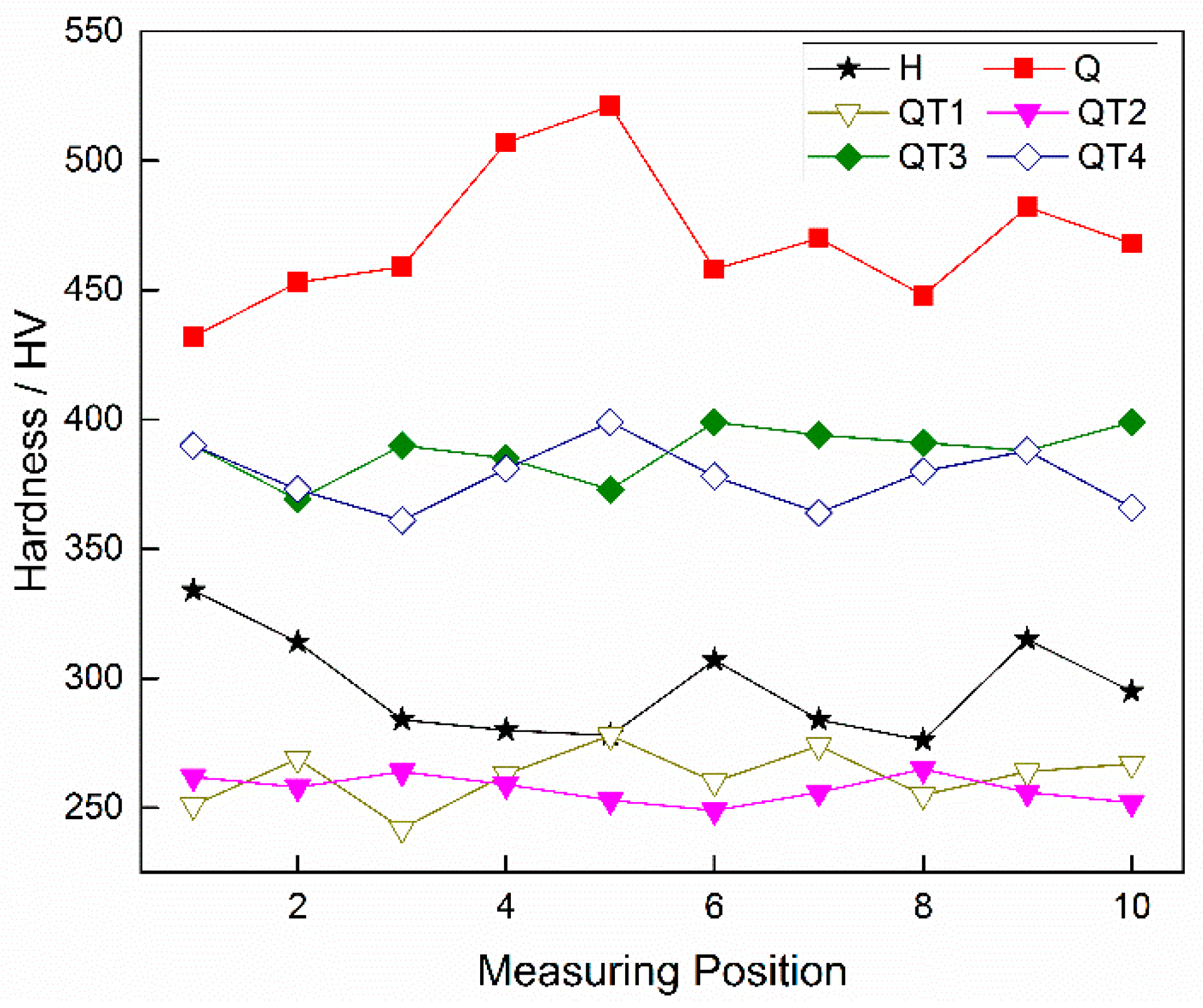
3.3. Hardness Evaluation under Different Heat Treatment Processes
4. Discussion
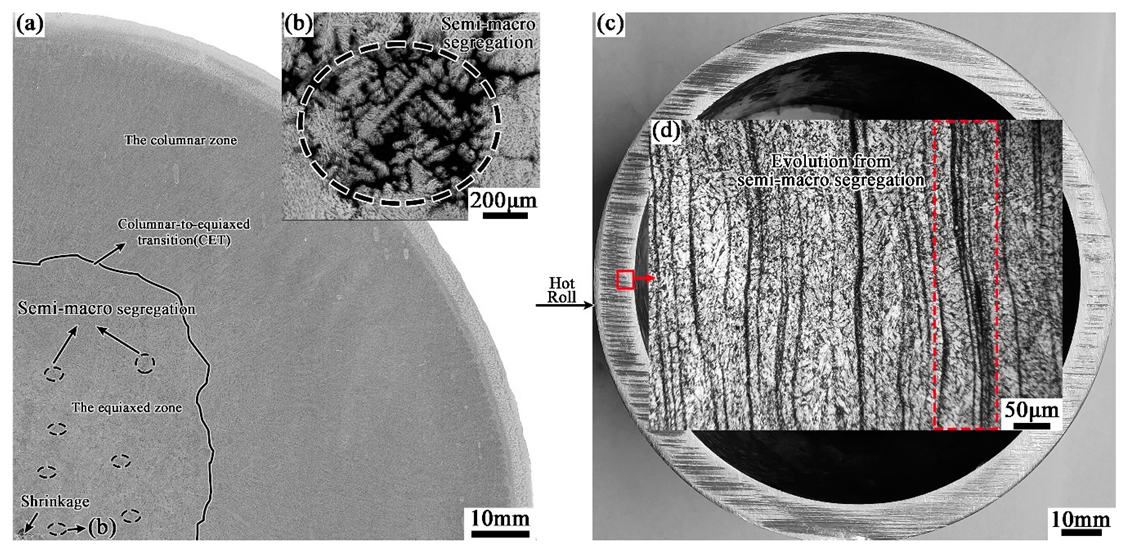
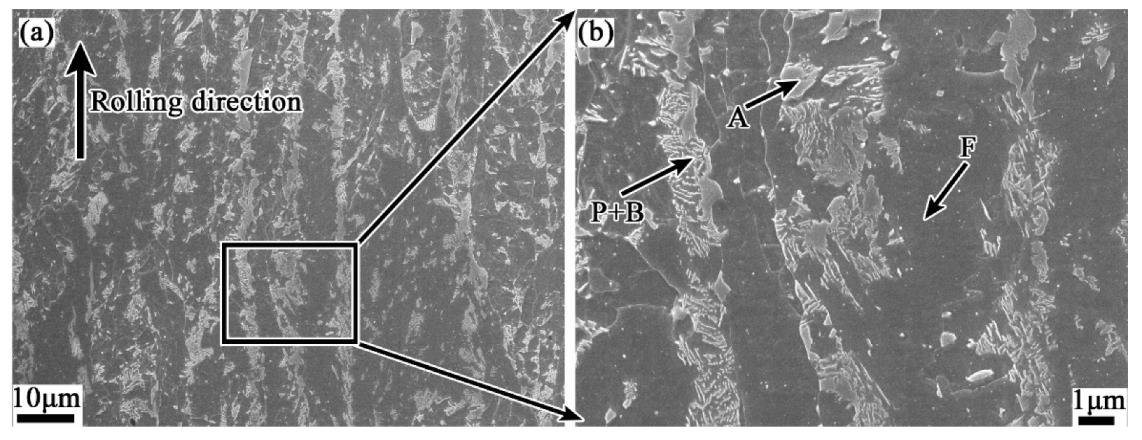
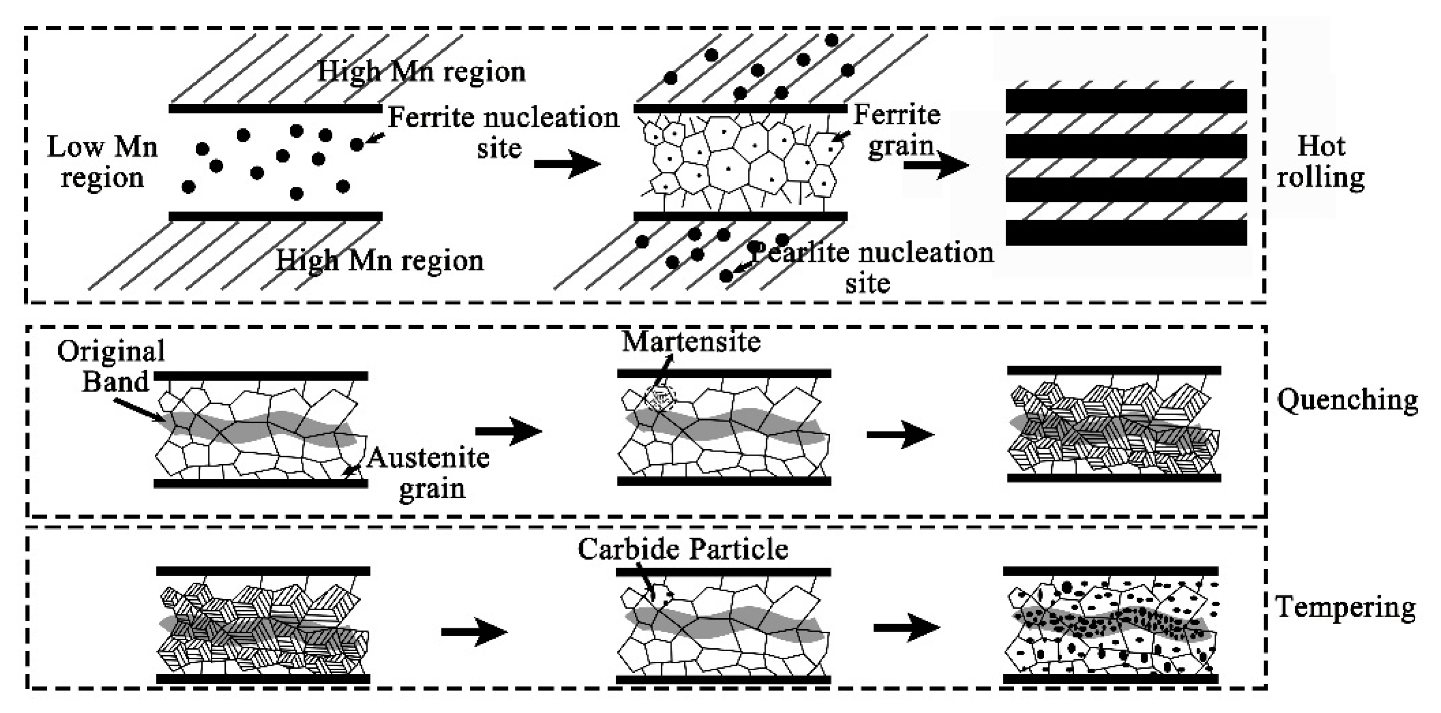
4.1. Evolution of Banded Structure and its Mechanism
4.2. Effect of Tempering Temperature and Cooling Rate on the Banded Structure
5. Conclusions
- The banded structure originated from as-cast semi-macro segregation will change significantly in subsequent hot working and heat treatment processes. The hot rolled state of the banded structure is mainly composed of pearlite, together with few bainite. After quenching during QT process, the C element diffuses obviously, but the morphology of the banded defect structure changes little. The microstructure, however, has been transformed into martensite with high concentration of alloy elements. After the following tempering treatment, the banded martensite structure will evolve into a kind of banded defect full of dense particles of various carbides.
- Compared with the conventional heat treatment process of these steel products, a lower tempering temperature and a quicker cooling rate after tempering can reduce the amount and density of carbide precipitation in the banded structure to a certain extent, thereby reducing the degree of the banded defects. However, the radial microstructure hardness test of the casing tube wall shows that, compared with a tempering temperature of 705 °C, both the overall hardness and the hardness variation of the sample are larger under 505 °C, which cannot meet the requirements of anti-SSC performance; however, under the condition of tempering at 705 °C and a following water cooling, instead of the conventional air cooling process, the banded structure defects can be decreased together with an obvious improvement of hardness uniformity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomohiko, O.; Kobayashi, K. Hydrogen embrittlement of OCTG and linepipes. Corros. Eng. 2011, 60, 156–163. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.H.; Liu, M.; Liu, Y.H.; Luo, M.; Zhang, C.X.; Wang, C.H.; Cao, G.H. A systematical analysis with respect to multiple hydrogen traps influencing sulfide stress cracking behavior of API-5CT-C110 casing steel. Mater. Sci. Eng. A 2018, 721, 81–88. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.H.; Dai, Y.C.; Lia, X.; Cao, G.H.; Russell, A.M.; Liu, Y.H.; Dong, X.M.; Zhang, Z.H. Effect of quenching and tempering process on sulfide stress cracking susceptibility in API-5CT-C110 casing steel. Mater. Sci. Eng. A 2017, 688, 378–387. [Google Scholar] [CrossRef]
- Kawashima, A.; Hashimoto, K.; Shimodaira, S. Hydrogen electrode reaction and hydrogen embrittlement of mild steel in hydrogen sulfide solutions. Corrosion 1976, 32, 321–331. [Google Scholar] [CrossRef]
- Grobner, P.J.; Sponseller, D.L.; Diesburg, D.E. The effects of processing variables on mechanical properties and sulfide stress cracking resistance of SAE 4135 steel modified with 0.75 percent Mo and 0.035 percent Cb. J. Eng. Ind. 1976, 98, 708–716. [Google Scholar] [CrossRef]
- Craig, B.D. The effect of nickel on hydrogen cracking resistance in low alloy steels—A review. Corrosion 1982, 38, 457–463. [Google Scholar] [CrossRef]
- Thompson, A.W.; Bernstein, I.M. The Role of Metallurgical Variables in Hydrogen-Assisted Environmental Fracture. In Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Springer: Boston, MA, USA, 1980; pp. 53–175. [Google Scholar] [CrossRef]
- Vollmer, L.W. The behavior of steels in hydrogen sulfide environments. Corrosion 1958, 14, 38–42. [Google Scholar] [CrossRef]
- Sponseller, D.L.; Garber, R.; Straatmann, J.A. Effect of microstructure on sulfide-stress-cracking resistance of high-strength casing steels. In MiCon 82: Optimization of Processing, Properties, and Service Performance Through Microstructural Control; ASTM International: West Conshohocken, PA, USA, 1983. [Google Scholar] [CrossRef]
- Verhoeven, J.D. A review of microsegregation induced banding phenomena in steels. J. Mater. Eng. Perform. 2000, 9, 286–296. [Google Scholar] [CrossRef]
- Thompson, S.W.; Howell, P.R. Factors influencing ferrite/pearlite banding and origin of large pearlite nodules in a hypoeutectoid plate steel. Mater. Sci. Technol. 1992, 8, 777–784. [Google Scholar] [CrossRef]
- Jatczak, C.F.; Girardi, D.J.; Rowland, E.S. On banding in steel. Trans. ASM 1956, 48, 279–305. [Google Scholar]
- Bastien, P.G. The mechanism of formation of banded structures. J. Iron Steel Inst. 1957, 187, 281–290. [Google Scholar]
- Li, B.; Zhang, Z.H.; Liu, H.S.; Luo, M.; Lan, P.; Tang, H.Y.; Zhang, J.Q. Characteristics and Evolution of the Spot Segregations and Banded Defects in High Strength Corrosion Resistant Tube Steel. Acta Metall. Sin. 2019, 55, 762–772. [Google Scholar] [CrossRef]
- Rivera, P.C.; Ramunni, V.P.; Bruzzoni, P. Hydrogen trapping in an API 5L X60 steel. Corros. Sci. 2012, 54, 106–118. [Google Scholar] [CrossRef]
- Urband, B.E.; Morey, S. High strength sour service C110 casing. In Proceedings of the Society of Petroleum Engineers SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 9–11 March 1999. [Google Scholar] [CrossRef]
- Krauss, G. Solidification, segregation, and banding in carbon and alloy steels. Metall. Mater. Trans. B 2003, 34, 781–792. [Google Scholar] [CrossRef]
- Ioffe, A.V.; Tetyueva, T.V.; Vyboishchik, M.A.; Knyazkin, S.A.; Zyryanov, A.O. Corrosion-mechanical fracture of tubing from carbon and alloy steels operating in environments containing hydrogen sulfide. Met. Sci. Heat Treat. 2013, 54, 492–497. [Google Scholar] [CrossRef]
- Rivera-Diaz-Del-Castillo, P.E.J.; Van Der Zwaag, S.; Sietsma, J. A model for ferrite/pearlite band formation and prevention in steels. Metall. Mater. Trans. A 2004, 35, 425–433. [Google Scholar] [CrossRef]
- Zhang, X.; Matsuura, K.; Ohno, M. Abnormal grain growth in austenite structure reversely transformed from ferrite/pearlite-banded Structure. Metall. Mater. Trans. A 2014, 45, 4623–4634. [Google Scholar] [CrossRef]
- Eckert, J.A.; Howell, P.R.; Thompson, S.W. Banding and the nature of large, irregular pearlite nodules in a hot-rolled low-alloy plate steel: A second report. J. Mater. Sci. 1993, 28, 4412–4420. [Google Scholar] [CrossRef]
- Ohmura, T.; Hara, T.; Tsuzaki, K. Evaluation of temper softening behavior of Fe-C binary martensitic steels by nanoindentation. Scr. Mater. 2003, 49, 1157–1162. [Google Scholar] [CrossRef]
- Hou, Z.; Hedström, P.; Xu, Y.B.; Odqvist, J. Microstructure of martensite in Fe–C–Cr and its implications for modelling of carbide precipitation during tempering. ISIJ Int. 2014, 54, 2649–2656. [Google Scholar] [CrossRef]
- Luo, Y.W.; Guo, H.J.; Guo, J. Effect of cooling rate on the transformation characteristics and precipitation behaviour of carbides in AISI M42 high-speed steel. Ironmak. Steelmak. 2019, 7, 642–648. [Google Scholar] [CrossRef]
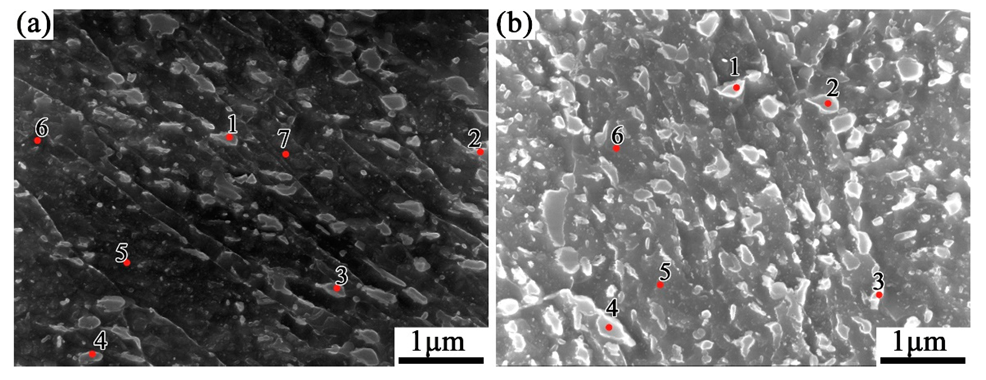
| Position | C | Fe |
|---|---|---|
| 1 | 1.63 | 98.37 |
| 2 | 1.71 | 98.29 |
| 3 | 1.90 | 98.10 |
| 4 | 1.82 | 98.18 |
| 5 | 1.23 | 98.77 |
| 6 | 1.37 | 98.63 |
| 7 | 1.16 | 98.84 |
| Position | C | Ti | Cr | Mn | Fe | Nb | Mo |
|---|---|---|---|---|---|---|---|
| 1 | 2.34 | 6.73 | - | - | 85.44 | 5.49 | - |
| 2 | 2.59 | - | 0.83 | 1.68 | 94.90 | - | - |
| 3 | 2.11 | - | 1.38 | - | 96.51 | - | - |
| 4 | 2.46 | - | - | - | 97.54 | - | - |
| 5 | 1.39 | - | - | - | 96.53 | - | 2.09 |
| 6 | 1.11 | - | - | - | 98.89 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Luo, M.; Yang, Z.; Yang, F.; Liu, H.; Tang, H.; Zhang, Z.; Zhang, J. Microstructure Evolution of the Semi-Macro Segregation Induced Banded Structure in High Strength Oil Tubes During Quenching and Tempering Treatments. Materials 2019, 12, 3310. https://doi.org/10.3390/ma12203310
Li B, Luo M, Yang Z, Yang F, Liu H, Tang H, Zhang Z, Zhang J. Microstructure Evolution of the Semi-Macro Segregation Induced Banded Structure in High Strength Oil Tubes During Quenching and Tempering Treatments. Materials. 2019; 12(20):3310. https://doi.org/10.3390/ma12203310
Chicago/Turabian StyleLi, Bo, Ming Luo, Zhanbing Yang, Feifei Yang, Huasong Liu, Haiyan Tang, Zhonghua Zhang, and Jiaquan Zhang. 2019. "Microstructure Evolution of the Semi-Macro Segregation Induced Banded Structure in High Strength Oil Tubes During Quenching and Tempering Treatments" Materials 12, no. 20: 3310. https://doi.org/10.3390/ma12203310
APA StyleLi, B., Luo, M., Yang, Z., Yang, F., Liu, H., Tang, H., Zhang, Z., & Zhang, J. (2019). Microstructure Evolution of the Semi-Macro Segregation Induced Banded Structure in High Strength Oil Tubes During Quenching and Tempering Treatments. Materials, 12(20), 3310. https://doi.org/10.3390/ma12203310





