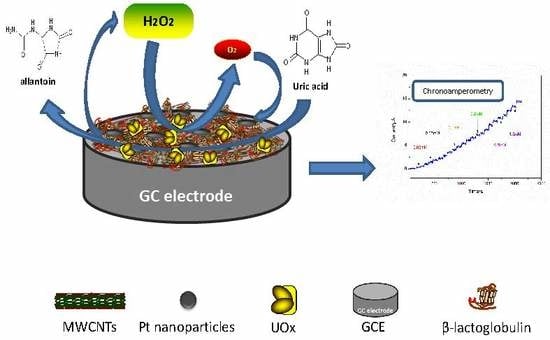
Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
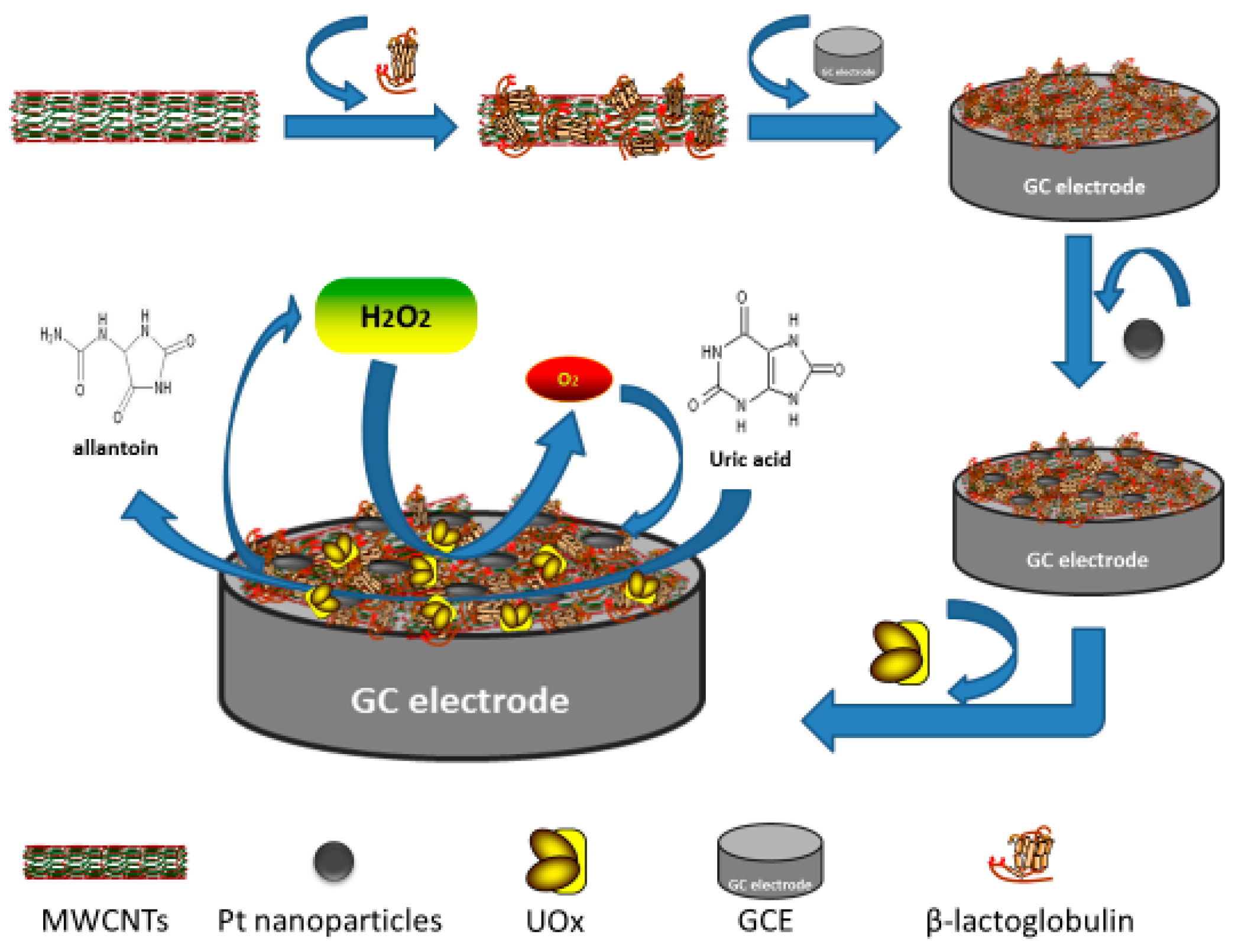
2.2. Preparation of the UOx/BSA/BLG-MWCNTs-PtNPs/GCE
2.3. Apparatus and Measurements
3. Results and Discussion
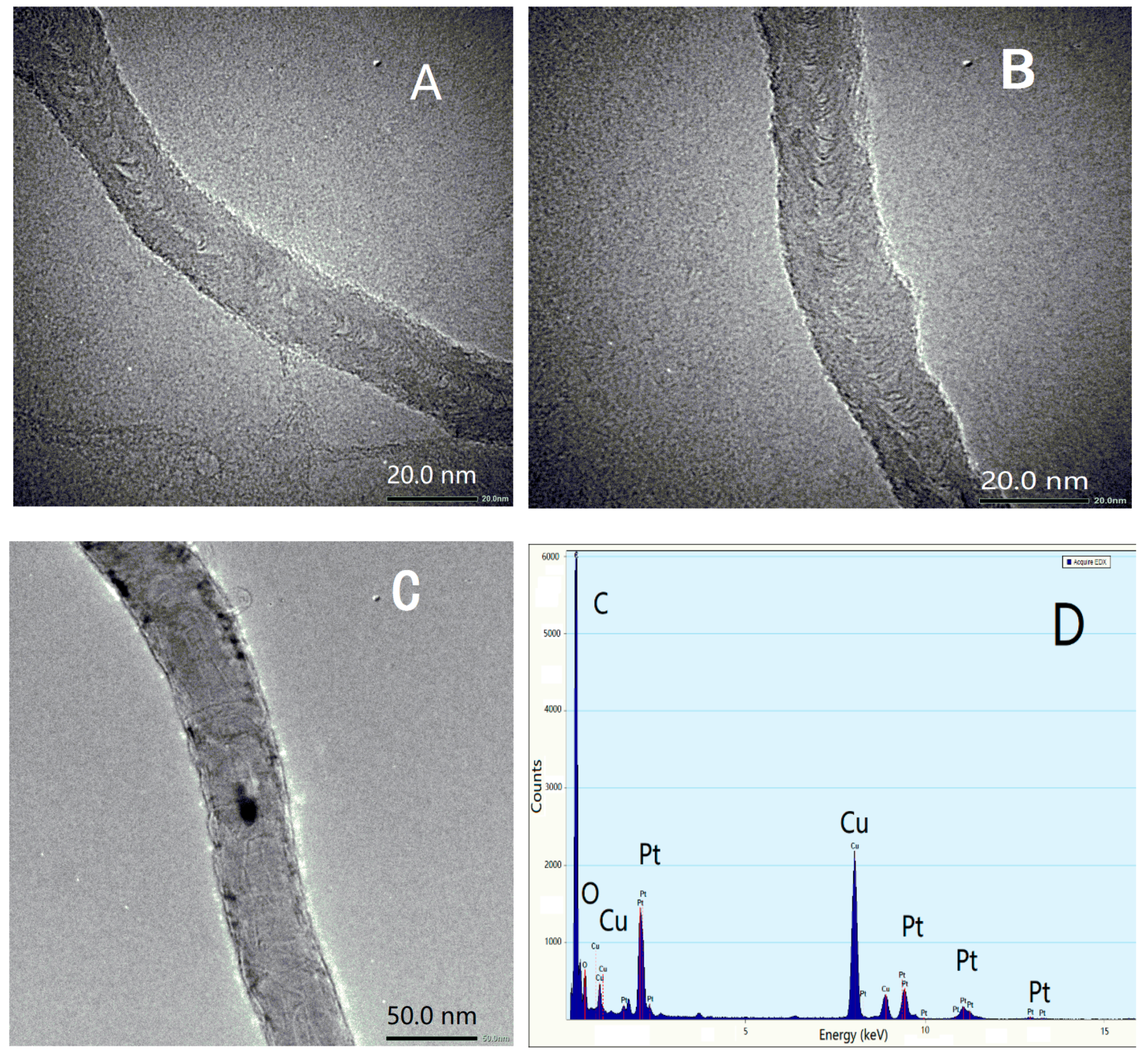
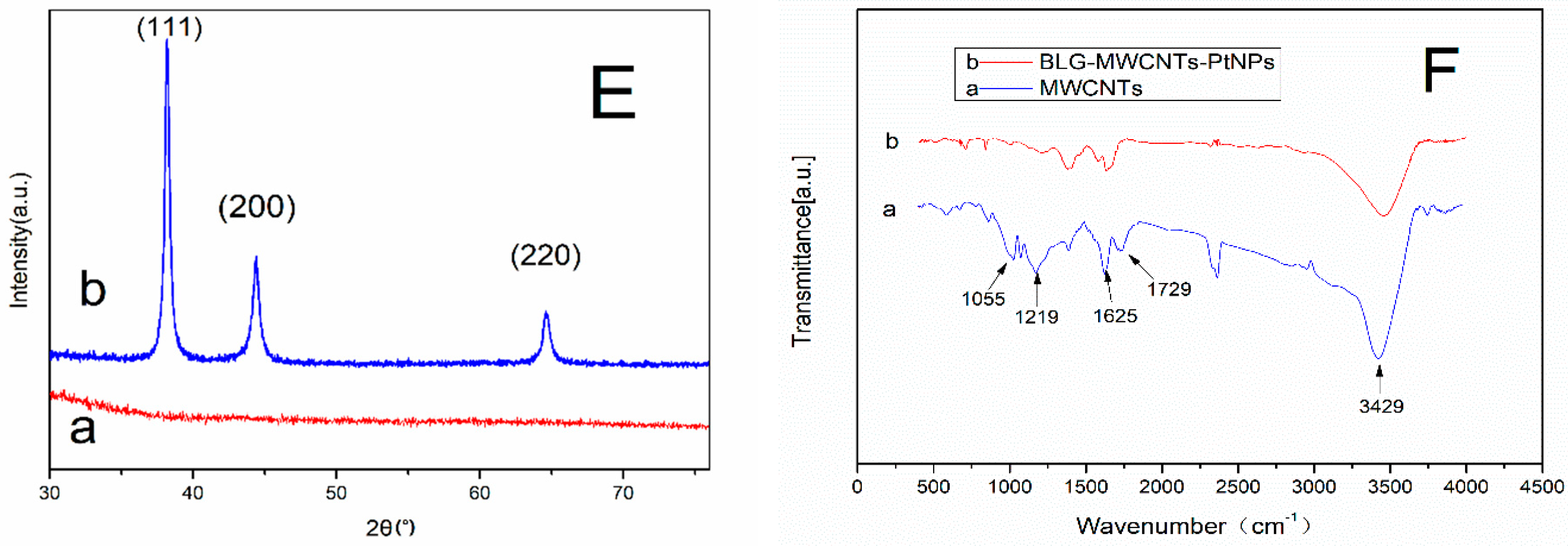
3.1. Morphologies of BLG-MWCNTs-PtNPs Nanocomposites
3.2. Electrochemical Activity of Nanocomposite Electrodes
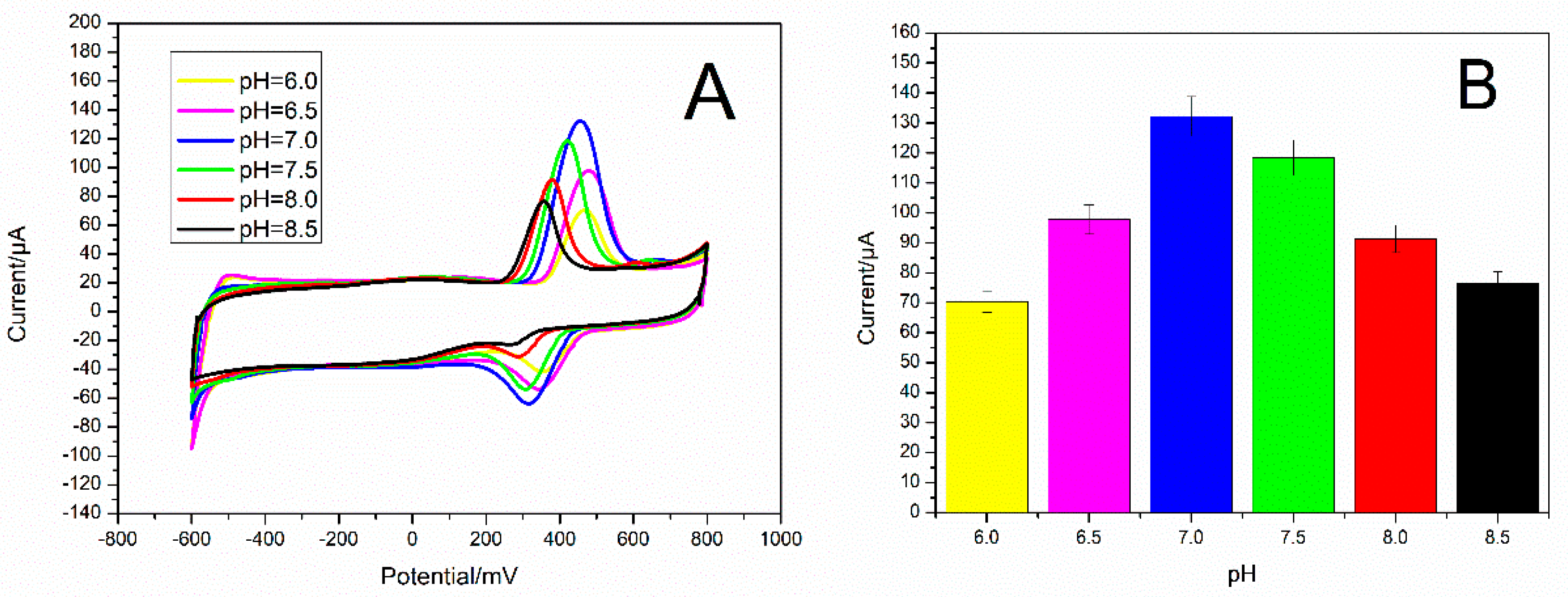
3.3. Optimization of the Testing Environment
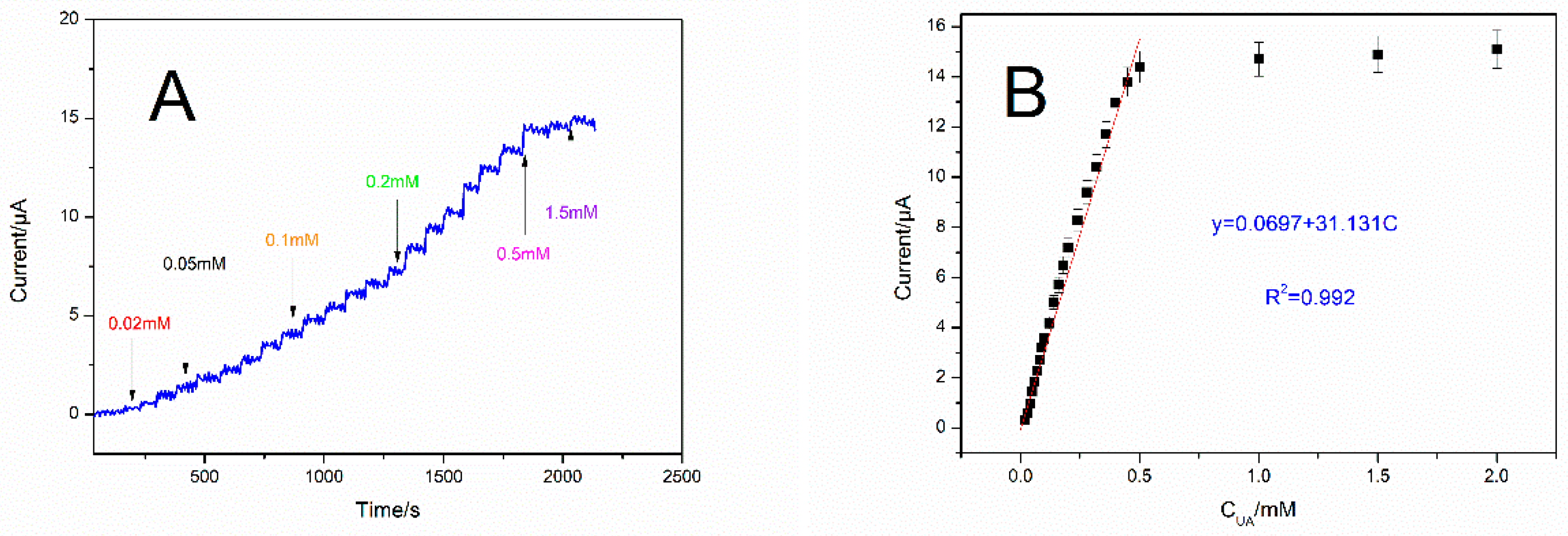
3.4. Response of UOx/BSA/BLG-MWCNTs-PtNPs/GCE to Uric Acid
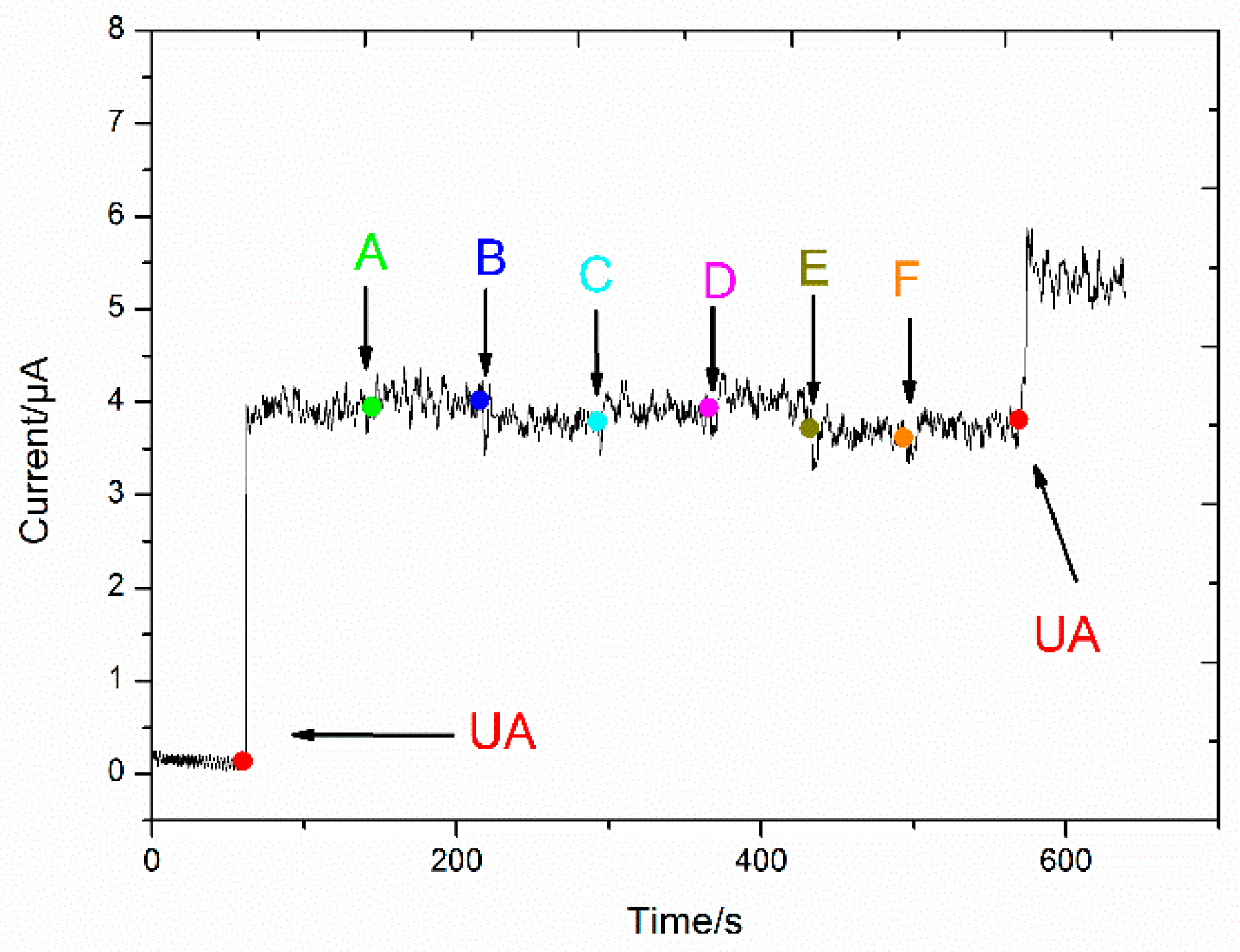
3.5. Reproducibility, Stability, and Selectivity of the UA Biosensor
3.6. Analysis of Uric Acid in Biological Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sinha, A.; Lu, X.; Wu, L.; Tan, D.; Li, Y.; Chen, J.; Jain, R. Voltammetric sensing of biomolecules at carbon based electrode interfaces: A review. Trac Trends Anal. Chem. 2018, 98, 174–189. [Google Scholar]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Narang, J.; Sharma, K.R.; Chauhan, N.; Mishra, A. Amplified electrochemical signal taking polyanline as sensing interface compared to polyindole carboxylic acid. Synth. Met. 2015, 203, 54–58. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Hasan, S.A.; Kapralov, A.A.; Ha, S.H.; Kim, K.; Shvedova, A.A.; Kagan, V.E.; Star, A. A Natural Vanishing Act: The Enzyme-Catalyzed Degradation of Carbon Nanomaterials. Acc. Chem. Res. 2012, 45, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; Ménard-Moyon, C.; Venturelli, E.; Gravel, E.; Marcolongo, G.; Meneghetti, M.; Doris, E.; Bianco, A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale 2011, 3, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Cho, K.J.; Choi, J.S.; Huh, K.M.; Lee, Y.-K. Targeted near-IR QDs-loaded micelles for cancer therapy and imaging. Biomaterials 2010, 31, 5436–5444. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Singhal, R.; Chaubey, A.; Sharma, S.K.; Kumar, A. Recent trends in biosensors. Curr. Appl. Phys. 2005, 5, 92–97. [Google Scholar] [CrossRef]
- Claussen, J.C.; Kumar, A.; Jaroch, D.B.; Khawaja, M.H.; Hibbard, A.B.; Porterfield, D.M.; Fisher, T.S. Nanostructuring Platinum Nanoparticles on Multilayered Graphene Petal Nanosheets for Electrochemical Biosensing. Adv. Funct. Mater. 2012, 22, 3399–3405. [Google Scholar] [CrossRef]
- Sharma, S.; Madou, M. Micro and nano patterning of carbon electrodes for bioMEMS, Bioinspired. Biomim. Nanobiomater. 2012, 1, 252–265. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, Synthesis, and Characterization of Graphene–Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, C.C.; Jannuzzi, S.A.V.; Santhiago, M.; Timm, R.A.; Formiga, A.L.B.; Kubota, L.T. Modified electrode using multi-walled carbon nanotubes and a metallopolymer for amperometric detection of l-cysteine. Electrochim. Acta 2013, 113, 332–339. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.M.; Wu, X.; Liu, X.C.; Dong, W.H.; Han, B.K.; Du, X.; Zhang, C.; Zhang, Y.Y.; Wang, H.T.; et al. An array of poly-l-histidine functionalized multi-walled carbon nanotubes on 4-aminothiophenol self-assembled monolayer and the application for sensitively glucose sensing. Electrochim. Acta 2017, 258, 988–997. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.-M.; Lou, B.-S. A simple strategy for the immobilization of catalase on multi-walled carbon nanotube/poly (l-lysine) biocomposite for the detection of H2O2 and iodate. Biosens. Bioelectron. 2014, 61, 639–647. [Google Scholar] [CrossRef]
- Rafati, A.A.; Afraz, A. Amperometric sensing of anti-HIV drug zidovudine on Ag nanofilm-multiwalled carbon nanotubes modified glassy carbon electrode. Mater. Sci. Eng. C 2014, 39, 105–112. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Gong, Z.; Wang, X.; Zhang, Y.; Gao, J. An Amperometric Immunosensor Based on Multi-Walled Carbon Nanotubes-Thionine-Chitosan Nanocomposite Film for Chlorpyrifos Detection. Sensors 2012, 12, 17247–17261. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, H.; Yang, Y.; He, Y.; Ding, N.; Wang, J.; Wu, Z.; Xiang, K.; Wang, G. Electrochemical immunosensor for casein based on gold nanoparticles and poly(l-Arginine)/multi-walled carbon nanotubes composite film functionalized interface. Biosens. Bioelectron. 2011, 26, 3469–3474. [Google Scholar] [CrossRef]
- Serafín, V.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Determination of prolactin hormone in serum and urine using an electrochemical immunosensor based on poly(pyrrolepropionic acid)/carbon nanotubes hybrid modified electrodes. Sens. Actuators B Chem. 2014, 195, 494–499. [Google Scholar] [CrossRef]
- Karadas, N.; Bozal-Palabiyik, B.; Uslu, B.; Ozkan, S.A. Functionalized carbon nanotubes—With silver nanoparticles to fabricate a sensor for the determination of zolmitriptan in its dosage forms and biological samples. Sens. Actuators B Chem. 2013, 186, 486–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, G.; Guo, Y.; Zhao, W.; Yang, Q.; Sun, X. An electrochemical biosensor based on au nanoparticles decorated reduced graphene oxide for sensitively detecting of hg2+. J. Electroanal. Chem. 2018, 824, 201–206. [Google Scholar] [CrossRef]
- De Souza Ribeiro, F.A.; Tarley, C.R.T.; Borges, K.B.; Pereira, A.C. Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract of Cucurbita pepo L. Sens. Actuators B Chem. 2013, 185, 743–754. [Google Scholar] [CrossRef]
- Alizadeh, T.; Mirzagholipur, S. A Nafion-free non-enzymatic amperometric glucose sensor based on copper oxide nanoparticles–graphene nanocomposite. Sens. Actuators B Chem. 2014, 198, 438–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhang, D.; Ma, M.; Wang, W.; Chen, Q. Nano-assemblies consisting of Pd/Pt nanodendrites and poly (diallyldimethylammonium chloride)-coated reduced graphene oxide on glassy carbon electrode for hydrogen peroxide sensors. Mater. Sci. Eng. C 2016, 58, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ren, Y.; Bai, Z.; Jiao, J.; Chen, Y.; Han, B.; Chen, Q. Synthesis of tetrahexahedral Au-Pd core–shell nanocrystals and reduction of graphene oxide for the electrochemical detection of epinephrine. J. Colloid Interface Sci. 2018, 512, 812–818. [Google Scholar] [CrossRef]
- Riihimäki-Lampén, L.H.; Vainio, M.J.; Vahermo, M.; Pohjala, L.L.; Heikura, J.M.; Valkonen, K.H.; Virtanen, V.T.; Yli-Kauhaluoma, J.T.; Vuorela, P.M. The Binding of Synthetic Retinoids to Lipocalin β-Lactoglobulins. J. Med. Chem. 2010, 53, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L.; Kontopidis, G. The core lipocalin, bovine β-lactoglobulin, Biochimica et Biophysica Acta (BBA). Protein Struct. Mol. Enzymol. 2000, 1482, 136–148. [Google Scholar] [CrossRef]
- Du, X.; Miao, Z.; Zhang, D.; Fang, Y.; Ma, M.; Chen, Q. Facile synthesis of β-lactoglobulin-functionalized multi-wall carbon nanotubes and gold nanoparticles on glassy carbon electrode for electrochemical sensing. Biosens. Bioelectron. 2014, 62, 73–78. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Miao, Z.; Ma, M.; Du, X.; Lin, J.; Han, B.; Takahashi, S.; Anzai, J.I.; Chen, Q. Dual-function amperometric sensors based on poly(diallydimethylammoniun chloride)-functionalized reduced graphene oxide/manganese dioxide/gold nanoparticles nanocomposite. Sens. Actuators B Chem. 2016, 222, 663–673. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, L. Polyvinyl pyrrolidone wrapped Sn nanoparticles/carbon Xerogel composite as anode material for high performance lithium ion batteries. Electrochim. Acta 2016, 212, 594–602. [Google Scholar] [CrossRef]
- Huang, L.; Jiao, S.; Li, M. Determination of uric acid in human urine by eliminating ascorbic acid interference on copper(II)-polydopamine immobilized electrode surface. Electrochim. Acta 2014, 121, 233–239. [Google Scholar] [CrossRef]
- Kumar, V.; Misra, N.; Paul, J.; Dhanawade, B.R.; Varshney, L. Uricase-immobilization on radiation grafted polymer support for detection of uric acid using Ag-nanoparticle based optical biosensor. Polymer 2014, 55, 2652–2660. [Google Scholar] [CrossRef]
- Azmi, N.E.; Ramli, N.I.; Abdullah, J.; Hamid, M.A.; Sidek, H.; Rahman, S.A.; Ariffin, N.; Yusof, N.A. A simple and sensitive fluorescence based biosensor for the determination of uric acid using H2O2-sensitive quantum dots/dual enzymes. Biosens. Bioelectron. 2015, 67, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, S.; Migliorelli, D.; Volpe, G.; Massoud, R.; Pierantozzi, A.; Cortese, C.; Palleschi, G. Uricase biosensor based on a screen-printed electrode modified with Prussian blue for detection of uric acid in human blood serum. Sens. Actuators B Chem. 2013, 179, 170–174. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2010, 144, 220–225. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Zou, X.; Dong, H.; Yao, P. Selective oxidation of styrene to 1,2-epoxyethylbenzene by hydrogen peroxide over heterogeneous phosphomolybdic acid supported on ionic liquid modified MCM-41. Chem. Eng. J. 2015, 260, 172–177. [Google Scholar] [CrossRef]
- Ghosh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar diffusion to macro disc electrodes—What electrode size is required for the Cottrell and Randles-Sevcik equations to apply quantitatively. J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Blanc, R.; González-Casado, A.; Navalón, A.; Vílchez, J.L. On the estimate of blanks in differential pulse voltammetric techniques: Application to detection limits evaluation as recommended by iupac. Anal. Chim. Acta 2000, 403, 117–123. [Google Scholar] [CrossRef]
- Wu, W.; Min, H.; Wu, H.; Ding, Y.; Yang, S. Electrochemical determination of uric acid using a multiwalled carbon nanotube platinum–nickel alloy glassy carbon electrode. Anal. Lett. 2017, 50, 91–104. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Lozano, M.L.; Galicia, L.; Ferreyra, N.F.; Rivas, G.A. Electrochemical sensing of uric acid using glassy carbon modified with multiwall carbon nanotubes dispersed in polyethylenimine. Electroanalysis 2014, 26, 2191–2196. [Google Scholar] [CrossRef]
- Omar, M.N.; Salleh, A.B.; Lim, H.N.; Ahmad Tajudin, A. Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal. Biochem. 2016, 509, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, F.S.; de Souza Paula, F.; Franco, D.L.; dos Santos, W.T.; Ferreira, L.F. Electrochemical detection of uric acid using graphite screen-printed electrodes modified with prussian blue/poly(4-aminosalicylic acid)/uricase. J. Electroanal. Chem. 2017, 806, 172–179. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of a sandwich format, amperometric screen-printed uric acid biosensor for urine analysis. Anal. Biochem. 2012, 428, 39–43. [Google Scholar] [CrossRef]

| Immobilization Materials | Detection Technique | Detection Limit (µM) | Linear Range (µM) | pH | Reference |
|---|---|---|---|---|---|
| Platinum–nickel/multiwalled carbon nanotubes | Cyclic voltammetry | 0.03 | 0.1–240.4 | 7.0 | [42] |
| Polyethylenimine-multiwall carbon nanotubes/GCE | Differential pulse voltammetry | 0.1 | 0.5–50 | 7.4 | [43] |
| Graphene oxide-uricase/GCE | Cyclic voltammetry | 3.45 | 20–490 | 6.5 | [44] |
| Graphite screen-printed electrodes/Prussian blue/poly(4-aminosalicylic acid)/uricase | Chronoamperometry | 3.0 | 10–200 | 8.27 | [45] |
| Cellulose acetate deposited screen-printed carbon electrode pre-modified with cobalt phthalocyanine | Chronoamperometry | 15 | 15–250 | 9.2 | [46] |
| Screen-printed electrode modified with Prussian blue/uricase | Chronoamperometry | 10 | 30–300 | 8.5 | [36] |
| UOx/BSA/BLG-MWCNTs-PtNPs/GCE | Chronoamperometry | 0.8 | 20–500 | 7.0 | This work |
| Sample | Added UA (mM) | Calculated UA (mM) | Recovery (%) UA | RSD (%) UA |
|---|---|---|---|---|
| 1 | 0.200 | 0.191 | 95.50 | 2.97 |
| 2 | 0.300 | 0.322 | 107.33 | 4.18 |
| 3 | 0.400 | 0.423 | 105.75 | 3.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Pan, M.; Liu, X.; Liu, J.; Cui, T.; Chen, Q. Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite. Materials 2019, 12, 214. https://doi.org/10.3390/ma12020214
Han B, Pan M, Liu X, Liu J, Cui T, Chen Q. Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite. Materials. 2019; 12(2):214. https://doi.org/10.3390/ma12020214
Chicago/Turabian StyleHan, Bingkai, Meixin Pan, Xinran Liu, Jian Liu, Teng Cui, and Qiang Chen. 2019. "Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite" Materials 12, no. 2: 214. https://doi.org/10.3390/ma12020214
APA StyleHan, B., Pan, M., Liu, X., Liu, J., Cui, T., & Chen, Q. (2019). Electrochemical Detection for Uric Acid Based on β-Lactoglobulin-Functionalized Multiwall Carbon Nanotubes Synthesis with PtNPs Nanocomposite. Materials, 12(2), 214. https://doi.org/10.3390/ma12020214