Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
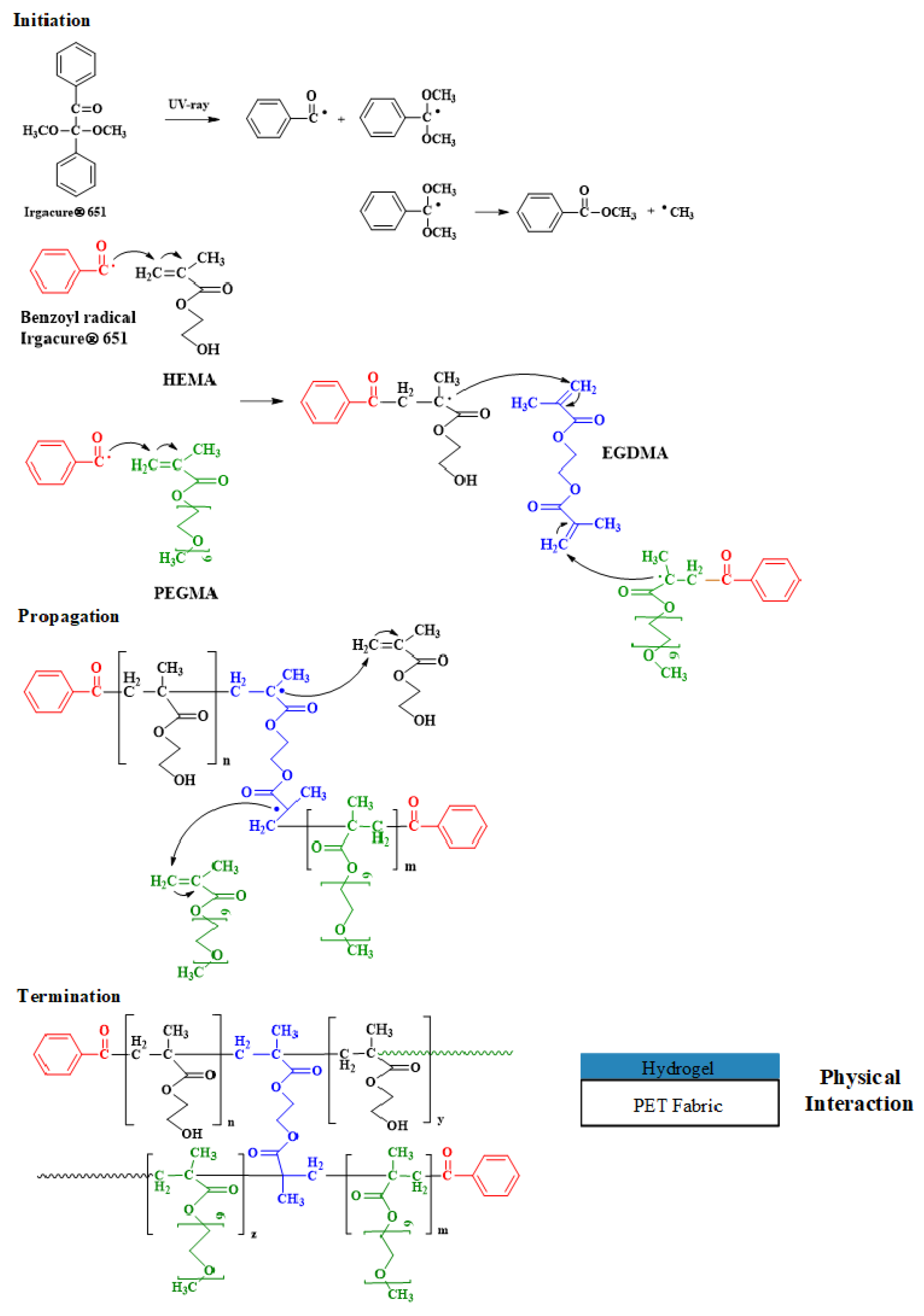
2.2. Photochemical Crosslinking/Grafting
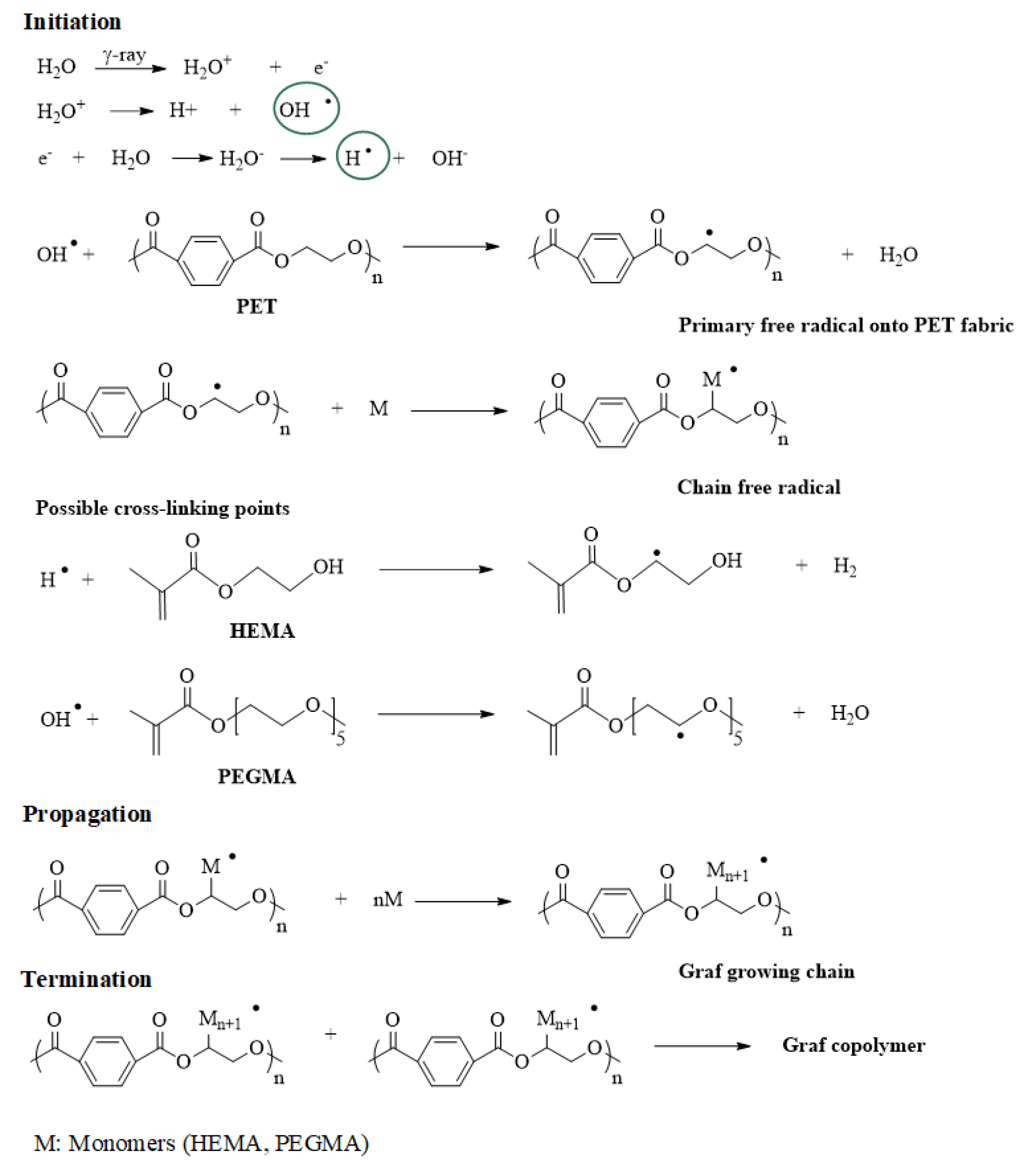
2.3. Gamma Radiation Grafting
2.4. Characterization of Grafted Hydrogels on PET Fabrics
2.5. In Situ Synthesis of Silver Nanoparticles
2.6. Antibacterial Activity
3. Results and Discussion
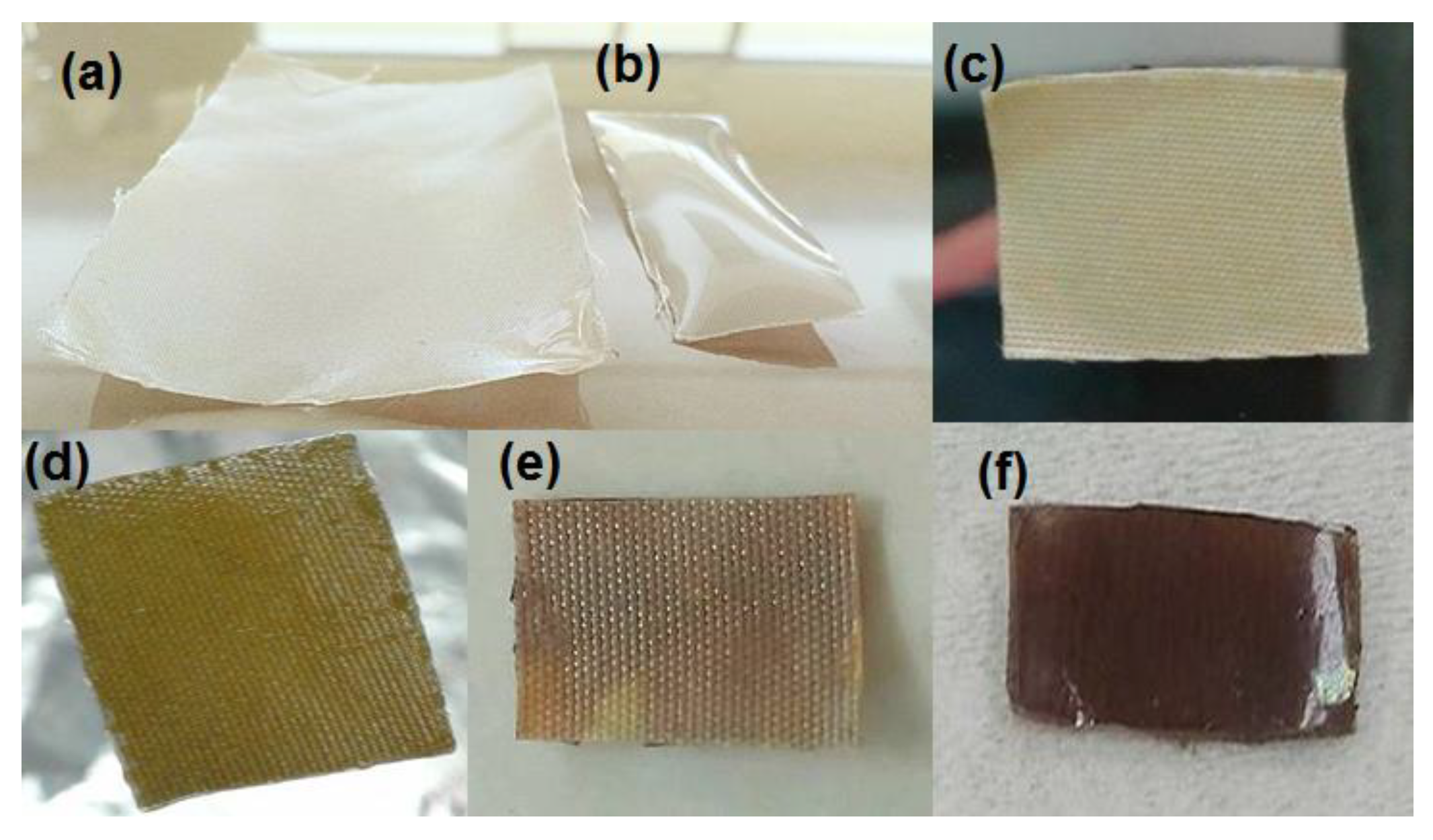
3.1. Grafting of Hydrogels onto PET-Fabrics
3.1.1. Characterization of Hydrogel-Grafted PET-Fabrics
Water Absorption
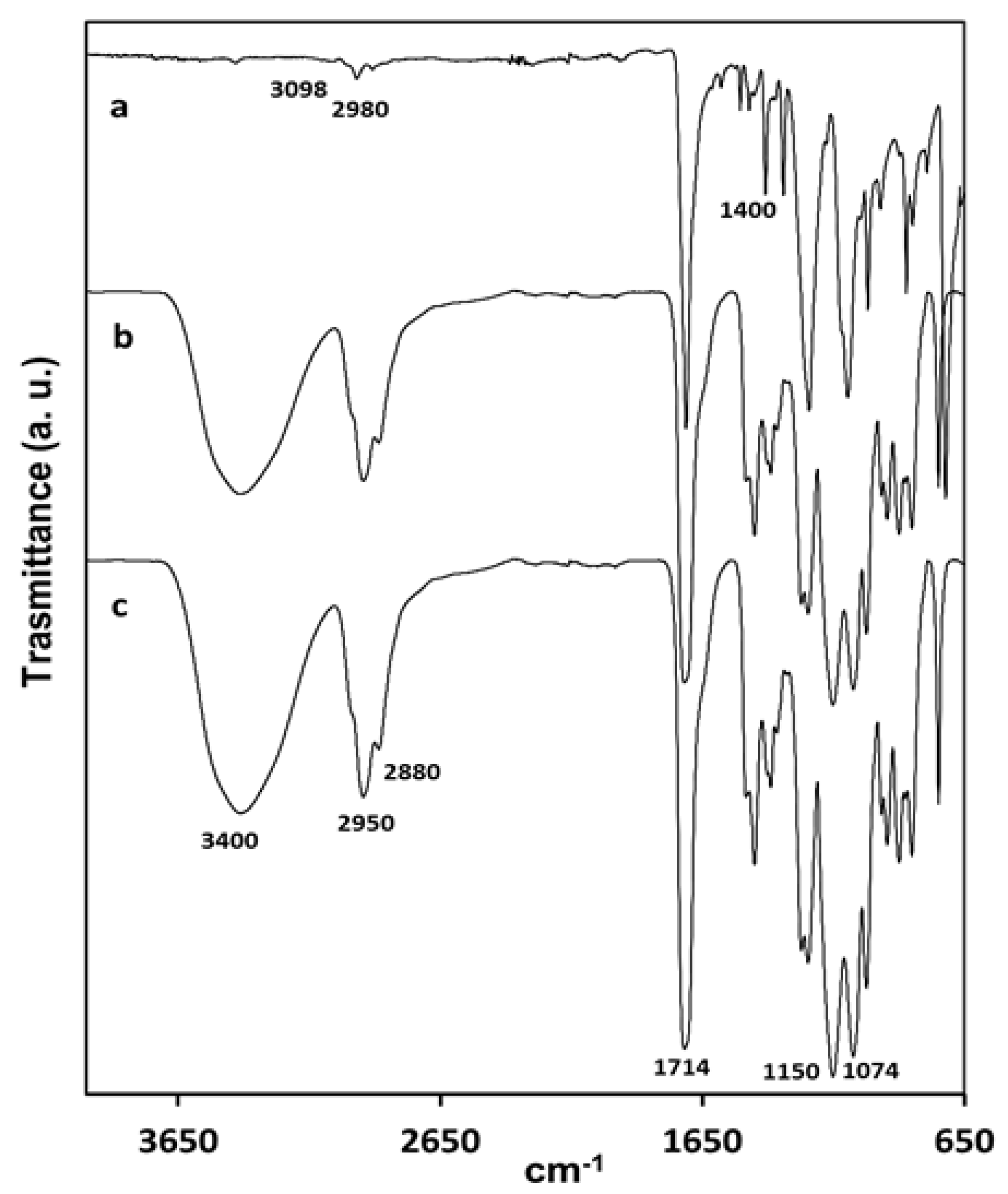
FTIR-ATR
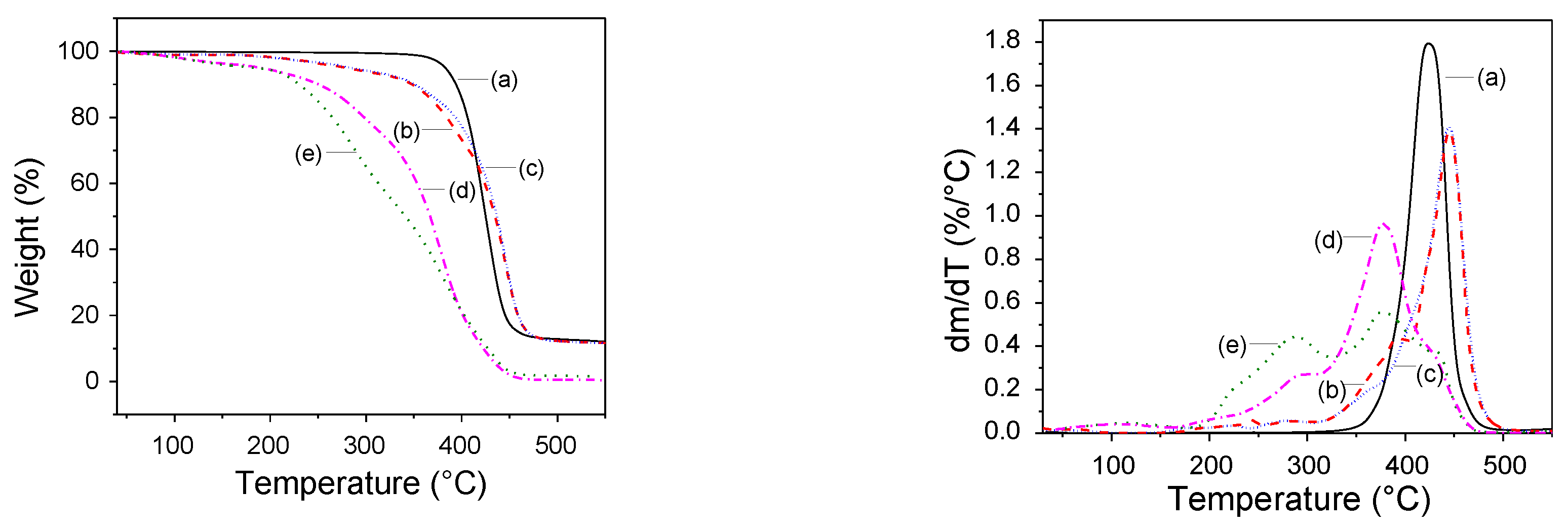
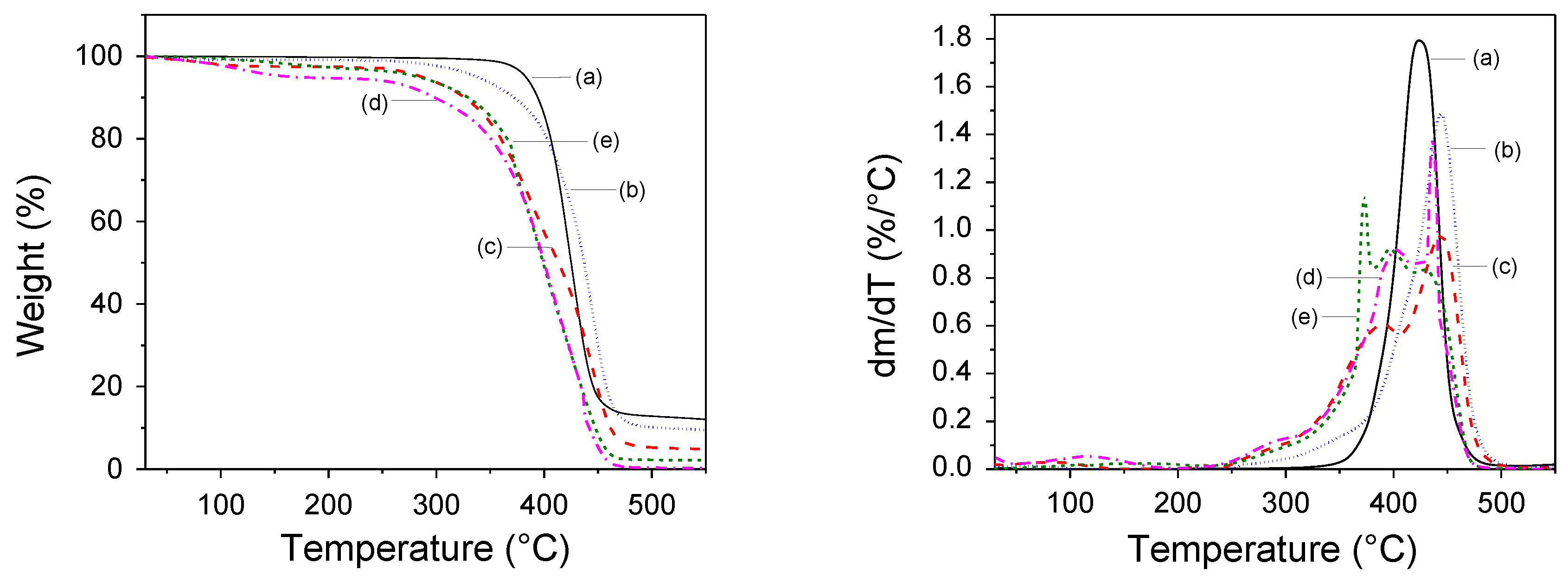
Thermal Stability
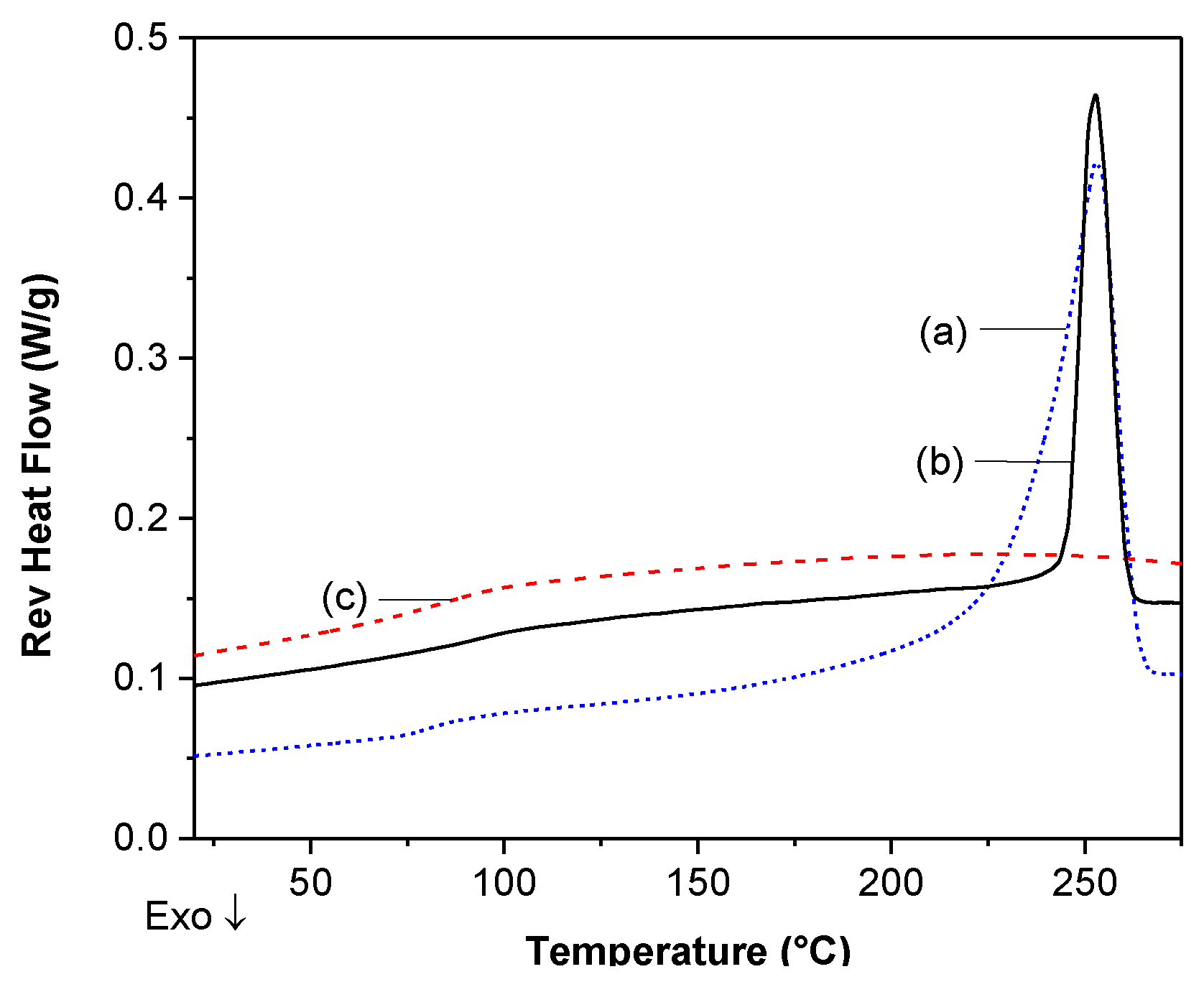
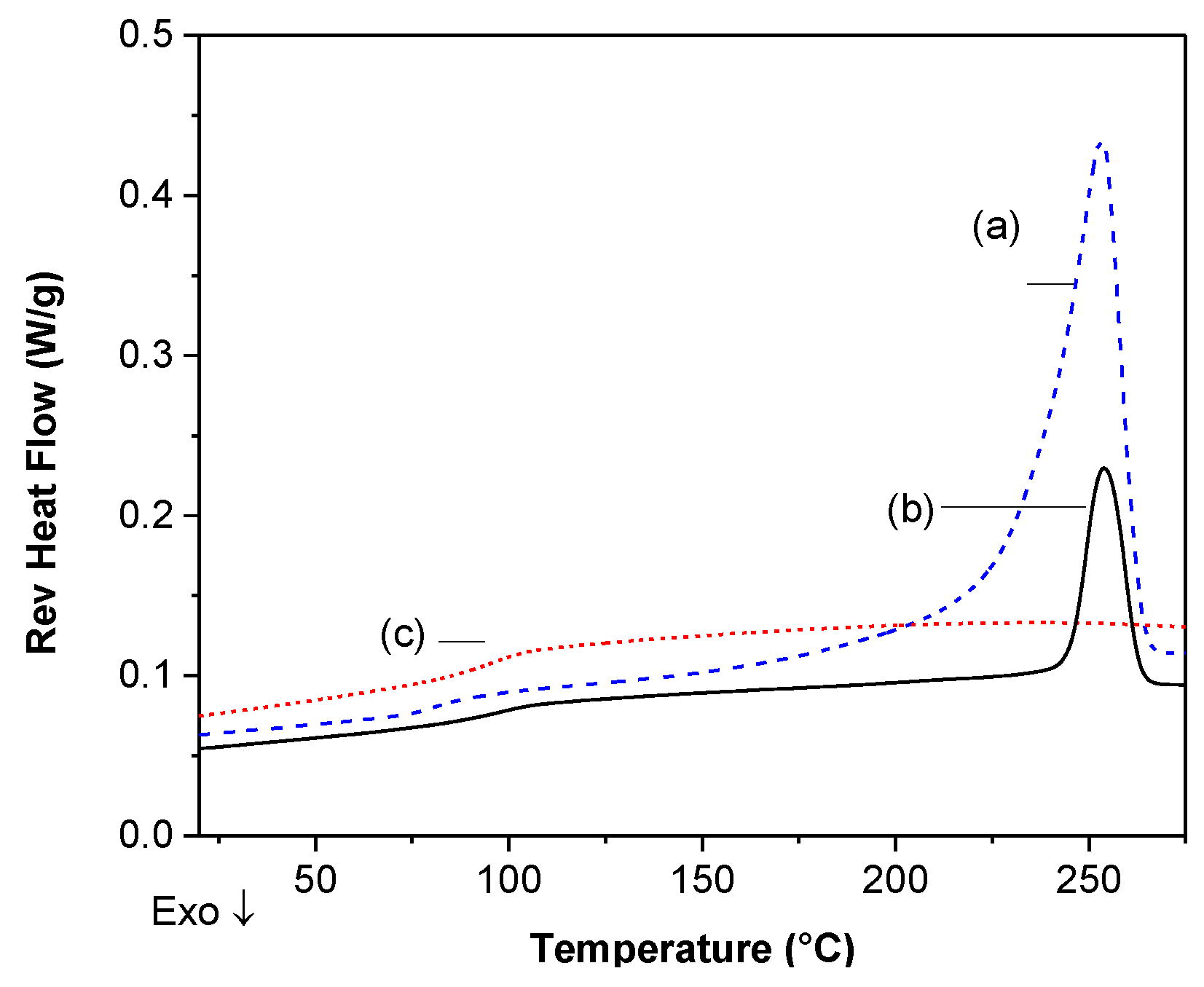
DSC Analysis
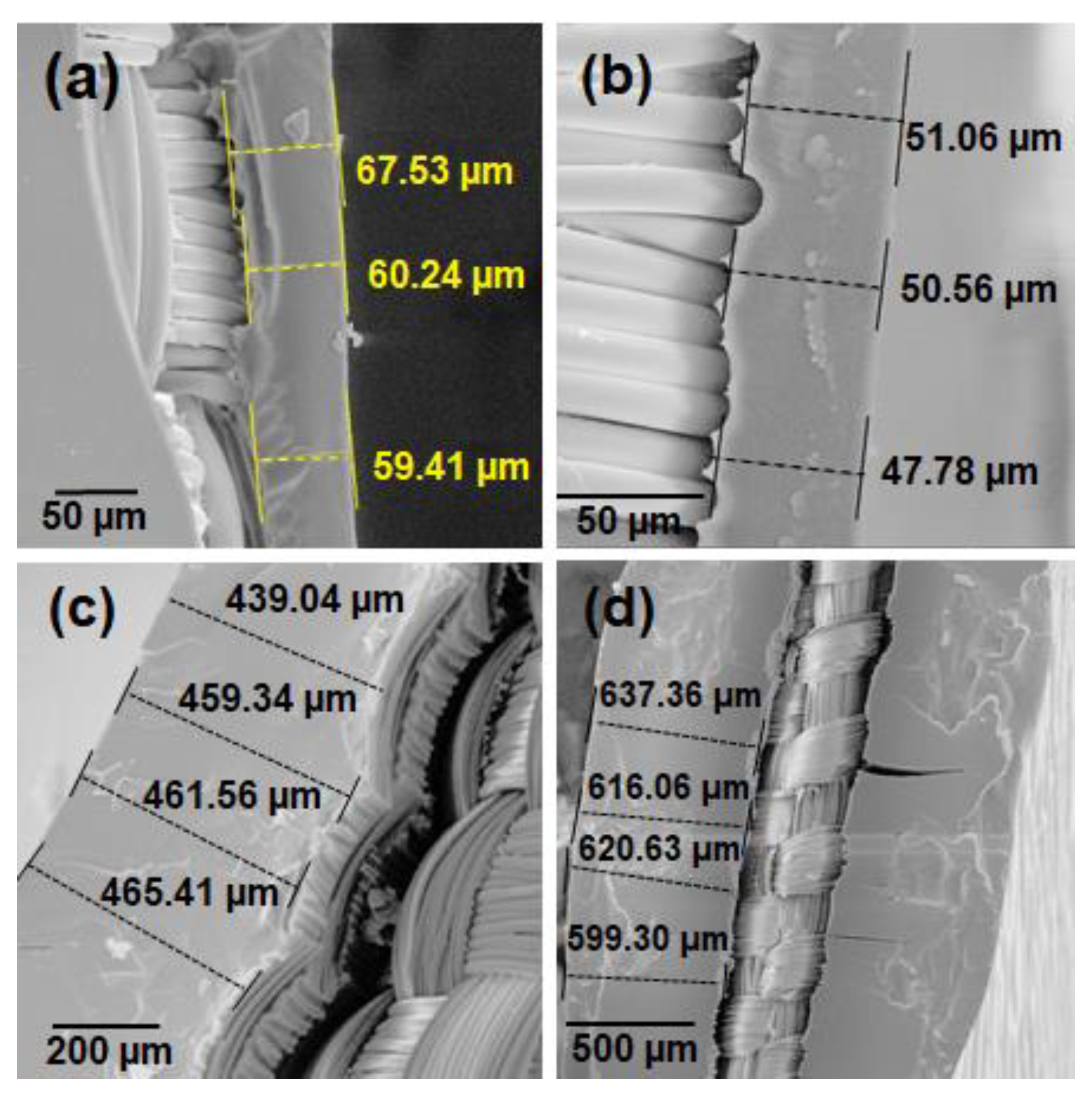
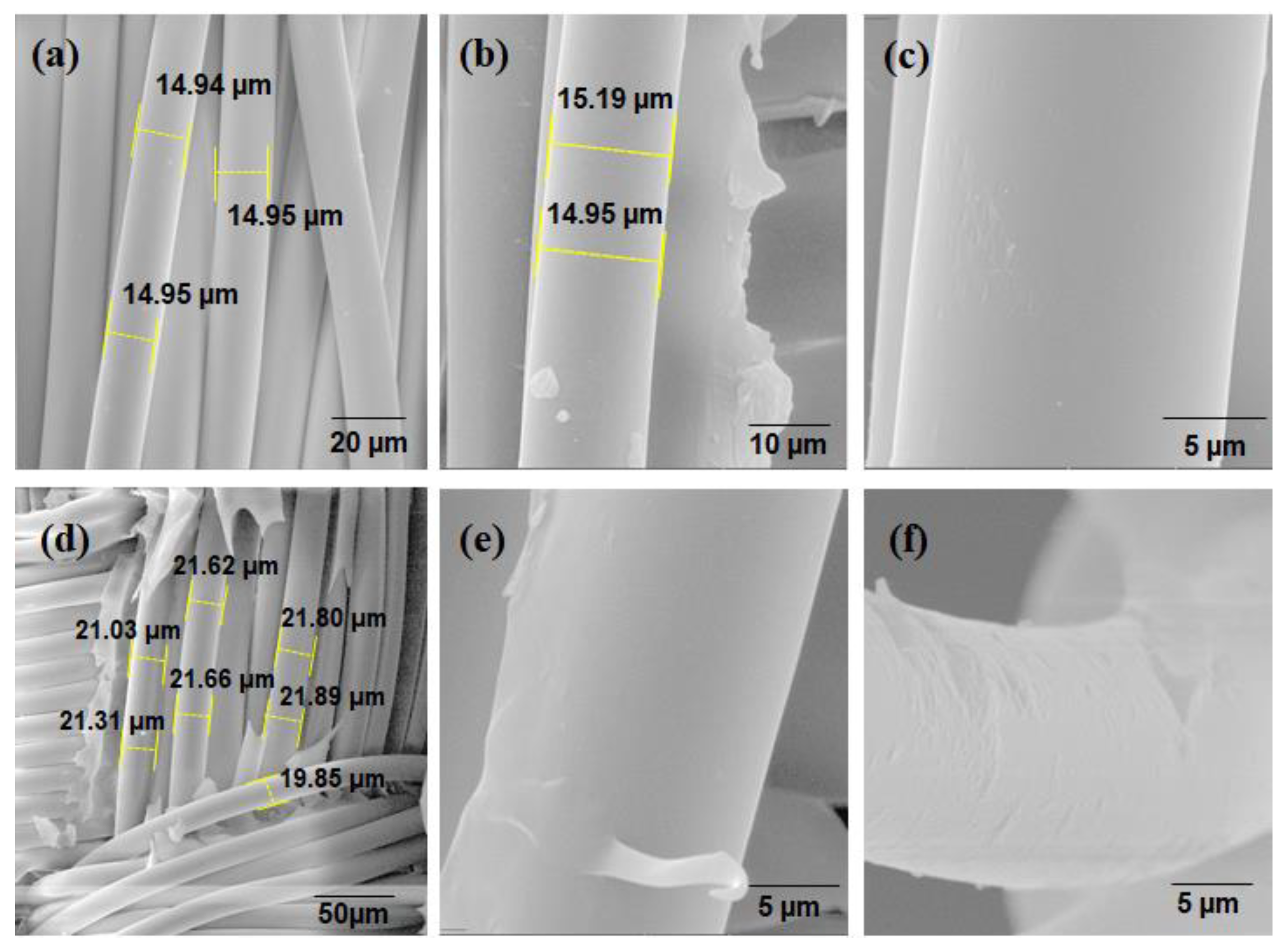
SEM
3.2. Imparting PET-Fabrics Antibacterial Properties
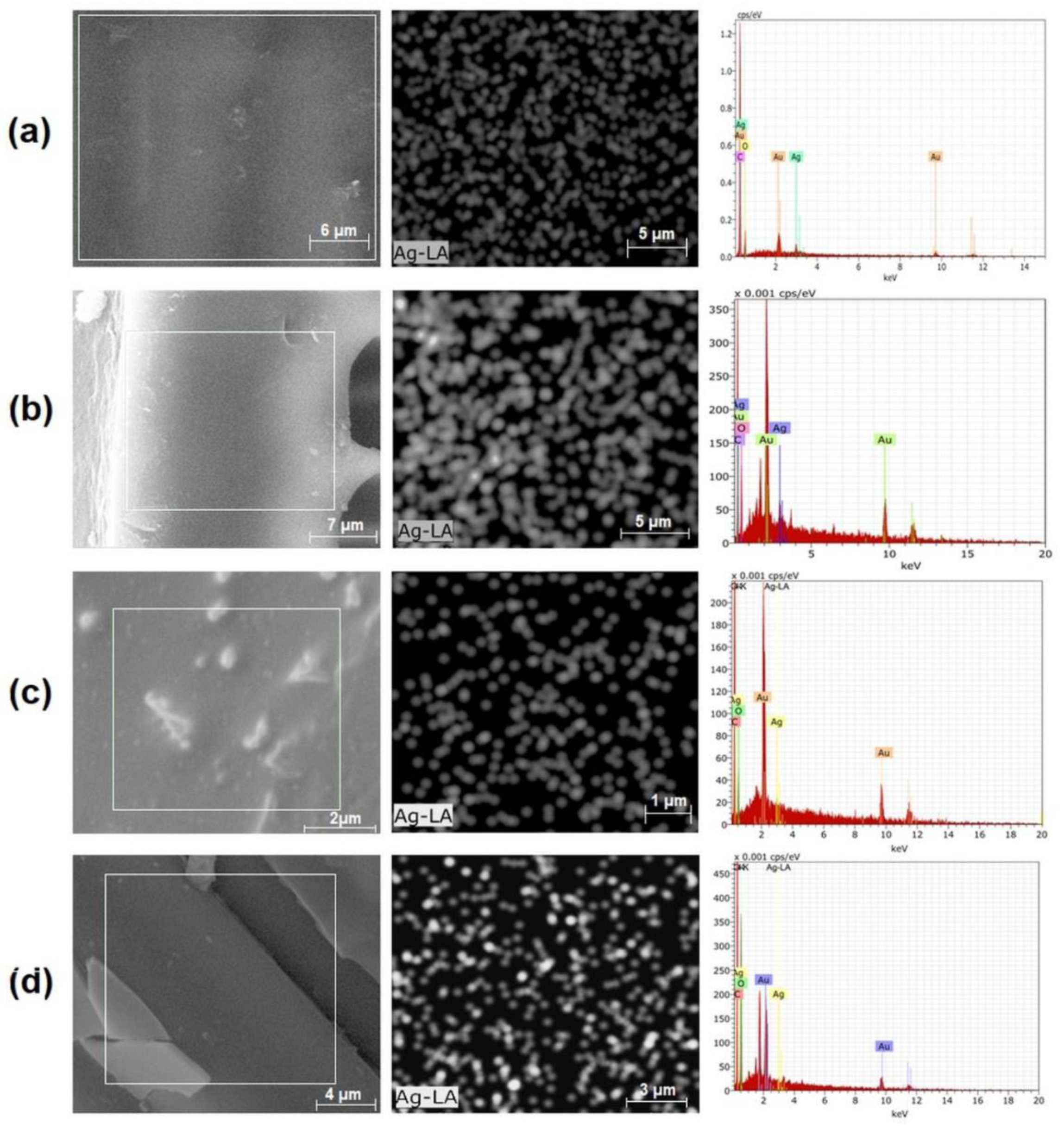
3.2.1. Incorporation of Silver Nanoparticles
3.2.2. Antibacterial Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 768–814. [Google Scholar] [CrossRef]
- Nedĕla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Ann. N.Y. Acad. Sci. 2001, 944, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Gregonis, D.E.; Russell, G.A.; Andrade, J.D.; Visser, A.C. Preparation and properties of stereoregular poly (hydroxyethyl methacrylate polymers and hydrogels. Polymer 1978, 19, 1279–1284. [Google Scholar] [CrossRef]
- Tomic, S.L.; Mićic, M.M.; Dobić, S.N.; Filipović, J.M.; Suljovrujić, E.H. Smart poly (2-hydroxyethyl methacrylate/itaconic acid) hydrogels for biomedical application. Radiat. Phys. Chem. 2010, 79, 643–664. [Google Scholar] [CrossRef]
- Sharrock, P.; Grégoire, G.J. HEMA reactivity with demineralized dentin. J. Dent. 2010, 38, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wu, T.; Xu, Ch.; Langenbach, K.J.; Elliot, J.T.; Vogt, B.D.; Beers, K.L.; Amis, E.J.; Washburn, N.R. Tuning Cell Adhesion on Gradient Poly (2-hydroxyethyl methacrylate)-Grafted Surfaces. Langmuir 2005, 21, 12309–12314. [Google Scholar] [CrossRef]
- Brahim, S.; Narinesingh, D.; Guiseppi-Eli, A. Synthesis and Hydration Properties of pH-Sensitive p(HEMA)-Based Hydrogels Containing 3-(Trimethoxysilyl)propyl Methacrylate. Biomolecules 2003, 4, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Aal, A.S.; Kupchishin, A.I.; Khutoryanskiy, V.V.; Mun, G.A.; Beksyrgaeva, A.G. Radiation grafting from binary monomer mixtures. II. Vinyl ether of monoethanolamine and N-vinylpyrrolidone. Radiat. Phys. Chem. 2003, 68, 793–798. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Bucio, E.; Burillo, G. Radiation-grafting of 4-vinylpyridine and N-isopropylacrylamide onto polypropylene to give novel pH and thermo-sensitive films. Radiat. Phys. Chem. 2009, 78, 1–7. [Google Scholar] [CrossRef]
- Contreras-Garcia, A.; Burillo, G.; Aliev, R.; Bucio, E. Radiation grafting of N, N′-dimethylacrylamide and N-isopropylacrylamide onto polypropylene films by two-step method. Radiat. Phys. Chem. 2008, 77, 936–940. [Google Scholar] [CrossRef]
- Ramírez-Fuentes, Y.S.; Bucio, E.; Burillo, G. Radiation-induced grafting of N-isopropylacrylamide and acrylic acid onto polypropylene films by two step method. Nucl. Instrum. Meth. B 2007, 265, 183–186. [Google Scholar] [CrossRef]
- Álvarez-Lorenzo, C.; Bucio, E.; Burillo, G.; Concheiro, A. Medical devices modified at the surface by γ-ray grafting for drug loading and delivery. Expert Opin. Drug Del. 2010, 7, 173–185. [Google Scholar] [CrossRef]
- Vahdat, A.; Bahramia, H.; Ansaria, N.; Ziaie, F. Radiation grafting of styrene onto polypropylene fibres by a 10 MeV electron beam. Radiat. Phys. Chem. 2007, 76, 787–793. [Google Scholar] [CrossRef]
- Hernández-Martínez, A.R.; Bucio, E. Novel pH and Temperature-Sensitive Behavior of Binary Graft DMAEMA/PEGMEMA onto LDPE Membranes. Des. Monomer Polym. 2009, 12, 543–552. [Google Scholar] [CrossRef]
- Bucio, E.; Contreras-García, A.; Meléndez-Ortiz, H.I.; Muñoz-Muñoz, F.D.; Alvarez-Lorenzo, C.; Concheiro, A. Smart polymers for biomedical applications and graft synthesis by gamma-rays. In Smart Polymeric Materials for Biomedical Applications; NOVA Science Publishers: New York, NY, USA, 2010; pp. 277–306. [Google Scholar]
- Ramírez-Jiménez, A.; Álvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of 2-hydroxyethylmethacrylate and oligo (ethylene glycol) methyl ether methacrylate onto polypropylene films by one step method. Radiat. Phys. Chem. 2012, 81, 27–32. [Google Scholar] [CrossRef]
- Stannet, V.T. Radiation grafting state-of-the-art. Radiat. Phys. Chem. 1990, 35, 82–87. [Google Scholar] [CrossRef]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: New York, NY, USA, 1960. [Google Scholar]
- Li, J.; Lin, F.; Li, L.; Li, J.; Liu, S. Surface Engineering of Poly (ethylene terephthalate) for Durable Hemocompatibility via a Surface Interpenetrating Network Technique. Macromol. Chem. Phys. 2012, 213, 2120–2129. [Google Scholar] [CrossRef]
- Liu, Y.X.; He, T.; Gao, C.Y. Surface modification of poly (ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf. B 2005, 46, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, N.; Rudenja, S. Surface Interpenetrating Networks of Poly (ethylene terephthalate) and Polyamides for Effective Biocidal Properties. Macromol. Chem. Phys. 2010, 211, 286–296. [Google Scholar] [CrossRef]
- Ping, X.; Wang, M.; Ge, X. Surface modification of poly (ethylene terephthalate) (PET) film by gamma-ray induced grafting of poly (acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572. [Google Scholar] [CrossRef]
- Aubert-Viard, F.; Martin, A.; Chai, F.; Neut, C.; Tabary, N.; Martel, B.; Blanchemain, N. Chitosan finishing nonwoven textiles loaded with silver and iodide for antibacterial wound dressing applications. Biomed. Mater. 2015, 10, 015023. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Z.; Qi, J.C.; Wu, J.H.; Tiang, T.; Hou, L.L.; Hao, L.M.; Yang, J.Q. One-pot fabrication and antimicrobial properties of novel PET nonwoven fabrics. Biomed. Mater. 2011, 6, 045009. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Mozetič, M.; Stana-Kleinschek, K.; Fröhlich, M.; Turk, B.; Vesel, A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials 2015, 8, 1526–1544. [Google Scholar] [CrossRef]
- Vesel, A.; Kovac, J.; Primc, G.; Junkar, I.; Mozetic, M. Effect of H2S Plasma Treatment on the Surface Modification of a Polyethylene Terephthalate Surface. Materials 2016, 9, 95. [Google Scholar] [CrossRef]
- Radetić, M.J. Functionalization of textile materials with silver nanoparticles. Mater. Sci. 2013, 48, 95–107. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Prasad, S.; Gambhir, I.S. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig. J. Nanomater. Biostruct. 2008, 3, 115–122. [Google Scholar]
- Chen, Y.H.; Su, C.H.; He, J.L. Antibacterial silver coating on poly (ethylene terephthalate) fabric by using high power impulse magnetron sputtering. Surf. Coat. Tech. 2013, 232, 868–875. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.H. Bacteriostasis of Nanosized Colloidal Silver on Polyester Nonwovens. Text. Res. J. 2004, 74, 442–447. [Google Scholar]
- Deng, X.; Nikiforov, A.Y.; Coenye, T.; Cools, P.; Aziz, G.; Morent, R.; Geyter, N.; Leys, C. Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci. Rep. 2015, 5, 10138. [Google Scholar] [CrossRef]
- Slepicka, P.; Slepickova-Kasalkova, N.; Siegel, J.; Kolska, Z.; Bacakova, L.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotech. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Molina, R.; Dimitrijević, S.; Jovančić, P.; Nedeljković, J.; Radetić, M. Antifungal efficiency of corona pretreated polyester and polyamide fabrics loaded with Ag nanoparticles. J. Mat. Sci. 2009, 44, 3983. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Guibert, G.; Mikhailov, S.; Gedanken, A. Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 2008, 19, 245705. [Google Scholar] [CrossRef] [PubMed]
- Milosević, M.; Radoicić, M.; Šaponjić, Z.; Nunney, T.; Marković, D.; Nedeljković, V.; Radetić, M. In situ generation of Ag nanoparticles on polyester fabrics by photoreduction using TiO2 nanoparticles. J. Mater. Sci. 2013, 48, 5447–5455. [Google Scholar] [CrossRef]
- D’Agostino, A.; Taglietti, A.; Grisoli, P.; Dacarro, G.; Cucca, L.; Patrini, M.; Pallavicini, P. Seed mediated growth of silver nanoplates on glass: Exploiting the bimodal antibacterial effect by near IR photo-thermal action and Ag+ release. RSC Adv. 2016, 6, 70414–70423. [Google Scholar] [CrossRef]
- Collins, C.H.; Lynes, P.M.; Granje, J.M. Antimicrobial sensibility and assay test. In Microbial Methods; Collin, C.H., Lynes, P.M., Eds.; Butterworth: London, UK, 1989; pp. 155–168. [Google Scholar]
- Contreras-Garcia, A.; Ramírez-Jiménez, A.; Bucio, E. Grafting polymerization induced by gamma-Rays. In Gamma Rays Technology, Applications and Health Implications; NOVA Science Publishers: New York, NY, USA, 2013; pp. 287–320. [Google Scholar]
- Kaczmarek, H.; Galka, P. Effect of Irgacure 6551 initiator on poly (methyl methacrylate) photostability studied by UV-vis spectroscopy. Open Proc. Chem. J. 2008, 1, 8–11. [Google Scholar] [CrossRef]
- Campbell, D.; Araki, K.; Turner, D.T. ESR study of free radicals formed by γ-irradiation of poly (ethylene terephthalate). J. Polym. Sci. Part A Polym. Chem. 1966, 4, 2597–2606. [Google Scholar] [CrossRef]
- Demirelli, k.; Coşkun, M.; Kaya, E. A detailed study of thermal degradation of poly (2-hydroxyethyl methacrylate). Polym. Degrad. Stabil. 2001, 72, 75–80. [Google Scholar] [CrossRef]
- Yuranova, T.; Rincon, A.G.; Bozzi, A.; Parra, S.; Pulgarin, C.; Albers, P.; Kiwi, J.J. Antibacterial textiles prepared by RF-plasma and vacuum-UV mediated deposition of silver. J. Photochem. Photobiol. A 2003, 161, 27–34. [Google Scholar] [CrossRef]
- Solomon, S.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, J.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Q.; Ye, L.H.; Huang, Y.C.; Yang, D.K.; Li, L.; Xu, G. In vitro model of bacterial biofilm formation on polyvinyl chloride biomaterial. Cell Biochem. Biophys. 2011, 61, 371. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
| Sample | EGDMA (% mol) 1 | PEGMA (% mol) 1 | Hydrogel (wt/wt %) 2 | Qwater 3 |
|---|---|---|---|---|
| UV_PET100_0_0.5 | 0.50 | – | 96 | 1.7 |
| UV_PET97_3_0.5 | 0.50 | 3 | 49 | 2.0 |
| UV_PET95_5_0.5 | 0.50 | 5 | 67 | 2.1 |
| UV_PET93_7_0.5 | 0.50 | 7 | 64 | 2.1 |
| UV_PET95_5_0.25 | 0.25 | 5 | 96 | 2.3 |
| UV_PET95_5_1 | 1.00 | 5 | 85 | 2.0 |
| Sample | Dose (kGy) | PEGMA (% Vol.) | Solvent (% Vol.) | Hydrogel (wt/wt%) 1 | Qwater 2 |
|---|---|---|---|---|---|
| γ60_PET100_0_50 | 60 | – | 50 | 257 | 2.5 |
| γ60_PET90_10_50 | 60 | 10 | 50 | 400 | 1.8 |
| γ60_PET90_10_40 | 60 | 10 | 40 | 400 | 1.8 |
| γ60_PET90_10_30 | 60 | 10 | 30 | 400 | 1.8 |
| γ60_PET80_20_50 | 60 | 20 | 50 | 213 | 1.9 |
| γ60_PET70_30_50 | 60 | 30 | 50 | 285 | 2.9 |
| γ50_PET90_10_50 | 50 | 10 | 50 | 285 | 1.8 |
| γ70_PET90_10_50 | 70 | 10 | 50 | 285 | 1.8 |
| Sample | HEMA:PEGMA (% mol) | EGDMA (% mol) | Hydrogel (wt/wt %) | Tg (°C) | Tm (°C) |
|---|---|---|---|---|---|
| Unmodified PET fabric | - | - | - | 80 | 253 |
| UV_PET100_0_0.5 | 100:0 | 0.5 | 96 | 79 | 254 |
| UV_PET97_3_0.5 | 97:3 | 0.5 | 49 | 108 | 255 |
| UV_PET95_5_0.5 | 95:5 | 0.5 | 67 | 98 | 253 |
| UV_PET93_7_0.5 | 93:7 | 0.5 | 64 | 58 | 255 |
| UV_PET95_5_0.25 | 95:5 | 0.25 | 96 | 91 | 253 |
| UV_PET95_5_1 | 95:5 | 1 | 85 | 110 | 254 |
| Sample | Dose (kGy) | H:P (v/v %) | Hydrogel (wt/wt%) | Tg (°C) | Tm (°C) |
|---|---|---|---|---|---|
| Unmodified PET fabric | - | - | - | 80 | 253 |
| γ60_PET100_0_50 | 60 | 100:0 | 257 | 95 | 255 |
| γ60_PET70_30_50 | 60 | 70:30 | 400 | 54 | 255 |
| γ60_PET80_20_50 | 60 | 80:20 | 400 | 76 | 255 |
| γ60_PET90_10_50 | 60 | 90:10 | 400 | 91 | 255 |
| γ60_PET90_10_40 | 60 | 90:10 | 213 | 96 | 254 |
| γ60_PET90_10_30 | 60 | 90:10 | 285 | 98 | 254 |
| γ50_PET90_10_50 | 50 | 90:10 | 285 | 88 | 255 |
| γ70_PET90_10_50 | 70 | 90:10 | 285 | 93 | 255 |
| Sample | Inhibition Distance (mm) | Inhibition Distance with Ag NP’s (mm) | Hydrogel (%) | ||
|---|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | ||
| UV_PET100:0:0.5 | 0 | 0 | 0.83 ± 0.3 | 1.00 ± 0.0 | 96 |
| UV_PET97:3:0.5 | 0 | 0 | 0.67 ± 0.6 | 1.33 ± 0.6 | 49 |
| UV_PET95:5:0.5 | 0 | 0 | 0.67 ± 0.6 | 0.67 ± 0.6 | 67 |
| UV_PET93:7:0.5 | 0 | 0 | 0.83 ± 0.3 | 1.83 ± 0.3 | 64 |
| UV_PET95:5:0.25 | 0 | 0 | 1.00 ± 0.5 | 1.33 ± 0.6 | 96 |
| γ60_PET100:0:50 | 0 | 0 | 0 | 2.0 ± 0.0 | 257 |
| γ60_PET90:10:50 | 0 | 0 | 0.83 ± 0.6 | 2.33 ± 0.6 | 400 |
| γ60_PET80:20:50 | 0 | 0 | 0 | 3.67 ± 0.8 | 213 |
| γ60_PET70:30:50 | 0 | 0 | 1.33 ± 1.3 | 3.33 ± 0.6 | 285 |
| γ70_PET90:10:50 | 0 | 0 | 0.67 ± 0.5 | 2.17 ± 0.8 | 285 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Villegas, K.A.; Ramírez-Jiménez, A.; Licea-Claverie, Á.; Pérez-Sicairos, S.; Bucio, E.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials 2019, 12, 3284. https://doi.org/10.3390/ma12203284
Montoya-Villegas KA, Ramírez-Jiménez A, Licea-Claverie Á, Pérez-Sicairos S, Bucio E, Bernáldez-Sarabia J, Licea-Navarro AF. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials. 2019; 12(20):3284. https://doi.org/10.3390/ma12203284
Chicago/Turabian StyleMontoya-Villegas, Kathleen A., Alejandro Ramírez-Jiménez, Ángel Licea-Claverie, Sergio Pérez-Sicairos, Emilio Bucio, Johanna Bernáldez-Sarabia, and Alexei F. Licea-Navarro. 2019. "Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods" Materials 12, no. 20: 3284. https://doi.org/10.3390/ma12203284
APA StyleMontoya-Villegas, K. A., Ramírez-Jiménez, A., Licea-Claverie, Á., Pérez-Sicairos, S., Bucio, E., Bernáldez-Sarabia, J., & Licea-Navarro, A. F. (2019). Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials, 12(20), 3284. https://doi.org/10.3390/ma12203284







