Investigation of Growth Mechanism of Plasma Electrolytic Oxidation Coating on Al-Ti Double-Layer Composite Plate
Abstract
1. Introduction
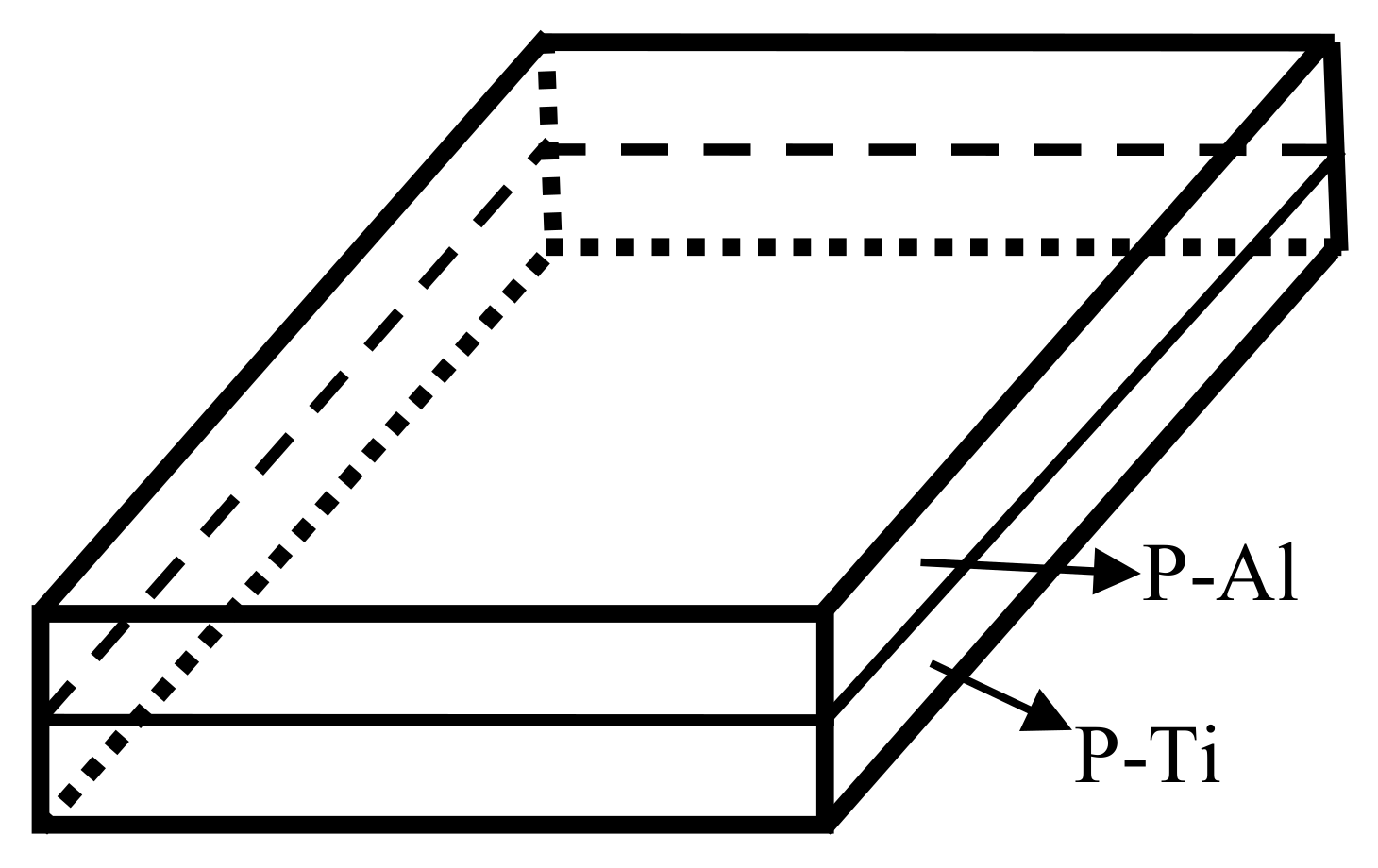
2. Materials and Methods
3. Results and Discussion
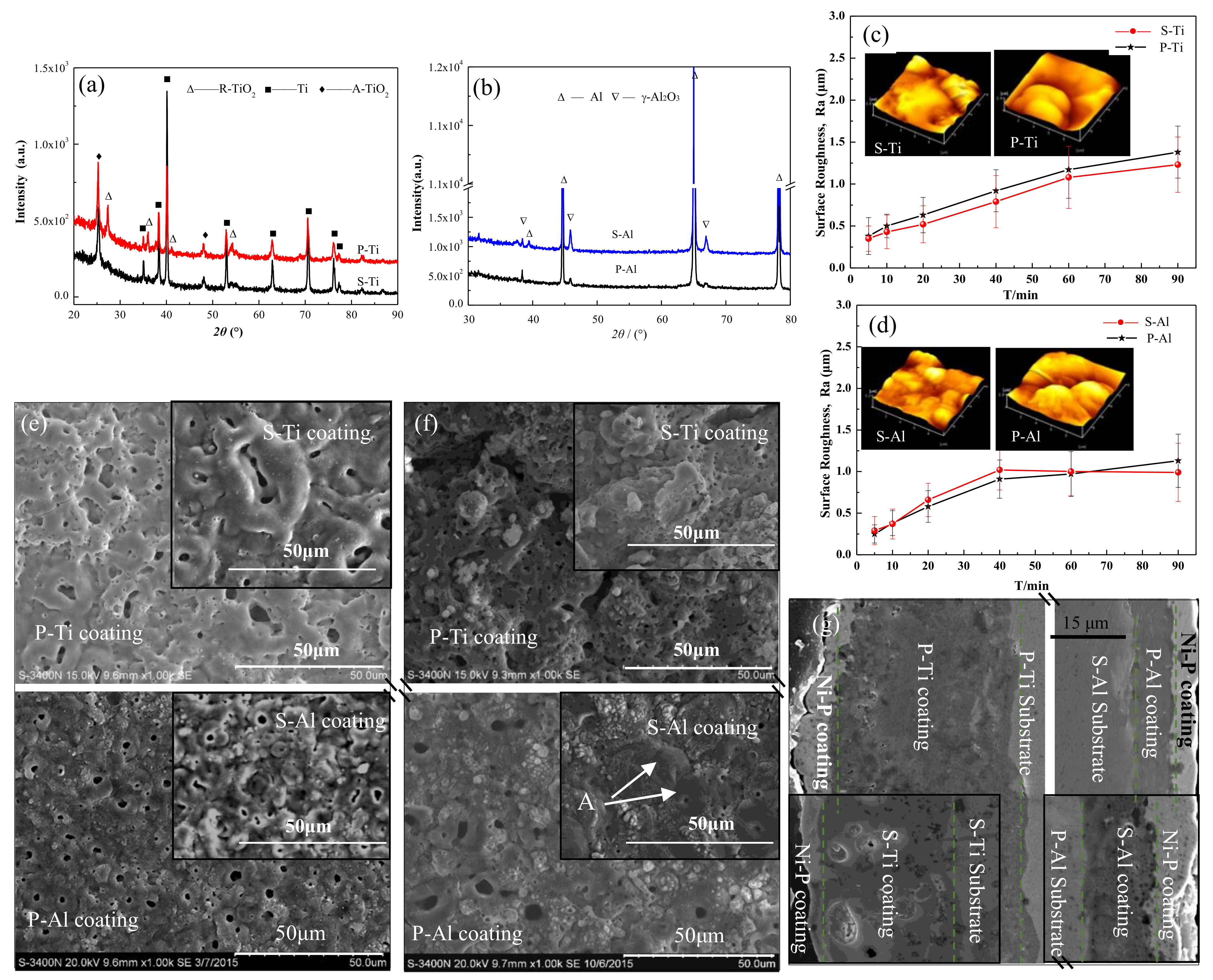
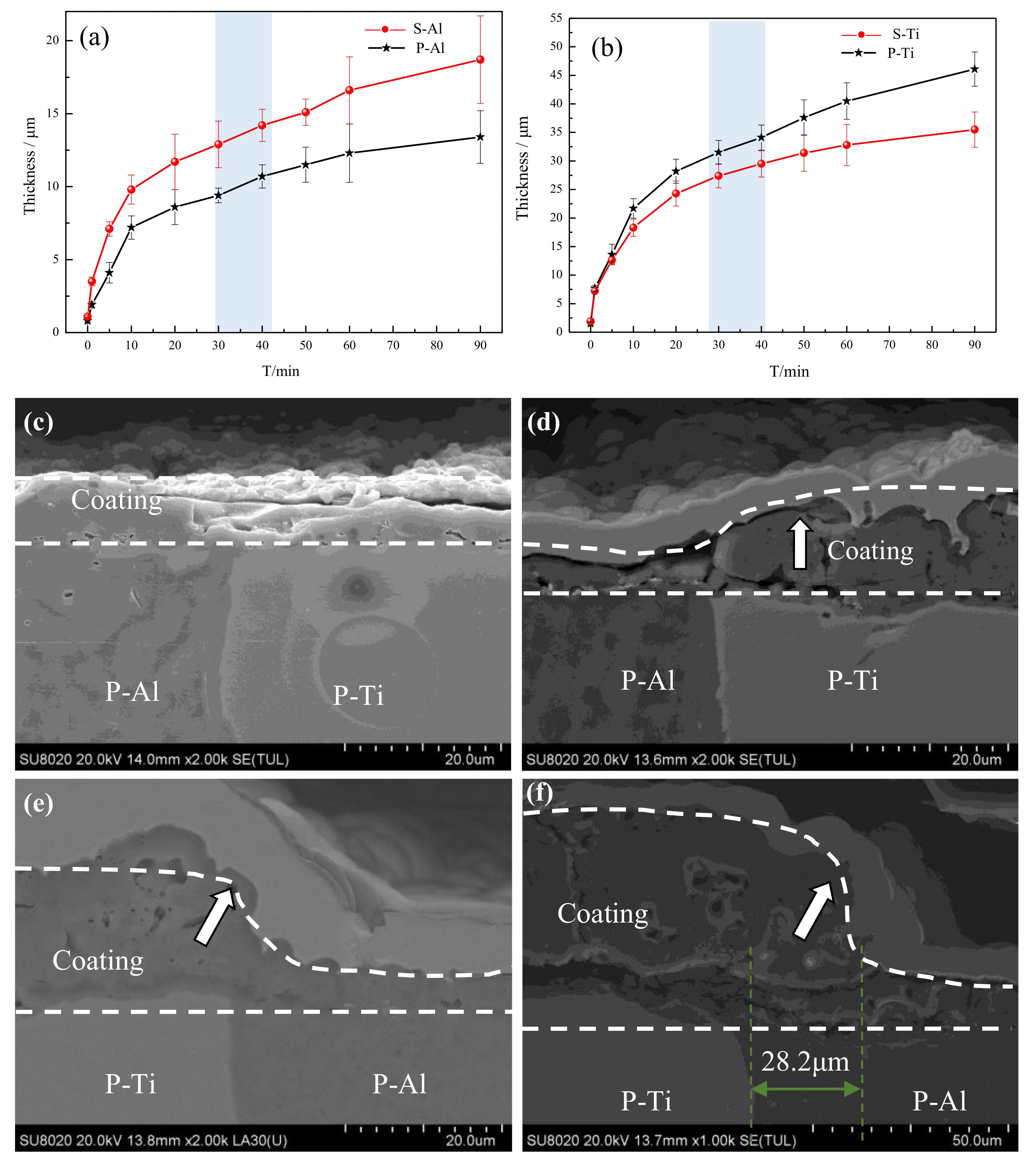
3.1. Phase Structure and Morphology Analysis
3.2. Electrochemical Performance Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- E, J.C.; Huang, J.Y.; Bie, B.X.; Sun, T.; Fezzaa, K.; Xiao, X.H.; Sun, W.; Luo, S.N. Deformation and fracture of explosion-welded Ti/Al plates: A synchrotron-based study. Mater. Sci. Eng. A 2016, 674, 308–317. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Silva Lima, Á.; Cavalcanti, E.B.; Brillas, E. Anodic oxidation, electro-Fenton and photoelectro-Fenton degradations of pyridinium- and imidazolium-based ionic liquids in waters using a BDD/air-diffusion cell. Electrochim. Acta 2016, 198, 268–279. [Google Scholar] [CrossRef]
- Li, G.L.; Cao, H.L.; Zhang, W.J.; Ding, X.; Yang, G.Z.; Qiao, Y.Q.; Liu, X.Y.; Jiang, X.Q. Enhanced osseointegration of hierarchical micro/nano-topographic titanium fabricated by micro-arc oxidation and electrochemical treatment. Acs Appl. Mater. Interf. 2016, 8, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Serdechnova, M.; Karpushenkov, S.A.; Karpushenkava, L.S.; Starykevich, M.; Ferreira, M.G.S.; Hack, T.; Luzviuk, M.H.; Zobkalo, L.A.; Blawert, C.; Zheludkevich, M.L. The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors. Materials 2018, 11, 2428. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.J.; Yao, S.W.; Luo, X.T.; Li, C.X.; Li, C.J. An effective approach for creating metallurgical self-bonding in plasma-spraying of NiCr-Mo coating by designing shell-core-structured powders. Acta Mater. 2016, 110, 19–30. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials 2018, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013. 112, 111–119. [CrossRef]
- Xue, W. Features of film growth during plasma anodizing of Al 2024/SiC metal matrix composite. Appl. Surf. Sci. 2006, 252, 6195–6200. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, X.; Li, G. The mechanism of PEO process on Al–Si alloys with the bulk primary silicon. Appl. Surf. Sci. 2009, 255, 9531–9538. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Lyubimov, V.V.; Ashitkov, R.V. Phase formation in ceramic coatings during plasma electrolytic oxidation of aluminium alloys. Ceram. Int. 1998, 24, 1–6. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110. [Google Scholar] [CrossRef]
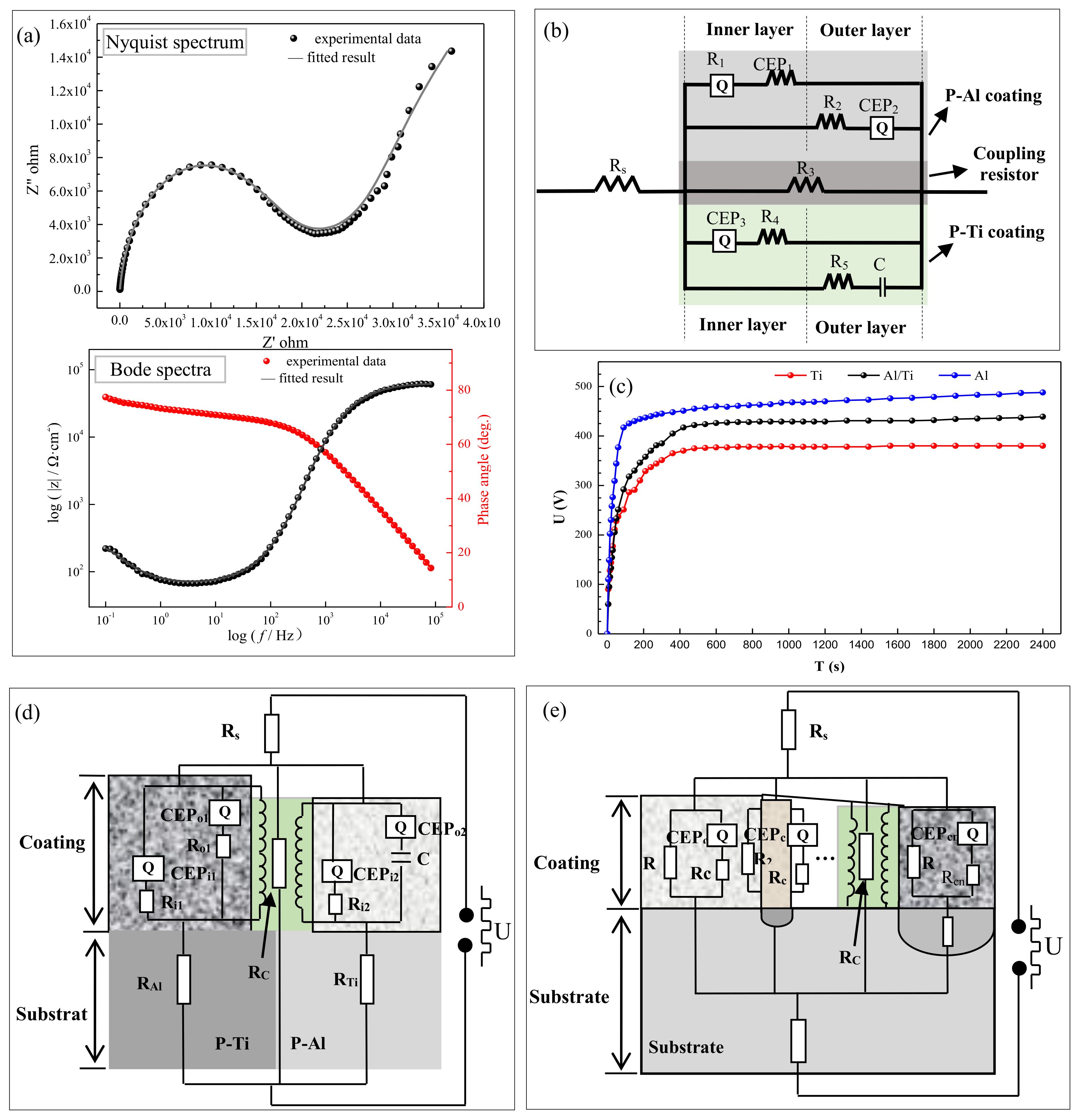
| Rs (Ω/cm2) | CPE1 | R1 (Ω/cm2) | CPE2 | R2 (Ω/cm2) | R3 (Ω/cm2) | CPE3 | R4 (Ω/cm2) | C (F/cm2) | R5 (Ω/cm2) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (F/cm2) | n1 | Q2 (F/cm2) | n2 | Q2 (F/cm2) | n2 | |||||||
| 1.14 | 4.34 × 10−8 | 0.14 | 3.92 × 106 | 1.61 × 10−5 | 0.93 | 1.15 × 106 | 1.08 × 103 | 2.02 × 10−5 | 0.96 | 8.69 × 104 | 3.73 × 106 | 8.83 × 1012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, W.; Ling, K.; Yang, R. Investigation of Growth Mechanism of Plasma Electrolytic Oxidation Coating on Al-Ti Double-Layer Composite Plate. Materials 2019, 12, 272. https://doi.org/10.3390/ma12020272
Chen Q, Li W, Ling K, Yang R. Investigation of Growth Mechanism of Plasma Electrolytic Oxidation Coating on Al-Ti Double-Layer Composite Plate. Materials. 2019; 12(2):272. https://doi.org/10.3390/ma12020272
Chicago/Turabian StyleChen, Quanzhi, Weizhou Li, Kui Ling, and Ruixia Yang. 2019. "Investigation of Growth Mechanism of Plasma Electrolytic Oxidation Coating on Al-Ti Double-Layer Composite Plate" Materials 12, no. 2: 272. https://doi.org/10.3390/ma12020272
APA StyleChen, Q., Li, W., Ling, K., & Yang, R. (2019). Investigation of Growth Mechanism of Plasma Electrolytic Oxidation Coating on Al-Ti Double-Layer Composite Plate. Materials, 12(2), 272. https://doi.org/10.3390/ma12020272





