Preparation and Characterization of Nanoporous Activated Carbon Derived from Prawn Shell and Its Application for Removal of Heavy Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
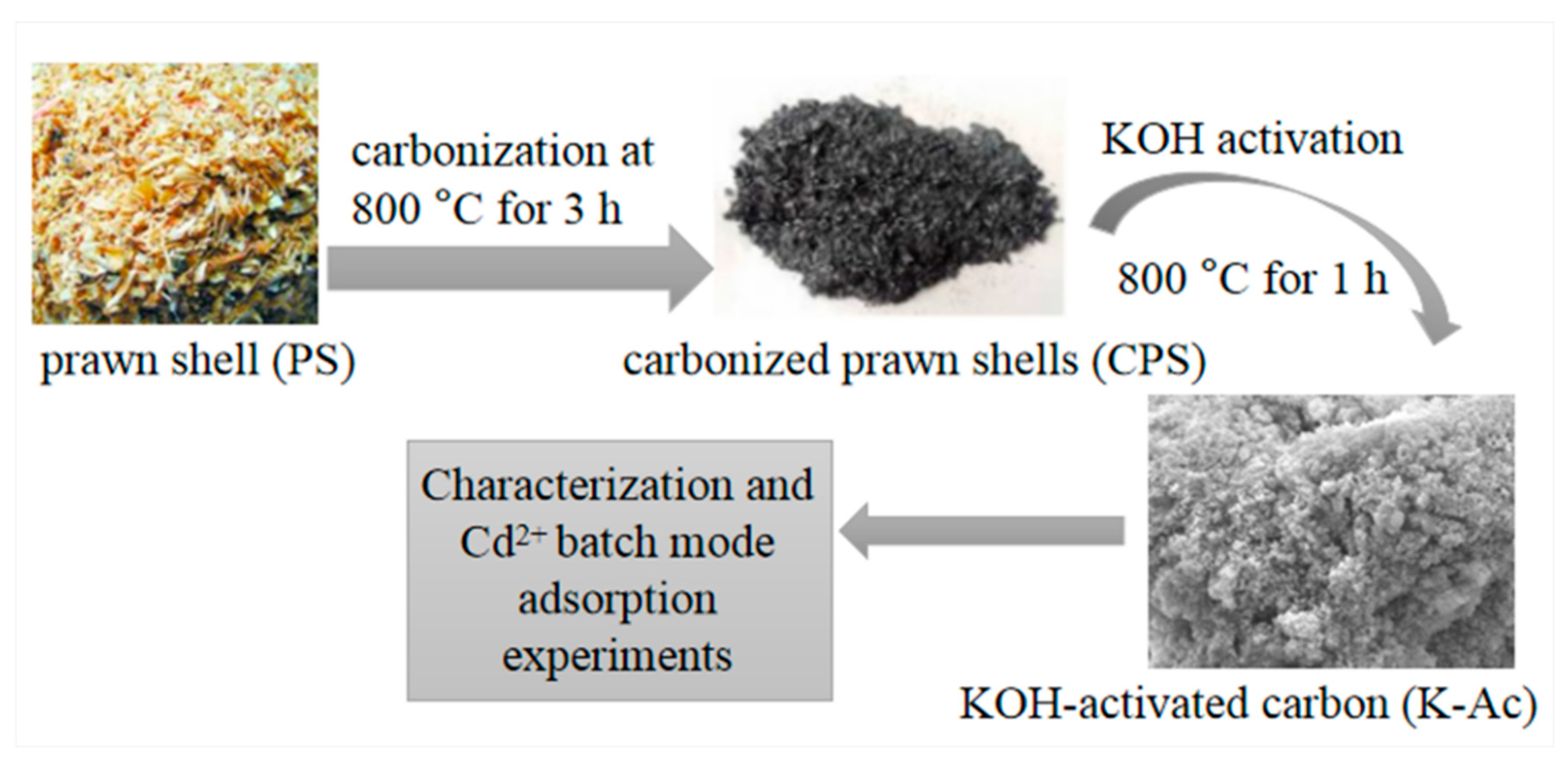
2.2. Preparation of K-Ac Materials
2.3. Adsorbent Characterization
2.4. Adsorption Experiments
2.5. Thermodynamics and Kinetics of K-Ac
2.5.1. Adsorption Thermodynamics
2.5.2. Adsorption Kinetics
3. Results and Discussion
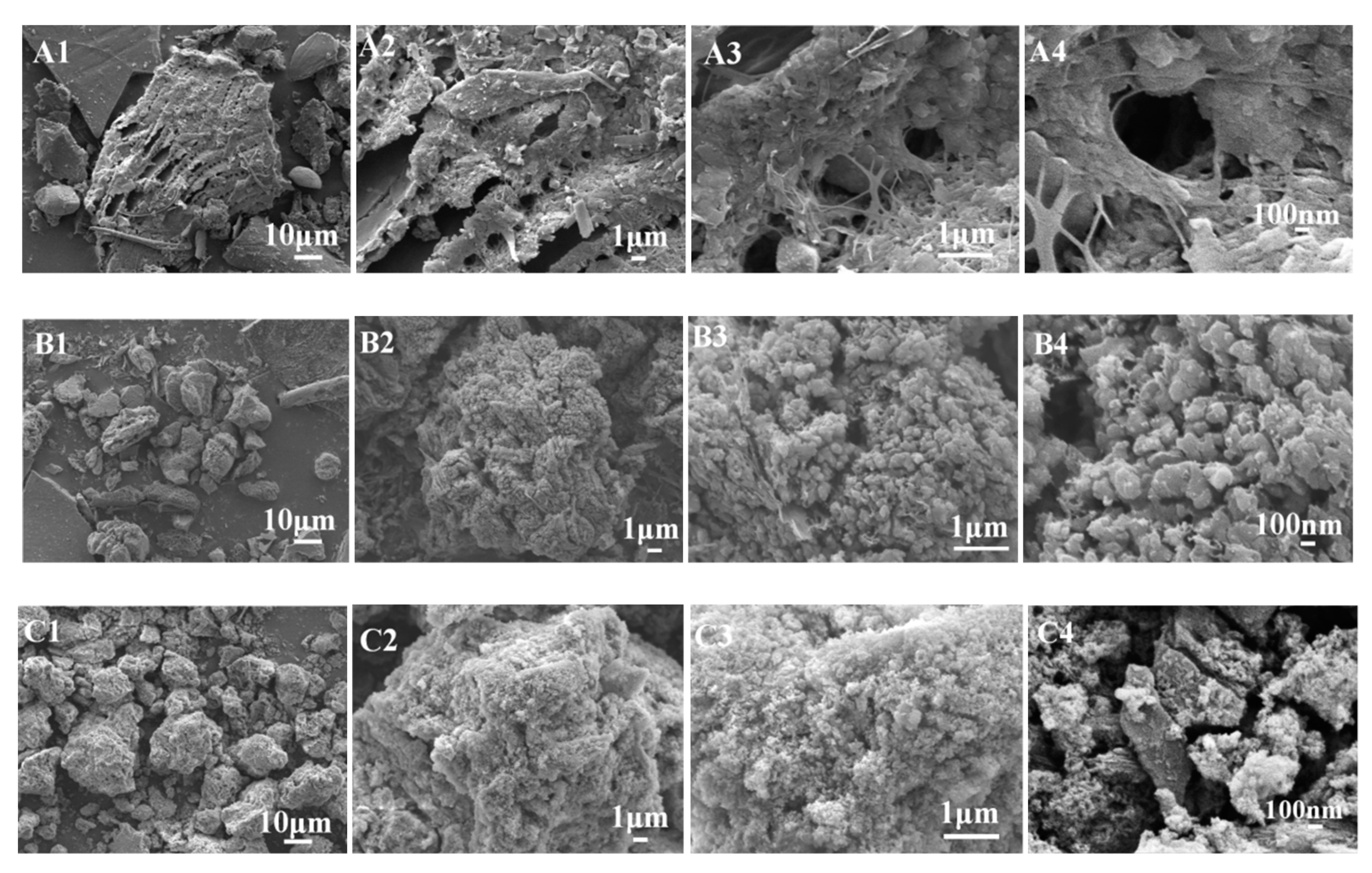
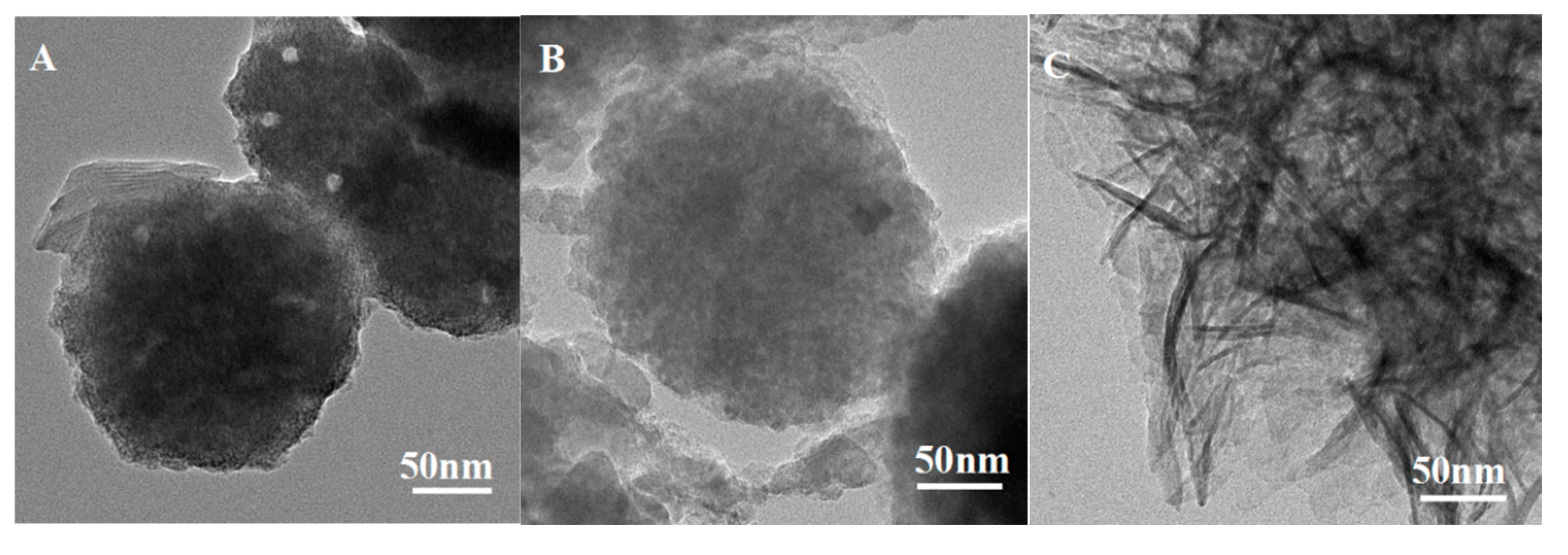
3.1. Surface Pore and Morphology Analysis
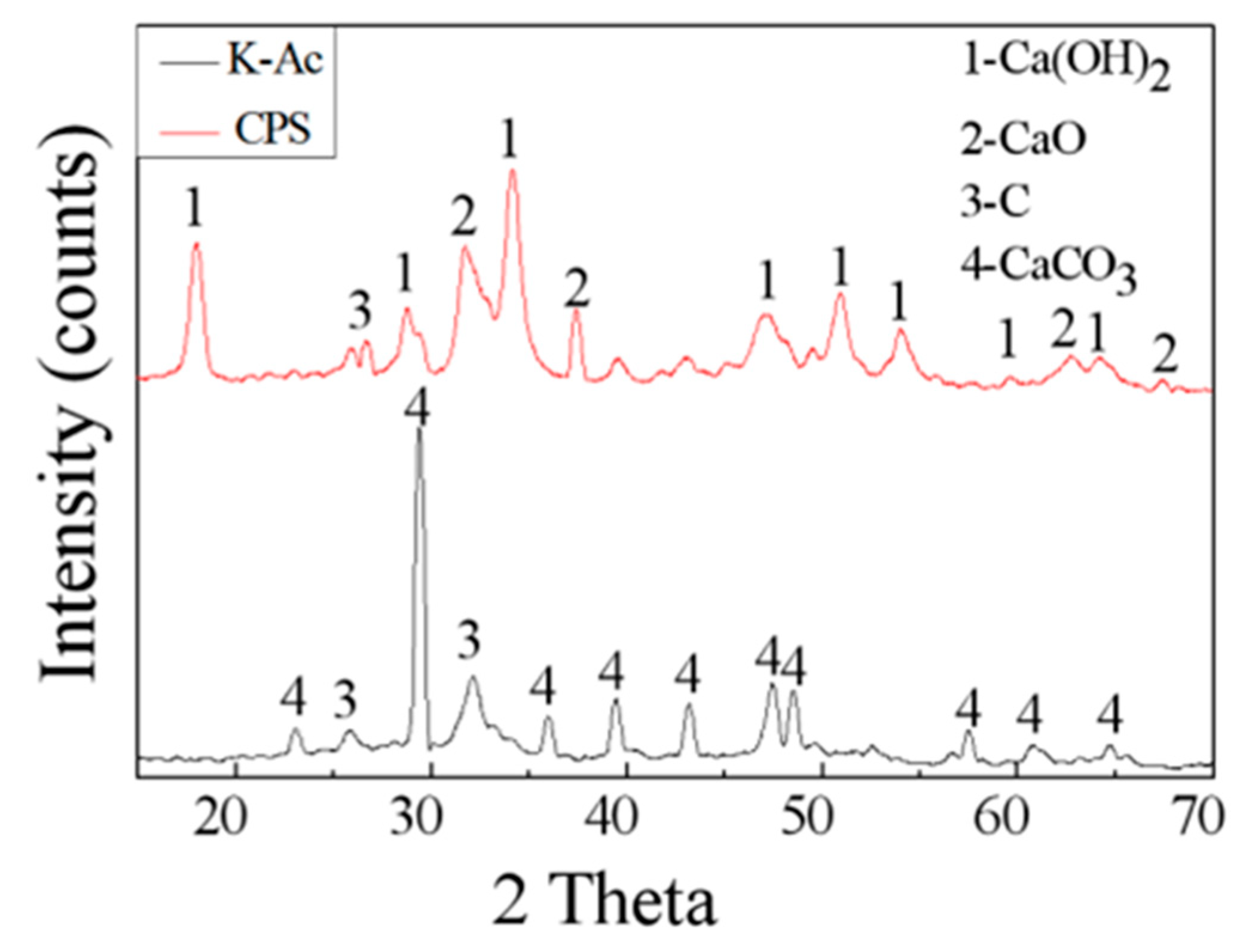
3.2. XRD Crystalline and FTIR Phase Analysis
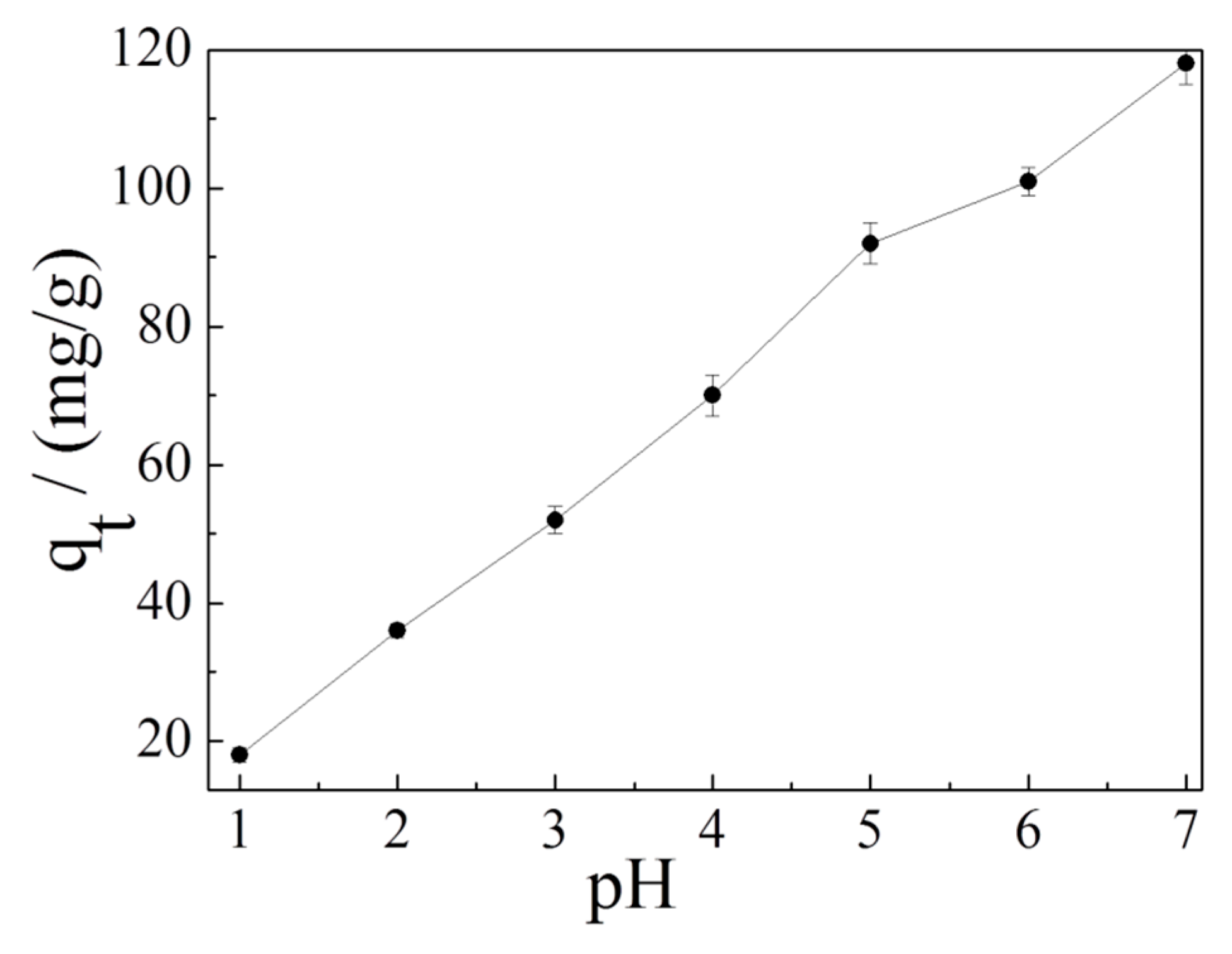
3.3. Effect of Solution pH
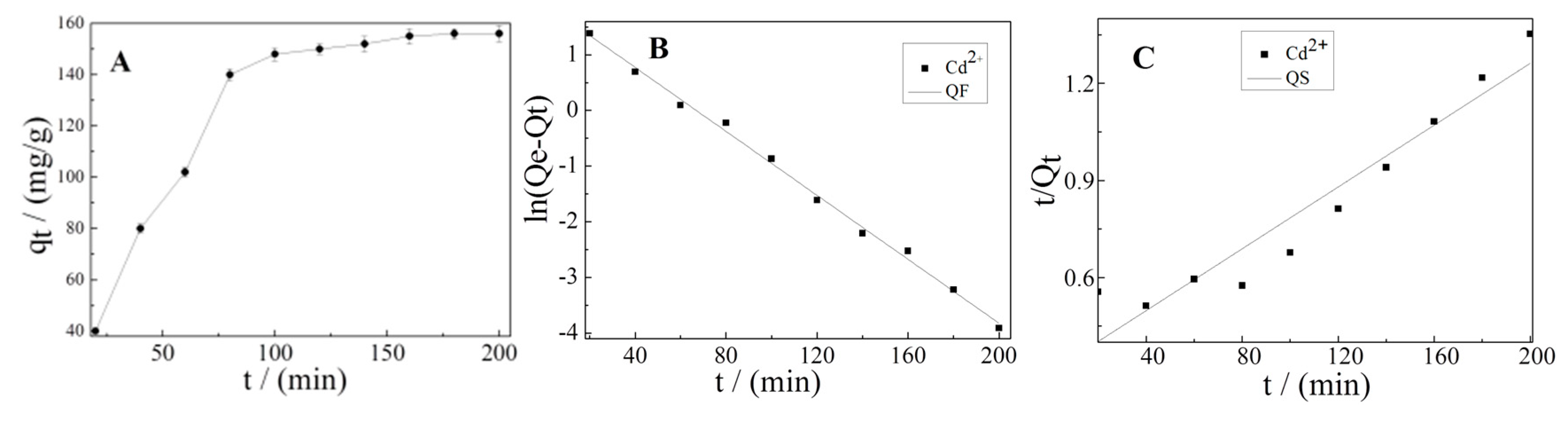
3.4. Effect of Contact Time and Adsorption Kinetics
3.5. Effect of Initial Cd2+ Concentration and Adsorption Isotherms
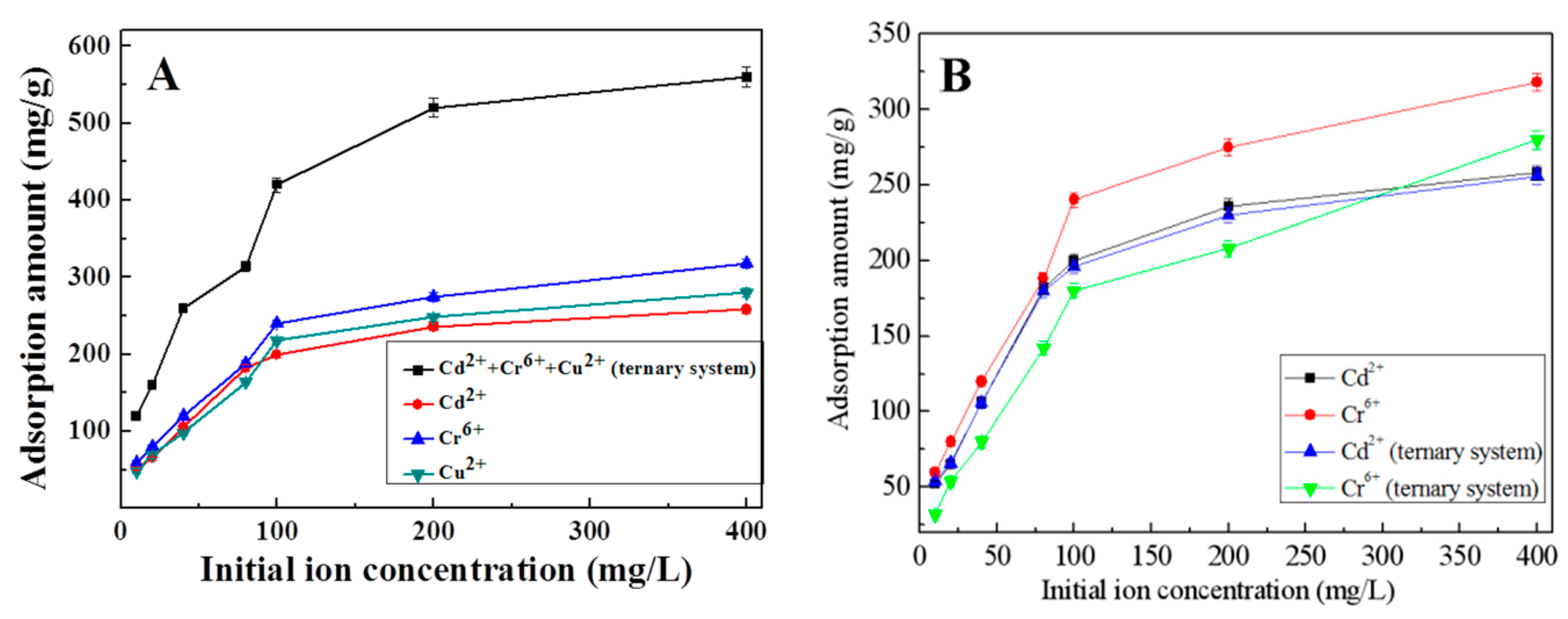
3.6. Adsorption Capacity in Cu2+, Cr6+, and Cd2+ Ternary System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, A.L.; Loh, M.M.; Aziz, J.A. Preparation and characterization of activated carbon from oil palm wood and its evaluation on methylene blue adsorption. Dyes Pigment. 2007, 75, 263–272. [Google Scholar] [CrossRef]
- Bestani, B.; Benderdouche, N.; Benstaali, B.; Belhakem, M.; Addou, A. Methylene blue and iodine adsorption onto an activated desert plant. Bioresour. Technol. 2008, 99, 8441–8444. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.N.M.E.; ObidulHuq, K.; Yahya, R.B. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Yeung, P.T.; Chung, P.Y.; Tsang, H.C.; Tang, J.O.; Cheng, G.Y.M.; Gambari, R.; Chui, C.H.; Lam, K.H. Preparation and characterization of bio-safe activated charcoal derived from coffee waste residue and its application for removal of lead and copper ions. RSC Adv. 2014, 4, 38839–38847. [Google Scholar] [CrossRef]
- Susan, E.; Bailey, T.J.; Olin, R.M.; Bricka, D.; Dean, A. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 11, 2469–2479. [Google Scholar]
- Zhang, Q.; Li, Y.; Phanlavong, P.; Wang, Z.; Jiao, T.; Qiu, H.; Peng, Q. Highly efficient and rapid fluoride scavenger using an acid/base tolerant zirconium phosphate nanoflake: Behavior and mechanism. J. Clean. Prod. 2017, 161, 317–326. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Yang, Q.; Chen, H.; Chen, X.; Jiao, T.; Peng, Q. Distinguished Cr (VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard Mater. 2017, 342, 732. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Yang, H.; Cui, Z. Biochemical mechanism of phytoremediation process of lead and cadmium pollution with Mucor circinelloides and Trichoderma asperellum. Ecotoxicol. Environ. Saf. 2018, 157, 21–28. [Google Scholar] [CrossRef]
- Feng, J.X.; Gao, Q.F.; Dong, S.L.; Sun, Z.L. Transference of heavy metals (Hg, Cu, Pb and Zn) with the trophic structure in a polyculture pond: Evidence from nitrogen stable isotope. Aquac. Res. 2016, 47, 1996–2003. [Google Scholar] [CrossRef]
- Mendoza-Carranza, M.; Sepulveda-Lozada, A.; Dias-Ferreira, C. Distribution and bioconcentration of heavy metals in a tropical aquatic food web: A case study of a tropical estuarine lagoon in SE Mexico. Environ. Pollut. 2016, 210, 155–165. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Stella, C.; Prearo, M.; Pastorino, P.; Serracca, L.; Ercolini, C.; Abete, M.C. Presence of trace metals in aquaculture marine ecosystems of the northwestern Mediterranean sea (Italy). Environ. Pollut. 2016, 215, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Cheng, Y.; Zhang, L.; Qiu, J. Efficient Removal of Cr(VI) from Aqueous Solution with Fe@Fe2O3Core−Shell Nanowires. Environ. Sci. Technol. 2008, 42, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Habibul, N.; Hu, Y.; Wang, Y.K.; Chen, W.; Yu, H.Q.; Sheng, G.P. Bioelectrochemical Chromium(VI) Removal in Plant-Microbial Fuel Cells. Environ. Sci. Technol. 2016, 50, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Ghani, A. Varietal differences in mungbean (Vigna radiata) for growth, yield, toxicity symptoms and cadmium accumulation. Ann. Appl. Biol. 2008, 152, 59–69. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.-H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Dynamic adsorption behavior of methylene blue onto oil plam shell granular activated carbon prepared by microwave heating. Chem. Eng. J. 2012, 203, 81–87. [Google Scholar] [CrossRef]
- Mahapatra, K.; Ramteke, D.S.; Paliwal, L.J. Production of activated carbon from sludge of food processing industry under controlled pyrolysis and its application for methylene blue removal. J. Anal. Appl. Pyrolysis 2012, 95, 79–86. [Google Scholar] [CrossRef]
- Nor, N.M.; Chung, L.L.; Teong, L.K.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Chem. Eng. 2013, 1, 658–666. [Google Scholar]
- Ruey-Shin, J.; Yao-Chung, Y.; Chien-Shiun, L.; Kuen-Song, L.; Hsi-Chuan, L.; Sea-Fue, W.; An-Cheng, S. Synthesis of magnetic Fe3O4/activated carbon nanocomposites with high surface area as recoverable adsorbents. J. Taiwan Inst. Chem. Eng. 2018, 90, 51–60. [Google Scholar]
- Omer, K.; Yasin, R.E.; Haluk, B.; Ali, T. Preparation of chemically-activated high surface area carbon from waste vinasse and its efficiency as adsorbent material. J. Mol. Liquids 2018, 272, 189–197. [Google Scholar]
- Junting, S.; Zhengping, Z.; Jing, J.; Meiling, D.; Feng, W. Removal of Cr6+ from wastewater via adsorption with high-specific-surface-area nitrogen-doped hierarchical porous carbon derived from silkworm cocoon. Appl. Surf. Sci. 2017, 405, 372–379. [Google Scholar]
- Ait Ahsaine, H.; Zbair, M.; Anfar, Z.; Naciri, Y.; El haouti, R.; El Alem, N.; Ezahri, M. Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Mater. Today Chem. 2018, 8, 121–132. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Optimization of microwave preparation conditions for activated carbon from Albizia lebbeck speed pods for methylene blue dye adsorption. J. Anal. Appl. Pyrol. 2014, 105, 199–208. [Google Scholar] [CrossRef]
- Song, M.; Jin, B.; Xiao, R.; Yang, L.; Wu, Y.; Zhong, Z.; Huang, Y. The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenergy 2013, 48, 250–256. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Thuana, T.V.; Quynha, B.T.P.; Nguyena, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- White, R.J.; Antonietti, M.; Titirici, M.M. Naturally inspired nitrogen doped porous carbon. J. Mater. Chem. 2009, 19, 8645–8650. [Google Scholar] [CrossRef]
- De Holanda, H.D.; Netto, F.M. Recovery of components from shrimp (Xiphopenoeous kryerl) processing waste by enzymatic hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Kandra, P.; Challa, M.M.; Kalangi Padma Jyothi, H. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Beaney, P.; Mendoza, J.L.; Healy, M. Comparison of chitins produced by chemical and bioprocedding methods. J. Chem. Technol. Biotechnol. 2005, 80, 145–150. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhou, Z. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol. J. Taiwan Inst. Chem. Eng. 2016, 62, 228–238. [Google Scholar] [CrossRef]
- Lillo-Rodenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amoros, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrol. 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Król, M.; Gryglewicz, G.; Machnikowski, J. KOH activation of pitch-derived carbonaceous materials—Effect of carbonization degree. Fuel Process. Technol. 2011, 92, 158–165. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Tseng, R.-L.; Tseng, S.-K. Pore structure and adsorption performance of the KOH-activated carbons prepared from corncob. J. Colloid Interface Sci. 2005, 287, 428–437. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Yang, J.; Lin, D. Adsorption and correlations of selected aromatic compounds on a KOH-activated carbon with large surface area. Sci. Total Environ. 2018, 618, 1677–1684. [Google Scholar] [CrossRef]
- Kanogwan, T.; Lupong, K.A. Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for methylene bule removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biocherm. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.I.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochem. 2003, 39, 179–183. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.I.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption characteristics of a submerged aquatic plant (Ceratophyllum deersum). Process Biochem. 2004, 92, 197–200. [Google Scholar]
- Ho, Y.S. Citation review of Lagerren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Ho, Y.S.; McKay, G.A. Kinetic study of dye sorption by biosorbent waste product pith. Resour. Conserv. Recyc. 1999, 25, 171–193. [Google Scholar] [CrossRef]
- Benquella, B.; Benaissa, H. Cadmium removal frm aqueous solution by chitin: Kinetic and equilibrium studies. Water Res. 2002, 36, 2463–2474. [Google Scholar] [CrossRef]
- Cheng, C.W.; Porter, C.F.; McKay, G. Sorption kinetics for the removal of copper and zinc from effluents using bone char. Sep. Purif. Technol. 1997, 19, 55–64. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood Trans. ICHemE 1998, 76, 183–191. [Google Scholar]
- Liu, X.; He, C.; Yu, X.; Bai, Y.; Ye, L.; Wang, B.; Zhang, L. Net-like porous activated carbon materials from shrimp shell by solution-processed carbonization and H3PO4 activation for methylene blue adsorption. Powder Technol. 2018, 326, 181–189. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lim, S.; Song, Y.; Ota, Y.; Qiao, W.; Tanaka, A.; Mochida, I. KOH activation of carbon nanofibers. Carbon 2004, 42, 1723–1729. [Google Scholar] [CrossRef]
- Cho, I.; No, K.; Meyers, P. Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J. Agric. Food Chem. 1998, 46, 3839–3843. [Google Scholar] [CrossRef]
- Sachindra, M.; Bhaskar, N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. Technol. 2008, 99, 9013–9016. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K.A. X-ray diffraction analysis on graphene layers of Assam coal. J. Chem. Sci. 2009, 121, 103–106. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Ng, E.-P. The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous Mesoporous Mater. 2014, 197, 316–323. [Google Scholar] [CrossRef]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous carbon spheres from hydrothermal carbonization and KOH activation on cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by KOH activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnikowski, J. Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 2017, 21, 436–443. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; ElGammal, M.H.; Rawash, E.-S.A. Rawash. Optimizing the preparation conditions of activated carbons from olive cake using KOH activation. New Carbon Mater. 2016, 31, 492–500. [Google Scholar] [CrossRef]
- Khosravi, L.; Yazdanbakhsh, M.; Eftekhar, M.; Haddadi, Z. Fabrication of nano Delafossite LiCo0.5Fe0.5O2 as the new adsorbent in efficient removal of reactive blue 5 from aqueous solutions. Mater. Res. Blue 2013, 48, 2213–2219. [Google Scholar] [CrossRef]
- Ma, J.; Shen, Y.; Shen, C.; Wen, Y.; Liu, W. Al-doping chitosan-Fe(III) hydrogel for the removal of fluoride from aqueous solutions. Chem. Eng. J. 2014, 248, 98–106. [Google Scholar] [CrossRef]
- Fang, L.; Kanggen, Z.; Quan, C.; Wang, A.; Wei, C. Preparation of magnetic ferrite by optimizing the synthetic pH and its application for the removal of Cd(II) from Cd-NH3-H2O system. Mol. Liquids 2018, 264, 215–222. [Google Scholar]
- Wang, X.R. Environmental Chemistry; Nanjing University Press: Nanjing, China, 1993. [Google Scholar]
- Ghorbani, F.; Younesi, H.; Seyed, G.; Ali, Z.; Amini, M.; Ali, D. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomy cescerevisiae. Chem. Eng. J. 2008, 145, 267–275. [Google Scholar] [CrossRef]
- Sharma, P.; Kumari, P.; Srivastava, M.M.; Srivastava, S. Ternary biosorption studies of Cd(II), Cr(III) and Ni(II) on shelled Moringa oleifera seeds. Bioresour. Technol. 2007, 98, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Mattuschka, B.; Straube, G. Biosorption of metals by a waste biomass. J. Chem. Technol. 1993, 58, 57–63. [Google Scholar] [CrossRef]
- Qin, F.; Wen, B.; Shan, X.Q.; Xie, Y.N.; Liu, T.; Zhang, S.Z.; Khan, S.U. Mechanisms of competitive adsorption of Pb, Cu, and Cd on peat. Environ. Pollut. 2006, 144, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, M., Jr.; Abia, A.A.; Spiff, A.I. Removal of Cu(II) and Zn(II) ions from waste water by cassava (Manihot esculenta Cranz) waste biomass. Afr. J. Biotechnol. 2003, 2, 360–364. [Google Scholar]
- He, I.; Li, Y.; Wang, C.; Zhang, K.; Lin, D.; Kong, L.; Liu, J. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by -cyclodextrin polymers. Appl. Surface Sci. 2017, 426, 29–39. [Google Scholar] [CrossRef]
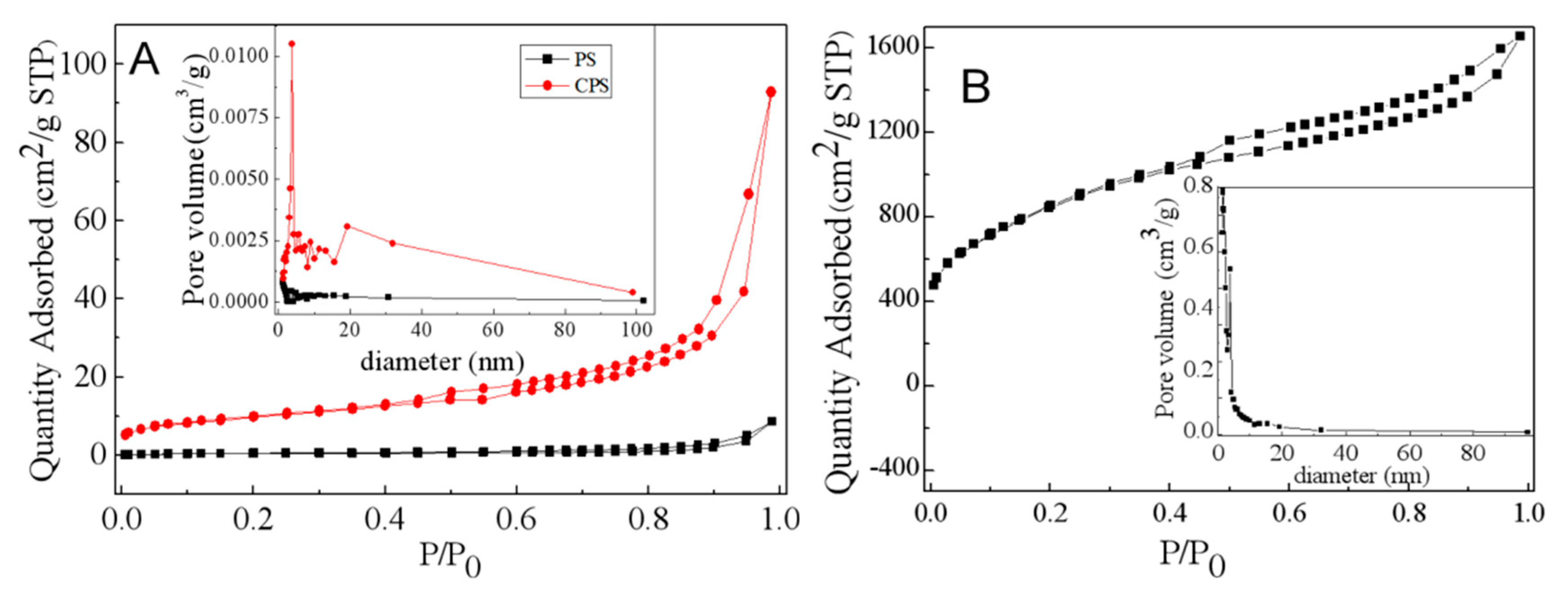


| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Radius (nm) |
|---|---|---|---|
| PS | 4 | 0.014 | 1.349 |
| CPS | 37 | 0.143 | 3.831 |
| K-Ac | 3160 | 2.382 | 1.351 |
| Sample | QF | QS | ||
|---|---|---|---|---|
| K-Ac | K1/(1/min) | R2 | K2/(mg/g·min) | R2 |
| 0.066 | 0.99587 | 19.723 | 0.91054 | |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| K-Ac | KL | R2 | RL | n | KF | R2 |
| 0.016 | 0.9737 | 0.862 | 1.011 | 1.829 | 0.93716 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and Characterization of Nanoporous Activated Carbon Derived from Prawn Shell and Its Application for Removal of Heavy Metal Ions. Materials 2019, 12, 241. https://doi.org/10.3390/ma12020241
Guo J, Song Y, Ji X, Ji L, Cai L, Wang Y, Zhang H, Song W. Preparation and Characterization of Nanoporous Activated Carbon Derived from Prawn Shell and Its Application for Removal of Heavy Metal Ions. Materials. 2019; 12(2):241. https://doi.org/10.3390/ma12020241
Chicago/Turabian StyleGuo, Jian, Yaqin Song, Xiaoyang Ji, Lili Ji, Lu Cai, Yaning Wang, Hailong Zhang, and Wendong Song. 2019. "Preparation and Characterization of Nanoporous Activated Carbon Derived from Prawn Shell and Its Application for Removal of Heavy Metal Ions" Materials 12, no. 2: 241. https://doi.org/10.3390/ma12020241
APA StyleGuo, J., Song, Y., Ji, X., Ji, L., Cai, L., Wang, Y., Zhang, H., & Song, W. (2019). Preparation and Characterization of Nanoporous Activated Carbon Derived from Prawn Shell and Its Application for Removal of Heavy Metal Ions. Materials, 12(2), 241. https://doi.org/10.3390/ma12020241





