Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
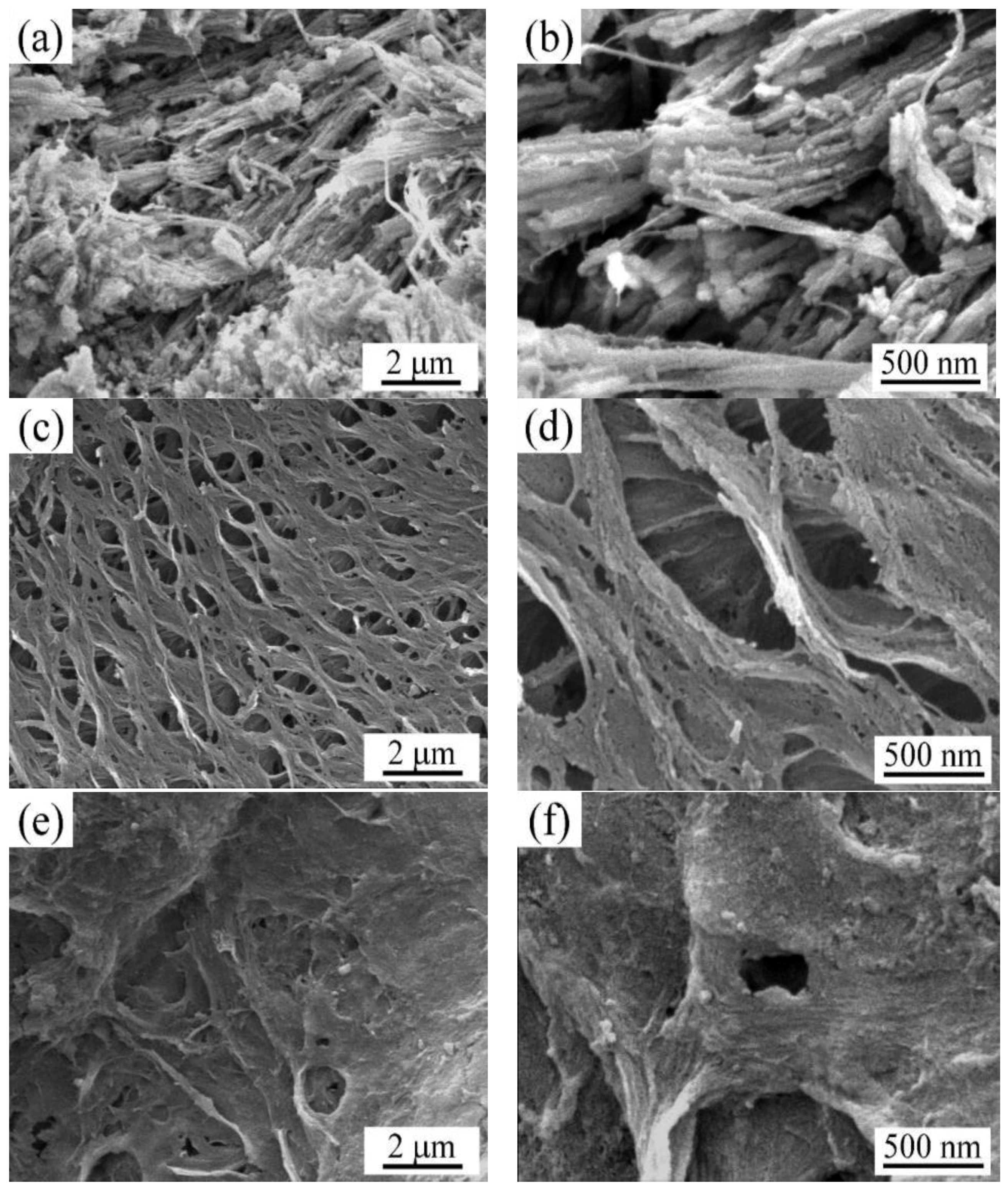
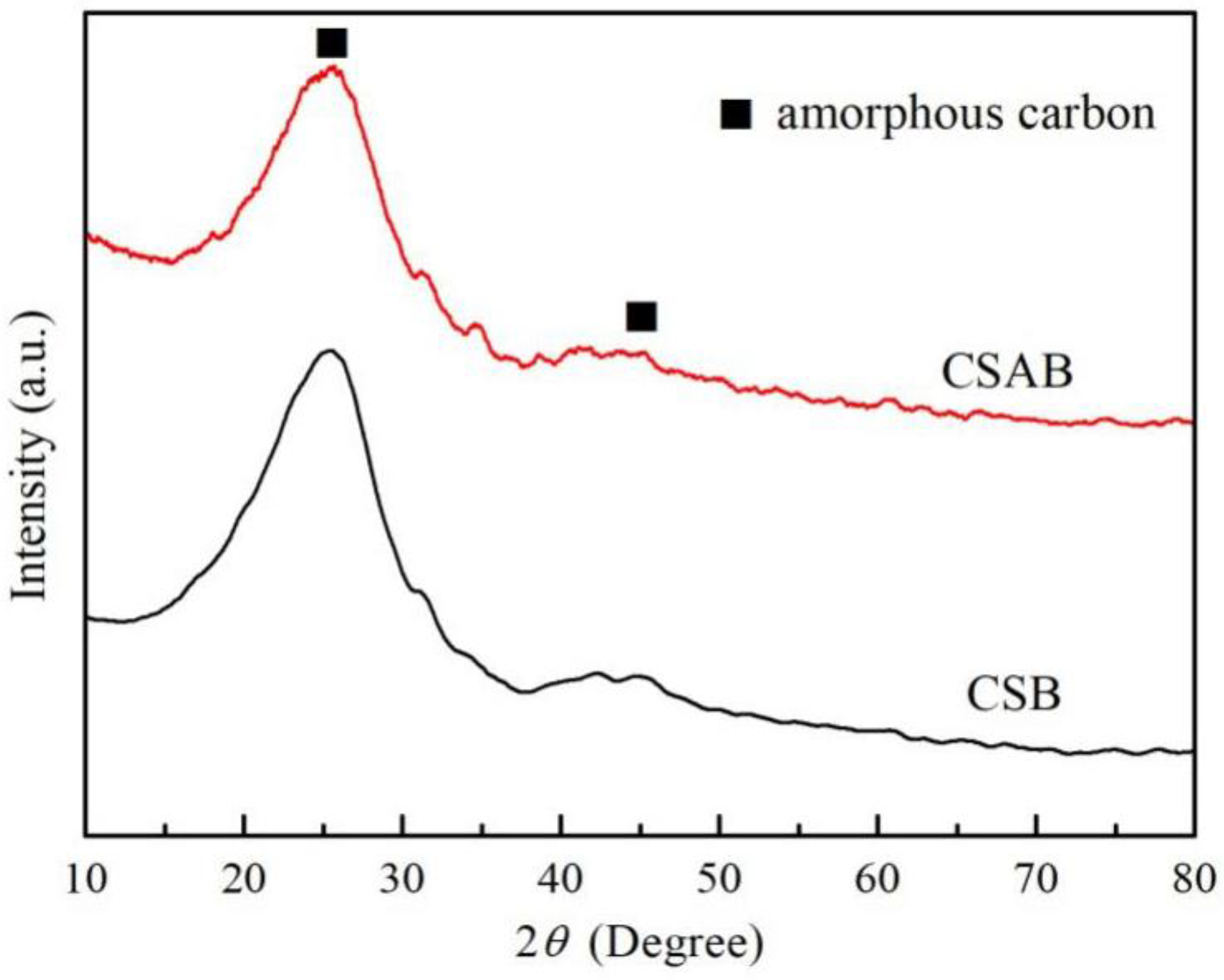
3.1. Structure Characterization of Crab Shell Biochar
3.2. Effect of Adsorbent Dosage
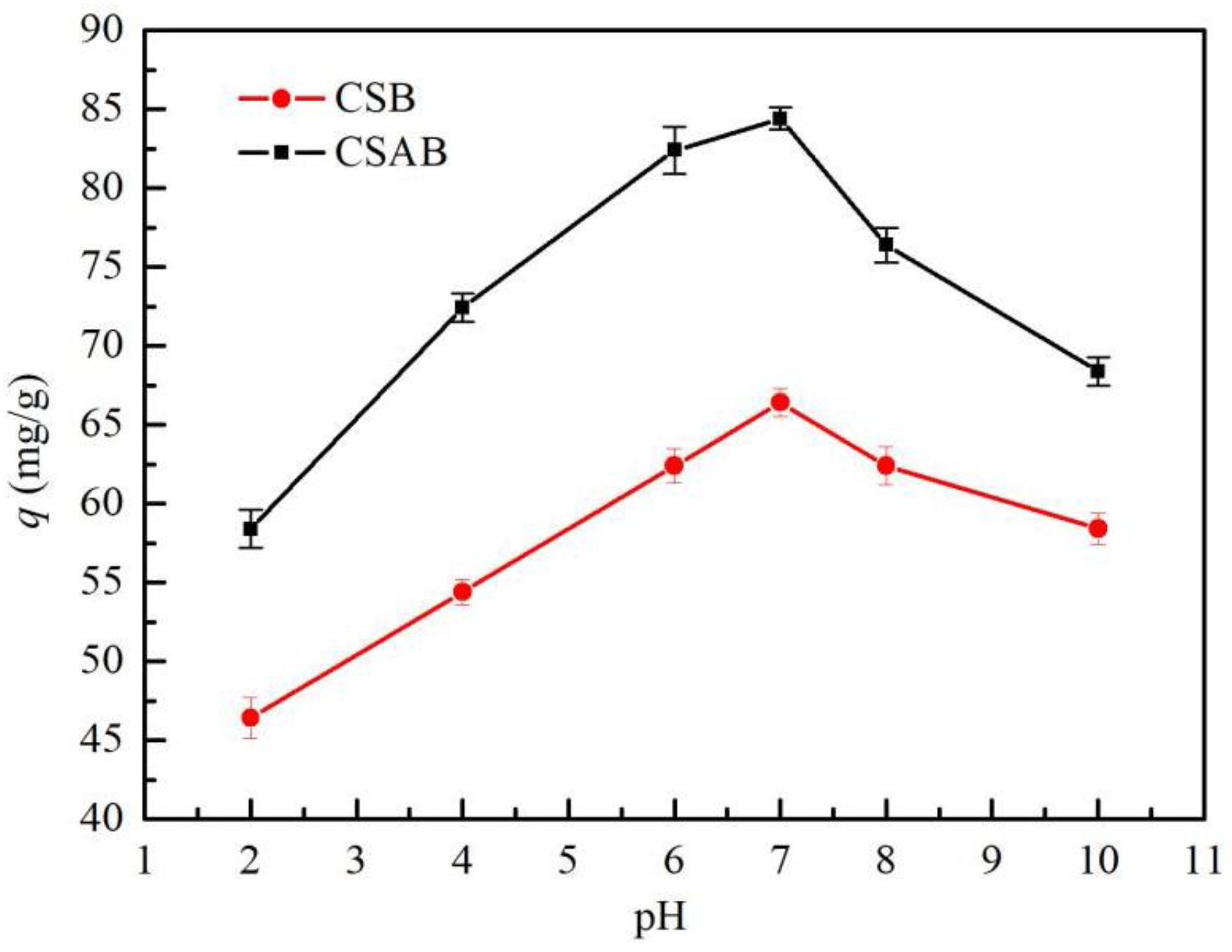
3.3. Effect of Initial pH
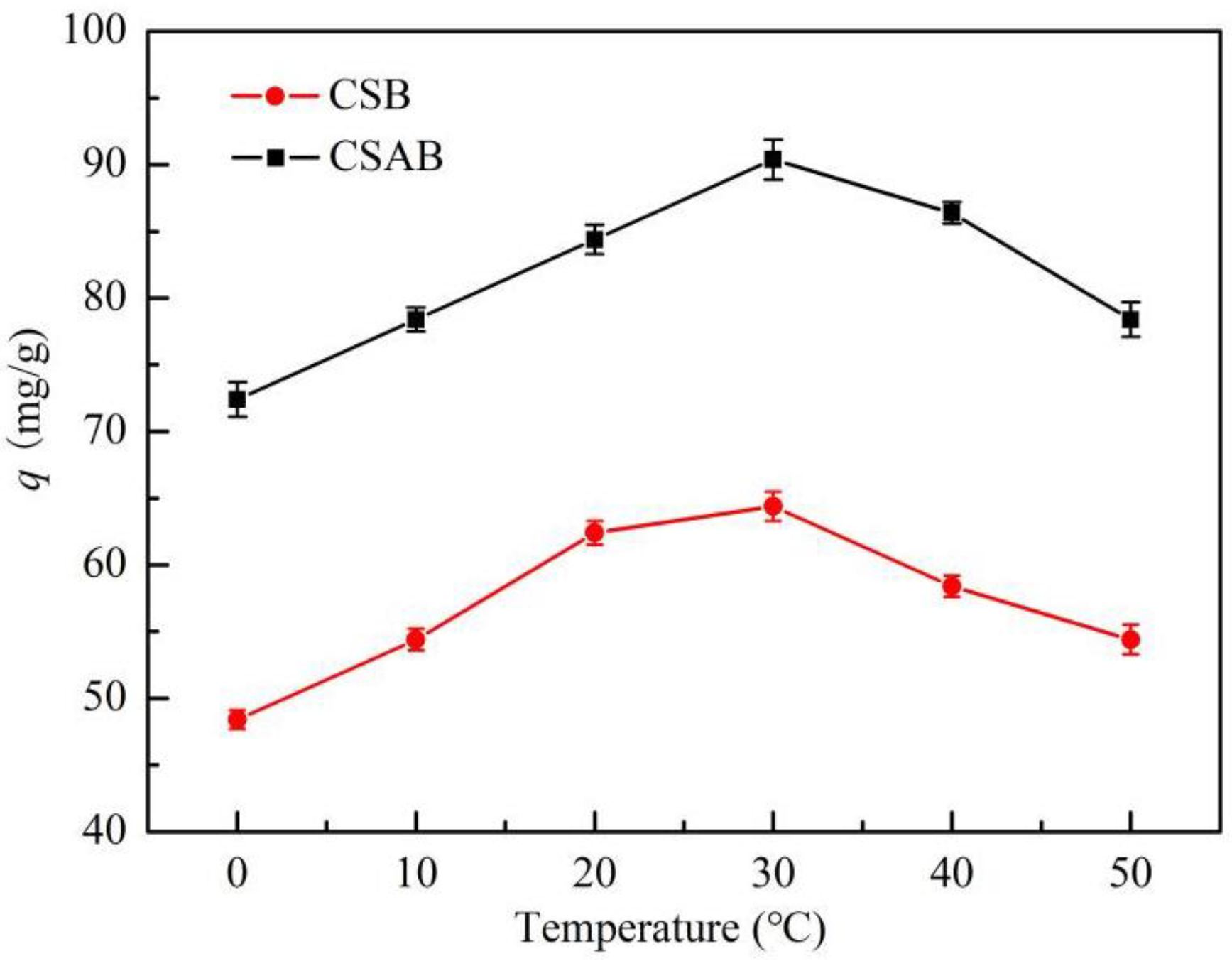
3.4. Effect of Adsorbent Temperature
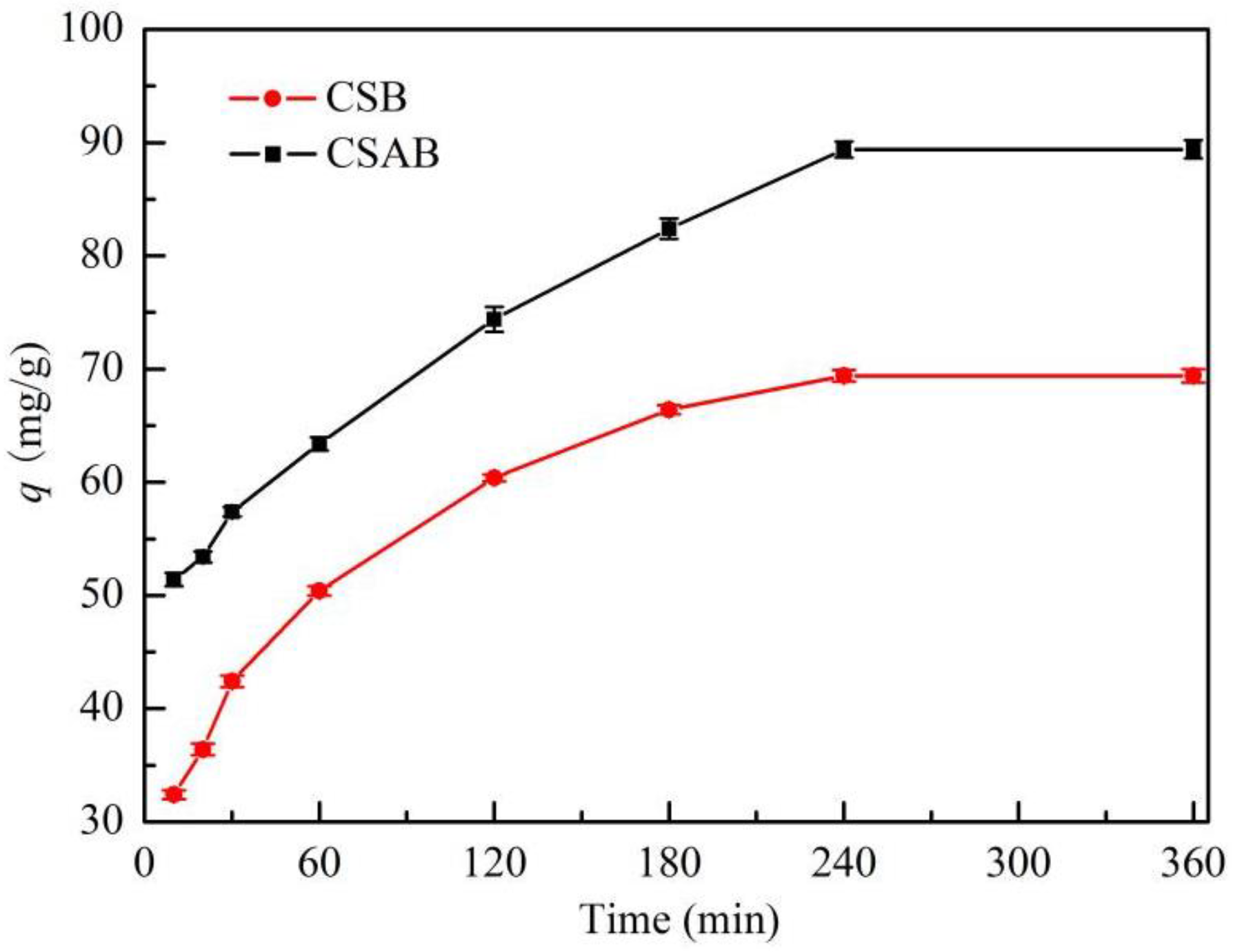
3.5. Effect of Contact Time
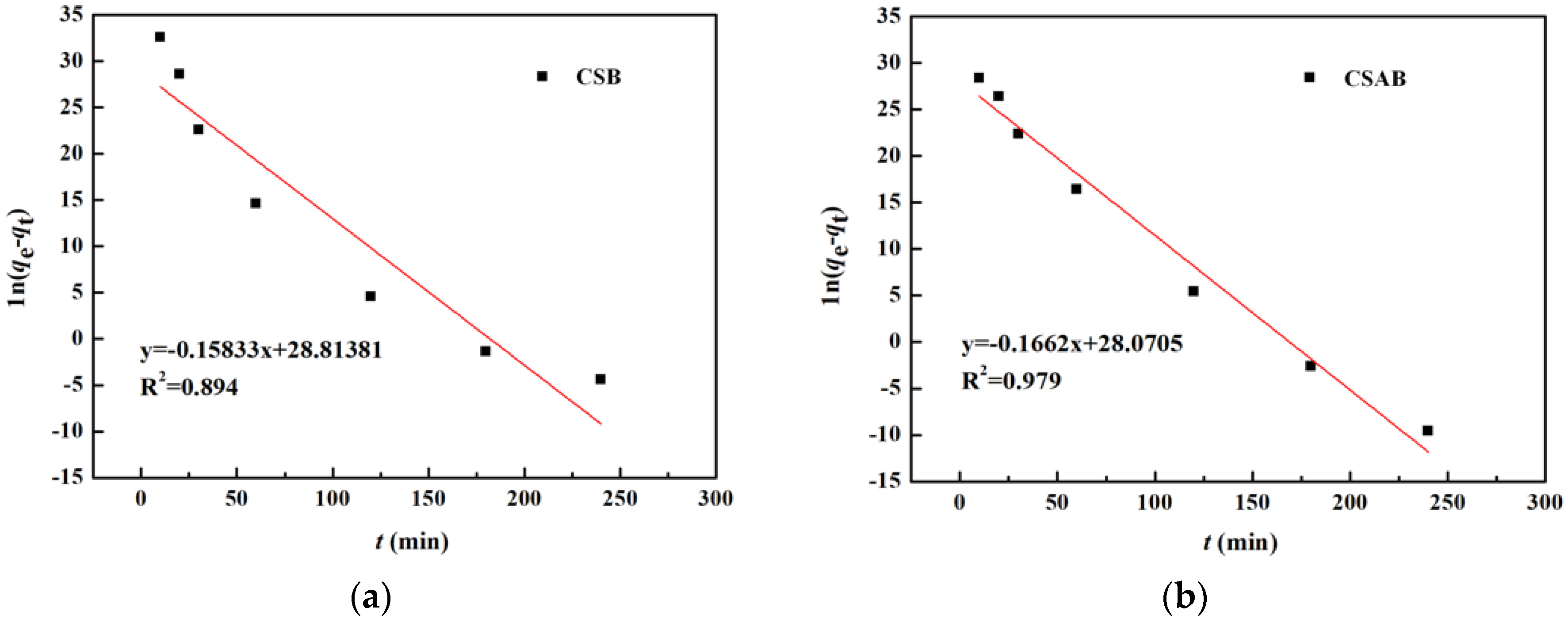
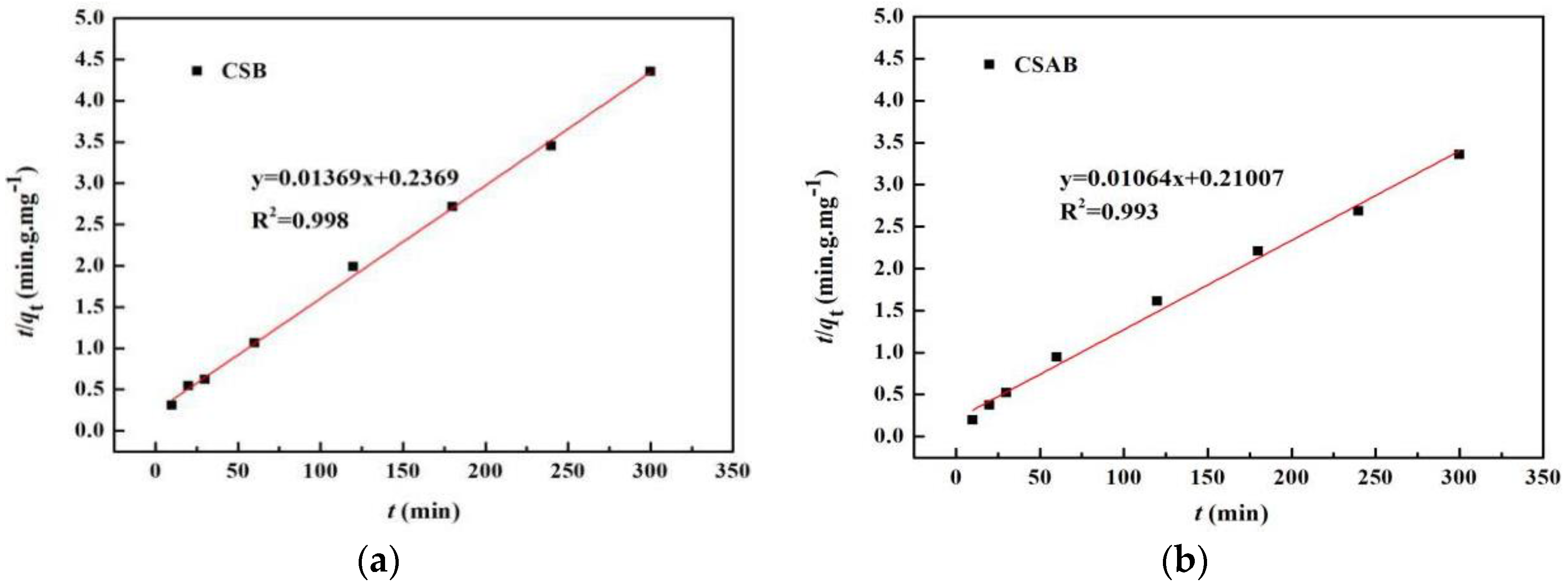
3.6. Adsorption Kinetics
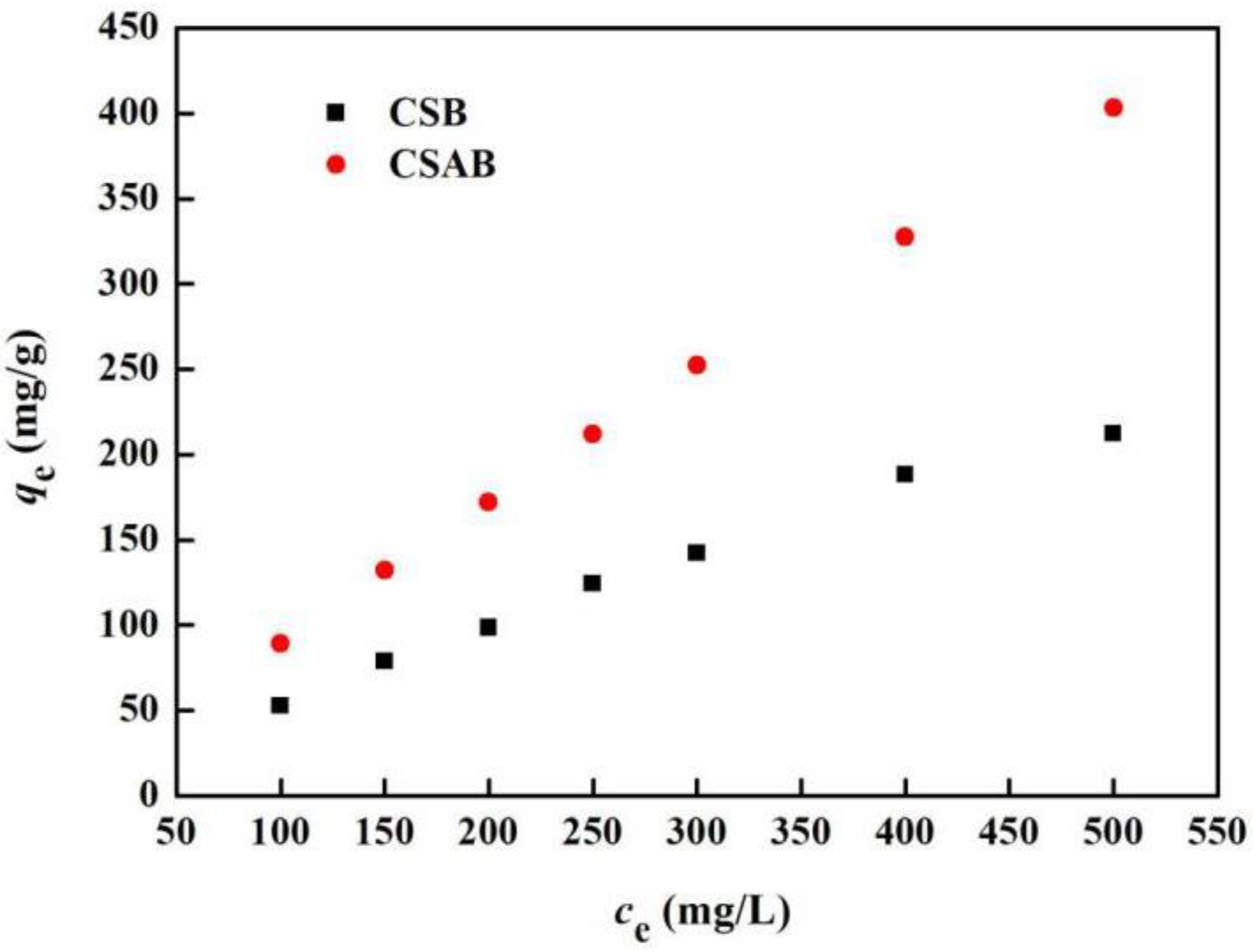
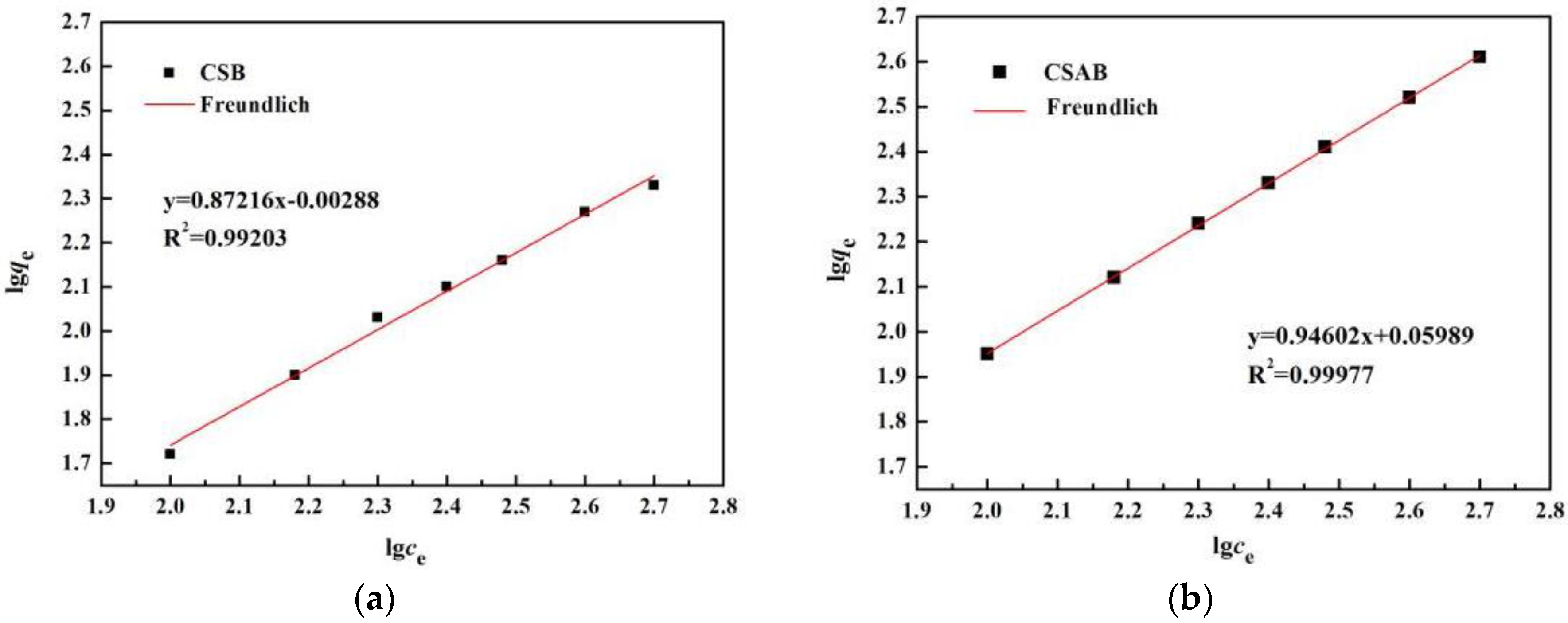
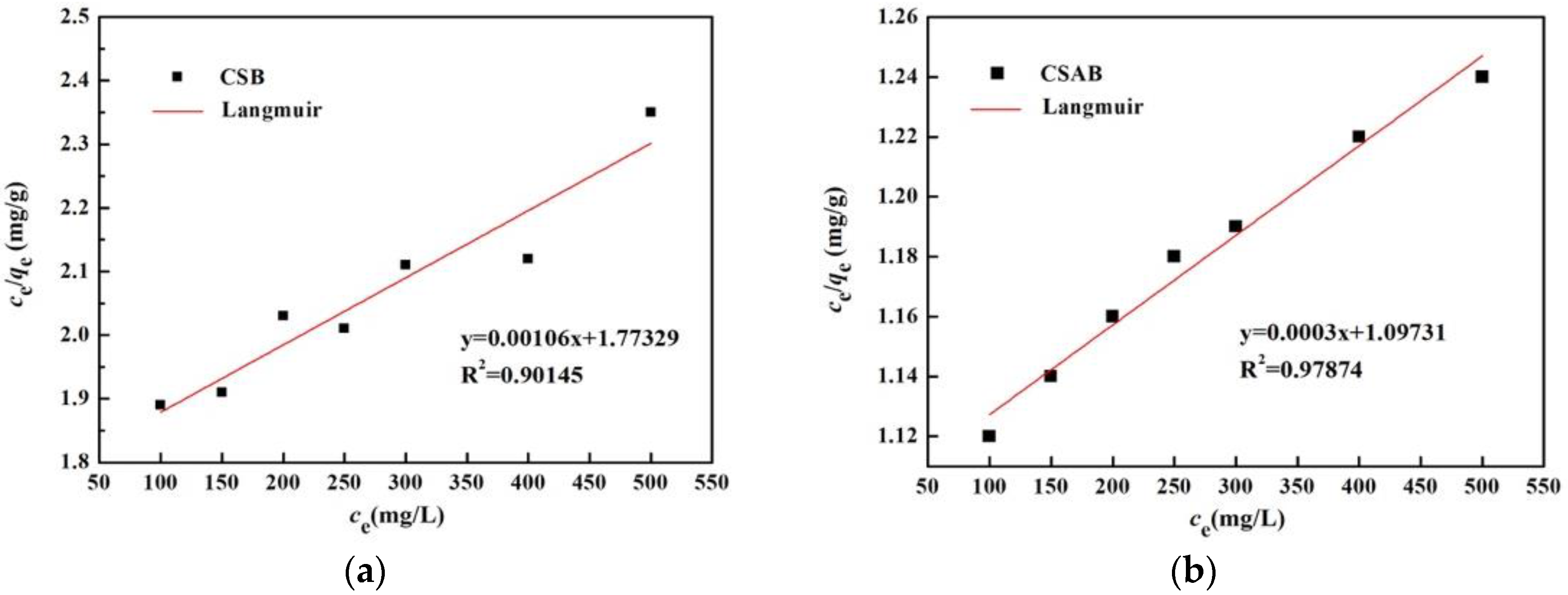
3.7. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasouli, Y.; Abbasi, M.; Hashemifard, S.A. Oily wastewater treatment by adsorption–membrane filtration hybrid process using powdered activated carbon, natural zeolite powder and low cost ceramic membranes. Water Sci. Technol. 2017, 76, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Al-Futaisi, A.; Jamrah, A.; Yaghi, B.; Taha, R. Assessment of alternative management techniques of tank bottom petroleum sludge in oman. J. Hazard. Mater. 2017, 141, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Machín-Ramírez, C.; Okoh, A.I.; Morales, D.; Mayolo-Deloisa, K.; Quintero, R.; Trejo-Hernández, M.R. Slurry-phase biodegradation of weathered oily sludge waste. Chemosphere 2008, 70, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Chaprão, M.J.; Ferreira, I.N.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, D.; Quan, L. Interactive factors leading to dying-off Carex tato in Momoge wetland polluted by crude oil, Western Jilin, China. Chemosphere 2006, 65, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Rocher, V.; Muller, M.; Moilleron, R.; Thevenot, D.R. Sources, distribution and variability of hydrocarbons and metals in atmospheric deposition in an urban area (Paris, France). Sci. Total. Environ. 2005, 337, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, Q.; Gong, P.; Sun, T. Ecotoxicity of soils contaminated with industrial and domestic wastewater in western Shenyang, China. Sci. China. Ser. C 2005, 48, 48–56. [Google Scholar] [CrossRef]
- Al-Shamrani, A.A.; James, A.; Xiao, H. Destabilisation of oil-water emulsions and separation by dissolved air flotation. Water Res. 2002, 36, 1503–1512. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Liu, M.; Bu, Y.; Zhang, J.; Chen, J.; Zhao, J. Adsorption-synergic biodegradation of diesel oil in synthetic seawater by acclimated strains immobilized on multifunctional materials. Mar. Pollut. Bull. 2015, 92, 195–200. [Google Scholar] [CrossRef]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. wastewater treatment and waste reduction. J. Membr. Sci. 2015, 151, 13–28. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Removal of Sudan IV from a simulated biphasic oily wastewater by using lipophilic carbon adsorbents. Chem. Eng. J. 2018, 347, 963–971. [Google Scholar] [CrossRef]
- Nekoo, S.H.; Fatemi, S. Experimental study and adsorption modeling of COD reduction by activated carbon for wastewater treatment of oil refinery. Iran. J. Chem. Chem. Eng. 2013, 32, 81–89. [Google Scholar]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Tseng, S.-K. Pore structure and adsorption performance of the KOH-activated carbons prepared from corncob. J. Colloid Interface Sci. 2005, 287, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, H.; Cai, L.; Gao, J.; Wang, Y.; Ji, L.; Song, D. Calcined mussel shell powder (CMSP) via modification with surfactants: Application for antistatic oil-removal. Materials 2018, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3, activation: Equilibrium, kinetics, and thermodynamic studies. J. Anal. Appl. Pyrolysis 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, F.; Tang, Q.; Wang, P.; Xu, Z.; Liang, J. Utilization of ultra-light carbon foams for the purification of emulsified oil wastewater and their adsorption kinetics. Chem. Phys. 2019, 516, 139–146. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef]
- Prajapati, Y.N.; Verma, N. Adsorptive desulfurization of diesel oil using nickel nanoparticle-doped activated carbon beads with/without carbon nanofibers: Effects of adsorbate size and adsorbent texture. Fuel 2017, 189, 186–194. [Google Scholar] [CrossRef]
- Rae, I.B.; Gibb, S.W.; Lu, S. Biosorption of Hg from aqueous solutions by crab carapace. J. Hazard. Mater. 2009, 164, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Gibb, S.W.; Cochrane, E. Effective removal of zinc ions from aqueous solutions using crab carapace biosorbent. J. Hazard. Mater. 2007, 149, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Shukla, S.S.; Dorris, K.L. Removal of nickel from aqueous solutions using crab shells. J. Hazard. Mater. 2005, 125, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C. Removal of As(V) from aqueous solutions by waste crab shells. Korean J. Chem. Eng. 2011, 28, 813–816. [Google Scholar] [CrossRef]
- Dai, L.; Tan, F.; Li, H.; Zhu, N.; He, M.; Zhu, Q.; Hu, G.; Wang, L.; Zhao, J. Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J. Environ. Manag. 2017, 198, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Yang, J.; Lin, D. Adsorption and correlations of selected aromatic compounds on a KOH-activated carbon with large surface area. Sci. Total Environ. 2018, 618, 1677–1684. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Liu, T.; Peng, N.; Gai, C. Removal of azo dye by a highly graphitized and heteroatom doped carbon derived from fish waste: Adsorption equilibrium and kinetics. J. Environ. Manag. 2016, 182, 446–454. [Google Scholar] [CrossRef]
- Tian, Z.; Xiang, M.; Zhou, J.; Hu, L.; Cai, J. Nitrogen and oxygen-doped hierarchical porous carbons from algae biomass: Direct carbonization and excellent electrochemical properties. Electrochim. Acta 2016, 211, 225–233. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Wu, Y.; Rong, M. Wood-Derived Carbons with Hierarchical Porous Structures and Monolithic Shapes Prepared by Biological-Template and Self-Assembly Strategies. ACS Sustain. Chem. Eng. 2015, 3, 1724–1731. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Mitome, T.; Uchida, Y.; Egashira, Y.; Hayashi, K.; Nishiura, A.; Nishiyama, N. Adsorption of indole on KOH-activated mesoporous carbon. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 89–95. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Jin, H.; Hanif, M.U.; Capareda, S.; Chang, Z.; Huang, H.; Ai, Y. Copper(ii) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of koh activation. J. Environ. Chem. Eng. 2016, 4, 365–372. [Google Scholar] [CrossRef]
- Li, S.; Han, K.; Li, J.; Li, M.; Lu, C. Preparation and characterization of super activated carbon produced from gulfweed by koh activation. Microporous Mesoporous Mater. 2017, 243, 291–300. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of koh-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; da Cunha, B.M.; Cardoso, N.F.; Brasil, J.L.; Dias, S.L.P. Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solutions. J. Hazard. Mater. 2009, 162, 270–280. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; Cardoso, N.F.; Martins, B.; Calvete, T. Pecan nutshell as biosorbent to remove toxic metals from aqueous solution. Sep. Sci. Technol. 2009, 44, 615–644. [Google Scholar] [CrossRef]
- Shahawy, A.E.; Heikal, G. Organic pollutants removal from oily wastewater using clean technology economically, friendly biosorbent (Phragmites australis). Ecol. Eng. 2018, 122, 207–218. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Bhatia, S.; Ibrahim, N.; Sumathi, S. Adsorption of residual oil from palm oil mill effluent using rubber powder. Braz. J. Chem. Eng. 2005, 22, 371–379. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Adsorption of residue oil from palm oil mill effluent using powder and flake chitosan: Equilibrium and kinetic studies. Water Res. 2005, 39, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, Z.; Sun, D.; Wu, T.; Li, Y. Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J. Ind. Eng. Chem. 2017, 49, 208–218. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mirna, M.M.; Sudaryanto, Y.; Ismadji, S. Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kinetics. Chem. Eng. J. 2007, 127, 121–129. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, G.; Deng, L.; Lu, B.; Li, J.; Zhang, J.; Shi, J.; Du, S. Ultra-deep desulfurization of diesel fuel via selective adsorption over modified activated carbon assisted by pre-oxidation. J. Clean. Prod. 2017, 161, 422–430. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Khan, E.; Virojnagud, W.; Ratpukdi, T. Use of biomass sorbents for oil removal from gas station runoff. Chemosphere 2004, 57, 681–689. [Google Scholar] [CrossRef]
- Ribeiro, T.H.; Rubio, J.; Smith, R.W. A dried hydrophobic aquaphyte as an oil filter for oil/water emulsions. Spill Sci. Technol. B 2003, 8, 483–489. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Ludwick, A.G.; Aglan, H.A. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components. Bioresour. Technol. 2009, 100, 2219–2222. [Google Scholar] [CrossRef]
- Sokker, H.H.; El-Sawy, N.M.; Hassan, M.A.; El-Anadouli, B.E. Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J. Hazard. Mater. 2011, 190, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ngarmkam, W.; Sirisathitkul, C.; Phalakornkule, C. Magnetic composite prepared from palm shell-based carbon and application for recovery of residual oil from POME. J. Environ. Manag. 2011, 92, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, G.; Caruel, H.; Borredon, M.-E.; Bonnin, C.; Vignoles, C. Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ. Sci. Technol. 2003, 37, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Sohaimi, K.S.A.; Ngadi, N.; Mat, H.; Inuwa, I.M.; Wong, S. Synthesis, characterization and application of textile sludge biochars for oil removal. J. Environ. Chem. Eng. 2017, 5, 1415–1422. [Google Scholar] [CrossRef]
- Gobi, K.; Mashitah, M.D.; Vadivelu, V.M. Adsorptive removal of Methylene Blue using novel adsorbent from palm oil mill effluent waste activated sludge: Equilibrium, thermodynamics and kinetics studies. Chem. Eng. J. 2011, 171, 1246–1252. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Fu, D.; Meng, X.; An, Q.; Chu, P.K. Removal of organic pollutants from super heavy oil wastewater by lignite activated coke. Colloid Surf. A 2014, 447, 120–130. [Google Scholar] [CrossRef]
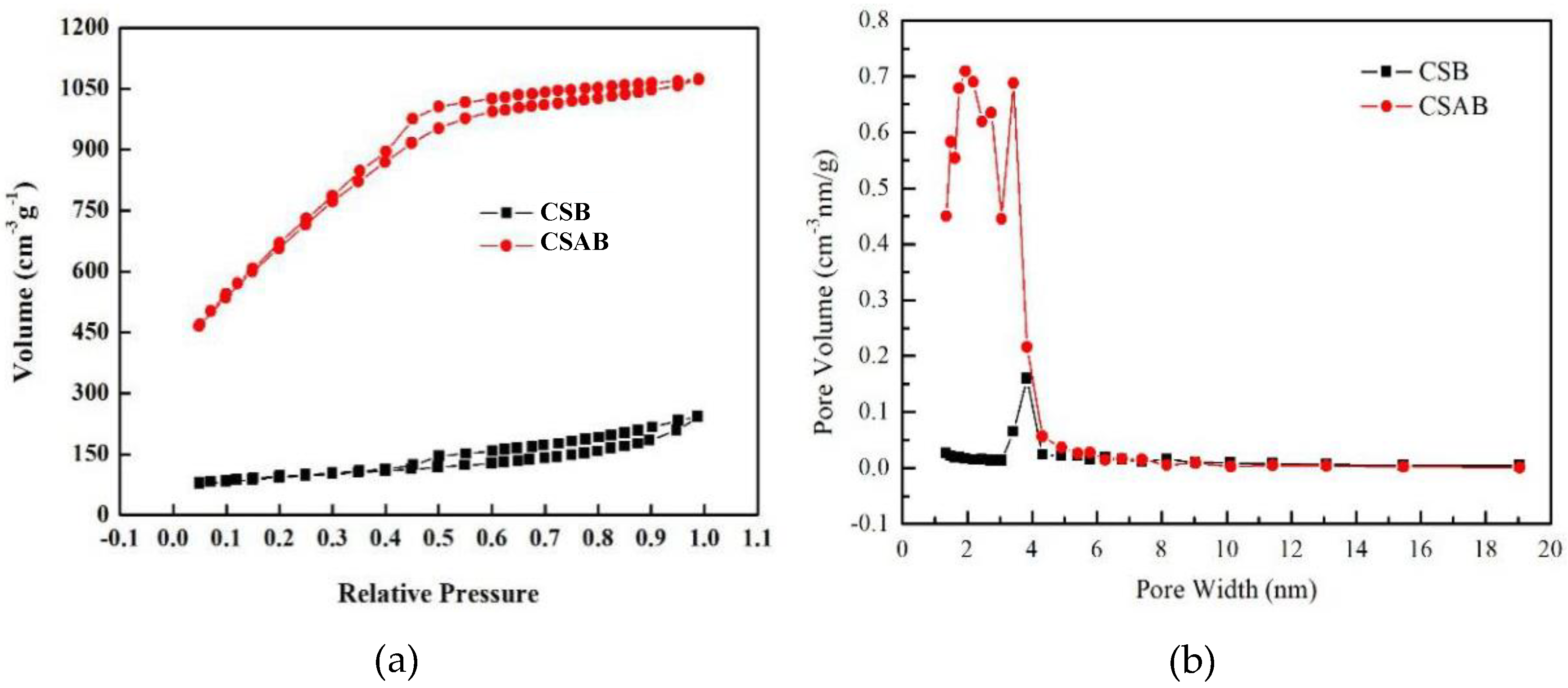


| Sample | Surface Area (m2/g) | Pore Volume (m3/g) | Pore Size (nm) |
|---|---|---|---|
| CSB | 307 | 0.324 | 3.840 |
| CSAB | 2441 | 1.682 | 1.937 |
| Sample | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe, 1 (mg/g) | k1 (min−1) | R2 | qe, 2 (mg/g) | k2 (g/(mg·min) | R2 | |
| CSAB | 79.82 | 0.1662 | 0.979 | 93.9 | 0.023 | 0.993 |
| CSB | 65.02 | 0.15833 | 0.894 | 73.1 | 0.089 | 0.998 |
| Sample | Freundlich Model | Langmuir Model | ||||
|---|---|---|---|---|---|---|
| n | KF | R2 | qm (mg/g) | KL | R2 | |
| CSAB | 1.057 | 1.0617 | 0.999 | 57.74 | 0.0158 | 0.978 |
| CSB | 1.146 | 0.993 | 0.992 | 30.71 | 0.0184 | 0.901 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Zhang, Y.; Zhou, Y.; Zhang, X.; Ji, L.; Song, W.; Zhang, H.; Liu, J. Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials. Materials 2019, 12, 236. https://doi.org/10.3390/ma12020236
Cai L, Zhang Y, Zhou Y, Zhang X, Ji L, Song W, Zhang H, Liu J. Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials. Materials. 2019; 12(2):236. https://doi.org/10.3390/ma12020236
Chicago/Turabian StyleCai, Lu, Yan Zhang, Yarui Zhou, Xiaodie Zhang, Lili Ji, Wendong Song, Hailong Zhang, and Jianshe Liu. 2019. "Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials" Materials 12, no. 2: 236. https://doi.org/10.3390/ma12020236
APA StyleCai, L., Zhang, Y., Zhou, Y., Zhang, X., Ji, L., Song, W., Zhang, H., & Liu, J. (2019). Effective Adsorption of Diesel Oil by Crab-Shell-Derived Biochar Nanomaterials. Materials, 12(2), 236. https://doi.org/10.3390/ma12020236





