Differential Bacterial Colonization and Biofilm Formation on Punctal Occluders
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Punctal Occluders
2.3. Occluder Exposure to Bacteria
2.4. Bacteria Biofilm Quantitation
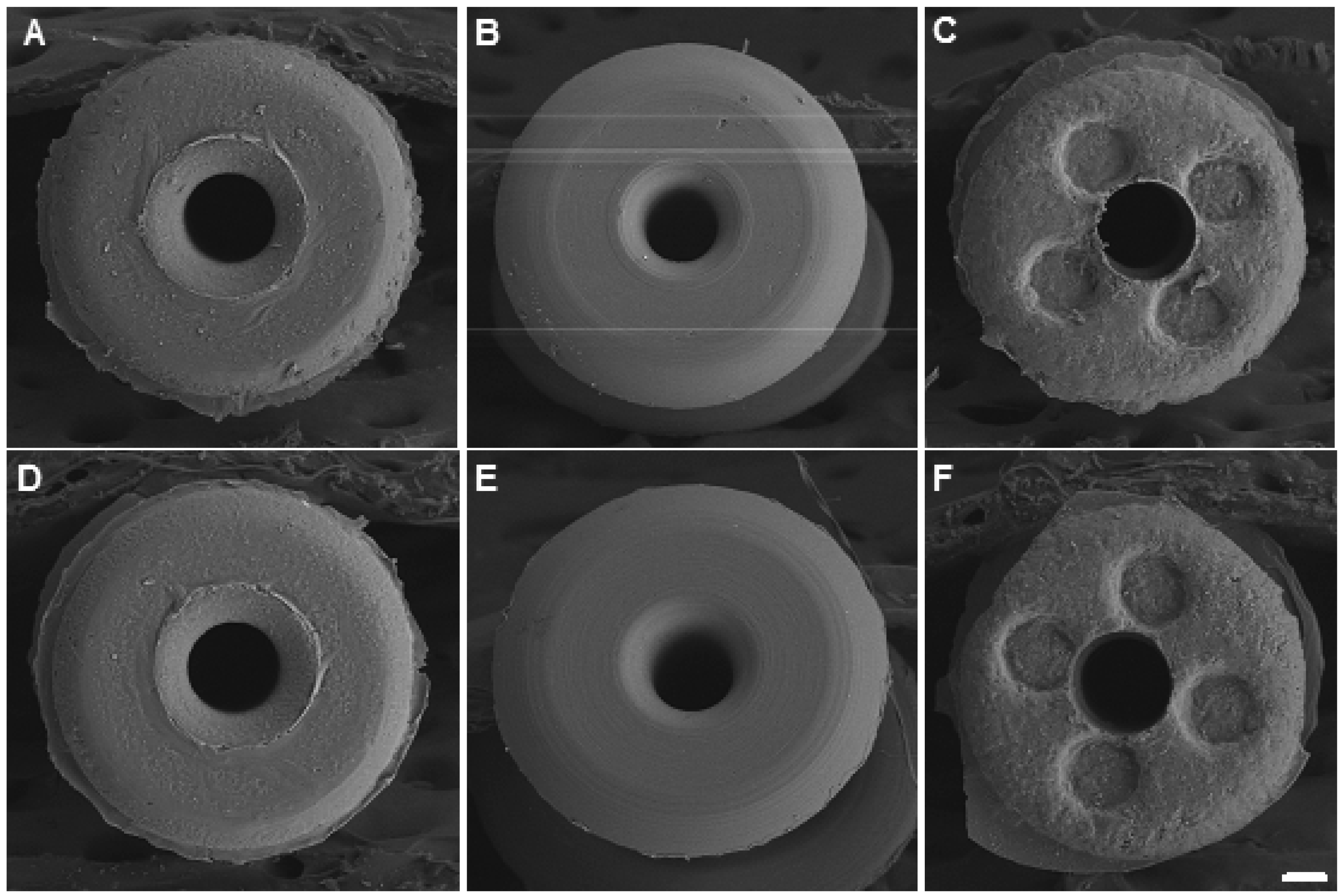
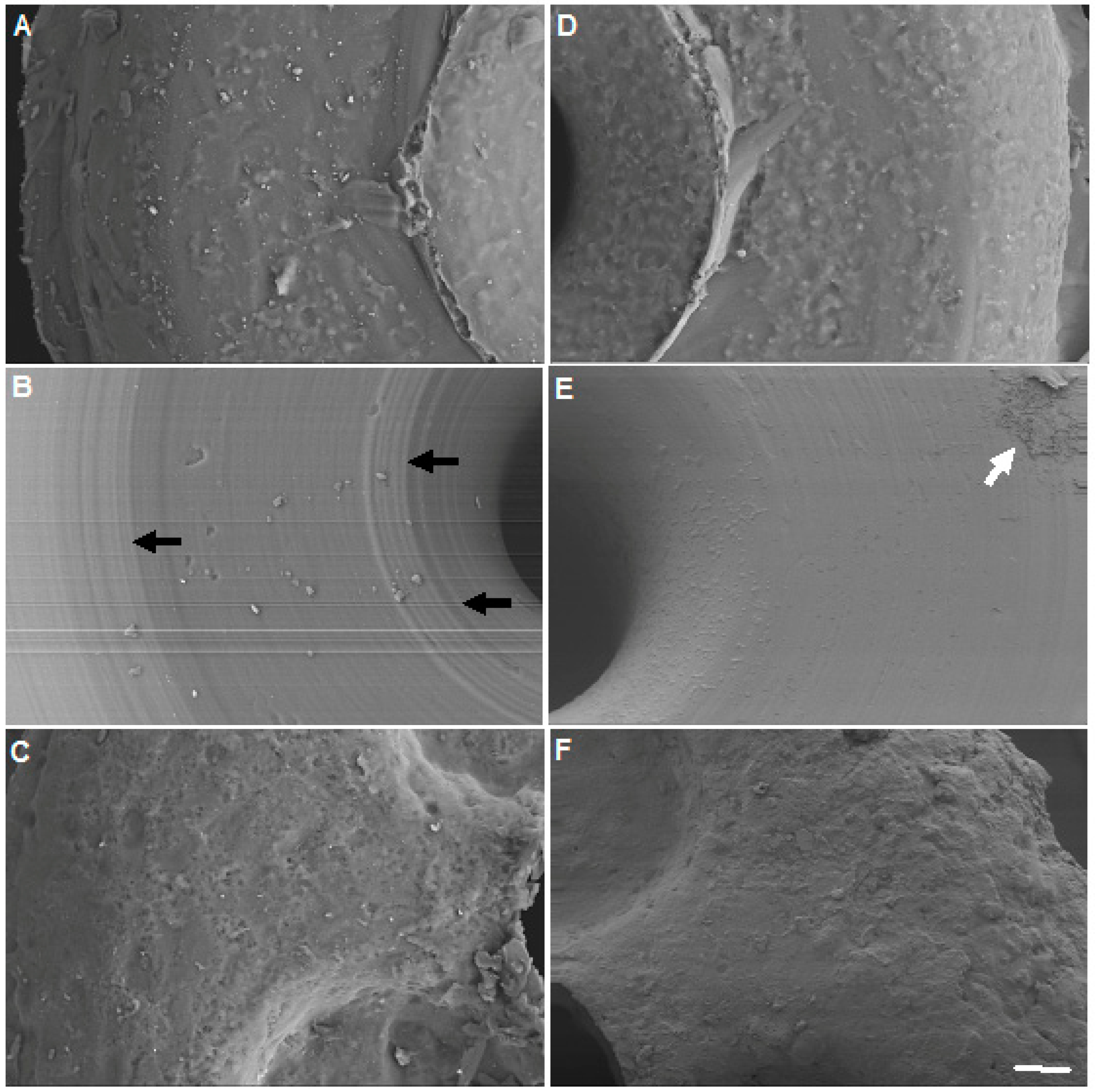
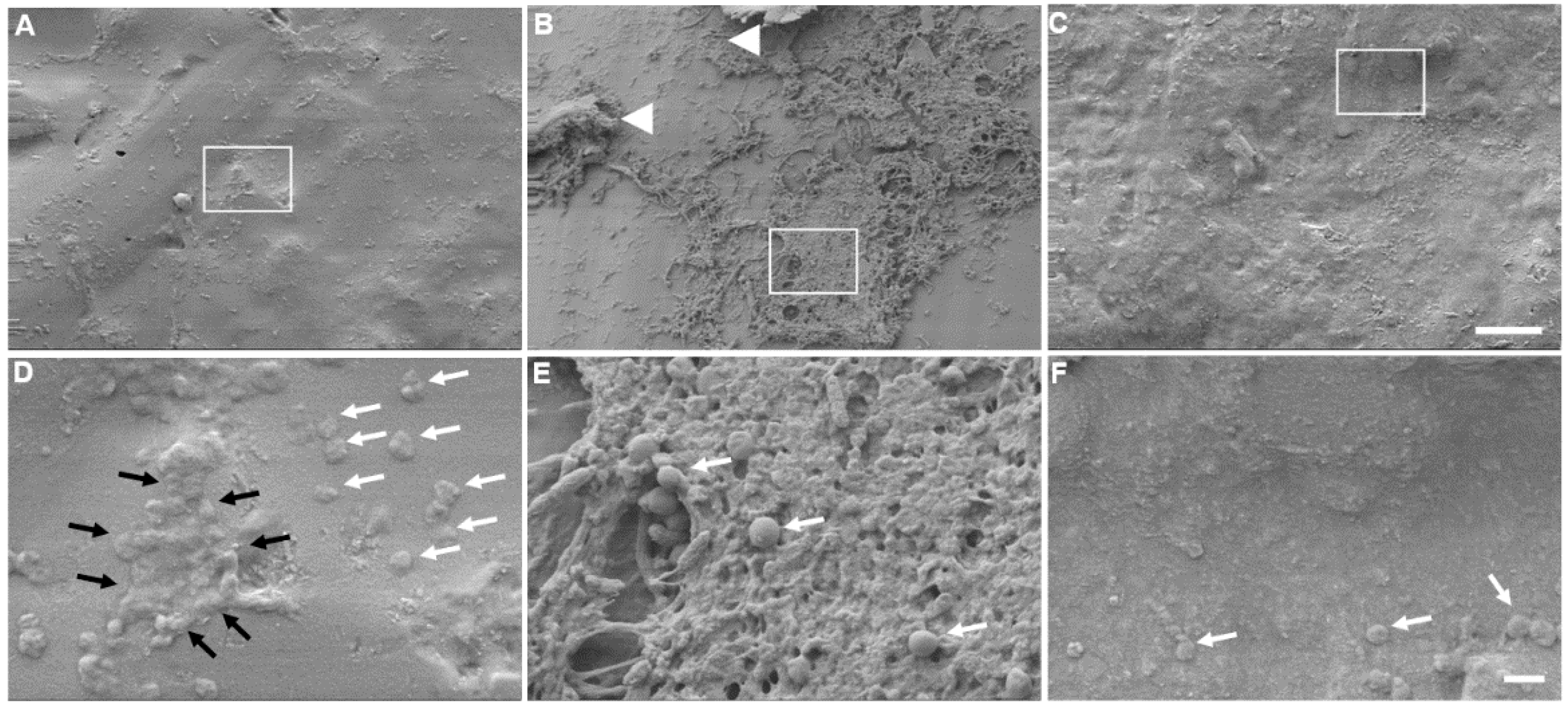
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results
3.1. Variation of Bacterial Growth on Different Occluders
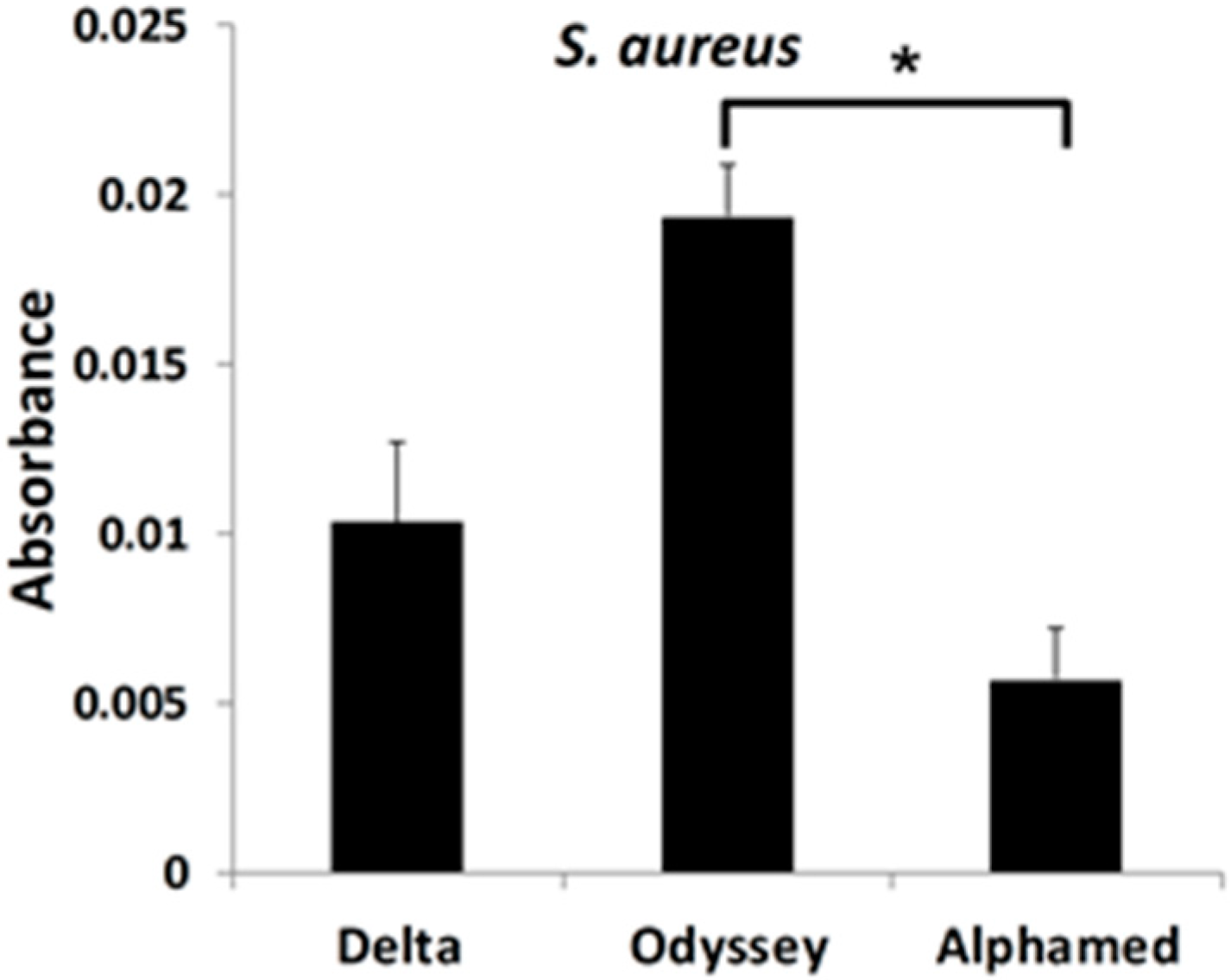
3.2. S. aureus Preferentially Forms a Biofilm on OdysseyR Occluder
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; et al. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Rynerson, J.M.; Perry, H.D. DEBS—A unification theory for dry eye and blepharitis. Clin. Ophthalmol. 2016, 10, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.D.; Collum, L.M.T. Dry eye: Diagnosis and current treatment strategies. Curr. Allergy Asthma Rep. 2004, 4, 314–319. [Google Scholar]
- Tost, F.; Geerling, G. Plugs for occlusion of the lacrimal drainage system. In Surgery for the Dry Eye; Geerling, G., Brewitt, H., Eds.; Karger Publishers: Basel, Switzerland, 2008; Volume 41, pp. 193–212. [Google Scholar]
- Jehangir, N.; Bever, G.; JafarMahmood, S.M.; Moshirfar, M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J. Ophthalmol. 2016, 2016, 9312340. [Google Scholar] [CrossRef] [PubMed]
- Horwath-Winter, J.; Thaci, A.; Gruber, A.; Boldin, I. Long-term retention rates and complications of silicone punctal plugs in dry eye. Am. J. Ophthalmol. 2007, 144, 441–444. [Google Scholar] [CrossRef]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Bowden, M.G.; Brown, E.L.; Laabei, M.; Massey, R.C. Staphylococcus aureus extracellular adherence protein triggers TNFα release, promoting attachment to endothelial cells via protein A. PLoS ONE 2012, 7, e43046. [Google Scholar] [CrossRef]
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Front. Microbiol. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Sugita, J.; Yokoi, N.; Fullwood, N.J.; Quantock, A.J.; Takada, Y.; Nakamura, Y.; Kinoshita, S. The detection of bacteria and bacterial biofilms in punctal plug holes. Cornea 2001, 20, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, B.; Abdi, P.; Akbari, M.; Fard, M.A. Microbiologic spectrum of acute and chronic dacryocystitis. Int. J. Ophthalmol. 2014, 7, 864–867. [Google Scholar] [PubMed]
- Laibson, P.R.; Donnenfeld, E.D. Corneal ulcers related to contact lens use. Int. Ophthalmol. Clin. 1986, 26, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar]
- Yokoi, N.; Okada, K.; Sugita, J.; Kinoshita, S. Acute conjunctivitis associated with biofilm formation on a punctal plug. Jpn. J. Ophthalmol. 2000, 44, 559–560. [Google Scholar] [CrossRef]
- Mayo, M.S.; Cook, W.L.; Schlitzer, R.L.; Ward, M.A.; Wilson, L.A.; Ahearn, D.G. Antibiograms, serotypes, and plasmid profiles of Pseudomonas aeruginosa associated with corneal ulcers and contact lens wear. J. Clin. Microbiol. 1986, 24, 372–376. [Google Scholar] [PubMed]
- Miller, M.J.; Ahearn, D.G. Adherence of Pseudomonas aeruginosa to hydrophilic contact lenses and other substrata. J. Clin. Microbiol. 1987, 25, 1392–1397. [Google Scholar] [CrossRef]
- Glastonbury, J.; Crompton, J.L. Pseudomonas aeruginosa corneal infection associated with disposable contact lens use. Aust. N. Z. J. Ophthalmol. 1989, 17, 451. [Google Scholar] [CrossRef]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of substratum topography on bacterial adhesion. J. Colloid Interface Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef]
- Bos, R.; van der Mei, H.C.; Gold, J.; Busscher, H.J. Retention of bacteria on a substratum surface with micropatterned hydrophobicity. FEMS Microbiol. Lett. 2000, 189, 311–315. [Google Scholar] [CrossRef]
- Medilanski, E.; Kaufmann, K.; Wick, L.; Wanner, O.; Harms, H. Influence of surface topography of stainless steel on bacterial adhesion. Biofoul 2002, 18, 193–203. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjiargyrou, M.; Donnenfeld, E.D.; Grillo, L.M.; Perry, H.D. Differential Bacterial Colonization and Biofilm Formation on Punctal Occluders. Materials 2019, 12, 274. https://doi.org/10.3390/ma12020274
Hadjiargyrou M, Donnenfeld ED, Grillo LM, Perry HD. Differential Bacterial Colonization and Biofilm Formation on Punctal Occluders. Materials. 2019; 12(2):274. https://doi.org/10.3390/ma12020274
Chicago/Turabian StyleHadjiargyrou, Michael, Eric D. Donnenfeld, Lola M. Grillo, and Henry D. Perry. 2019. "Differential Bacterial Colonization and Biofilm Formation on Punctal Occluders" Materials 12, no. 2: 274. https://doi.org/10.3390/ma12020274
APA StyleHadjiargyrou, M., Donnenfeld, E. D., Grillo, L. M., & Perry, H. D. (2019). Differential Bacterial Colonization and Biofilm Formation on Punctal Occluders. Materials, 12(2), 274. https://doi.org/10.3390/ma12020274




