Crystallization Products and Structural Characterization of CaO-SiO2-Based Mold Fluxes with Varying Al2O3/SiO2 Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Differential Scanning Calorimetry Analysis
2.3. X-ray Diffraction and Scanning Electron Microscope Analysis
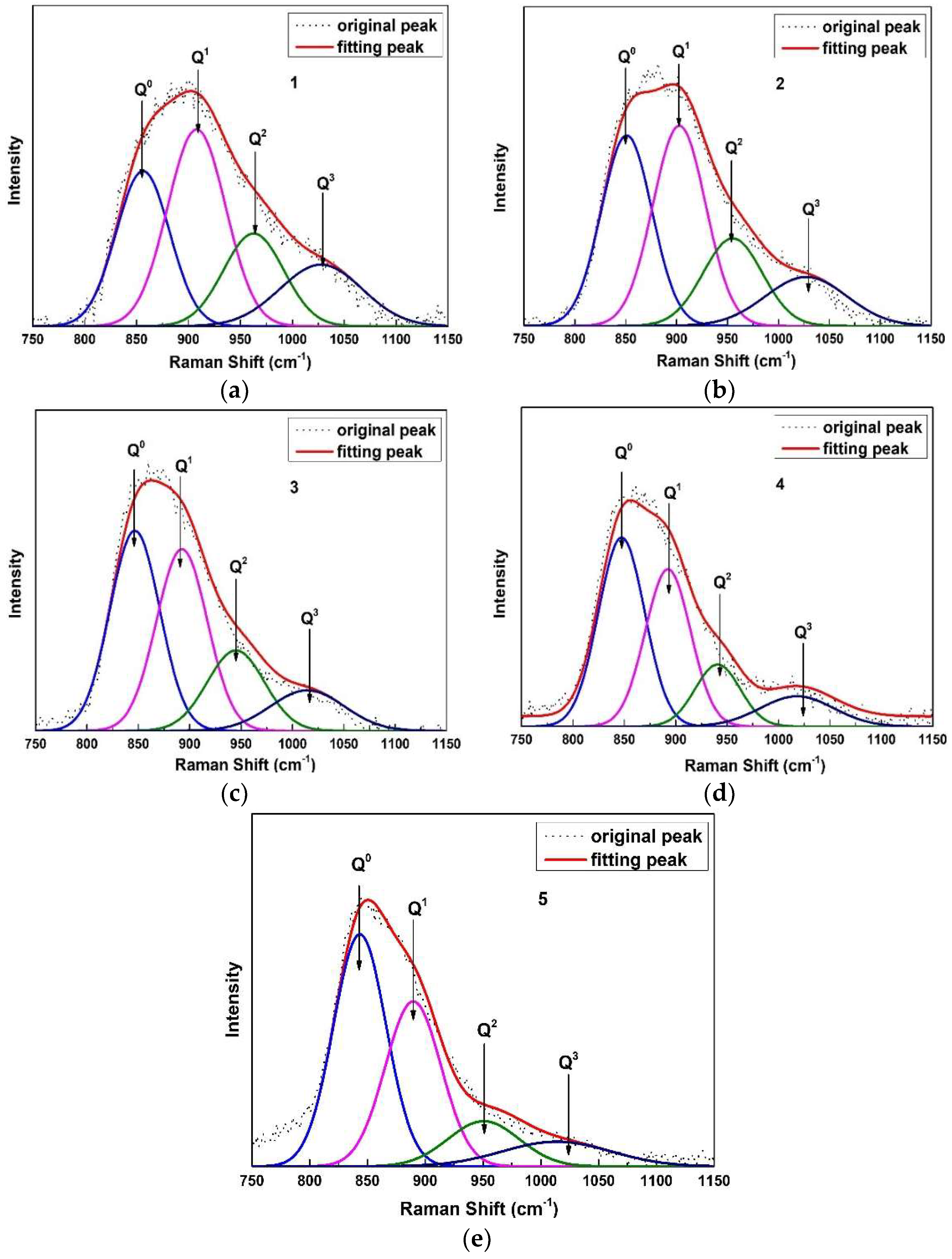
2.4. Raman Spectroscopy Analysis
3. Results and Discussion
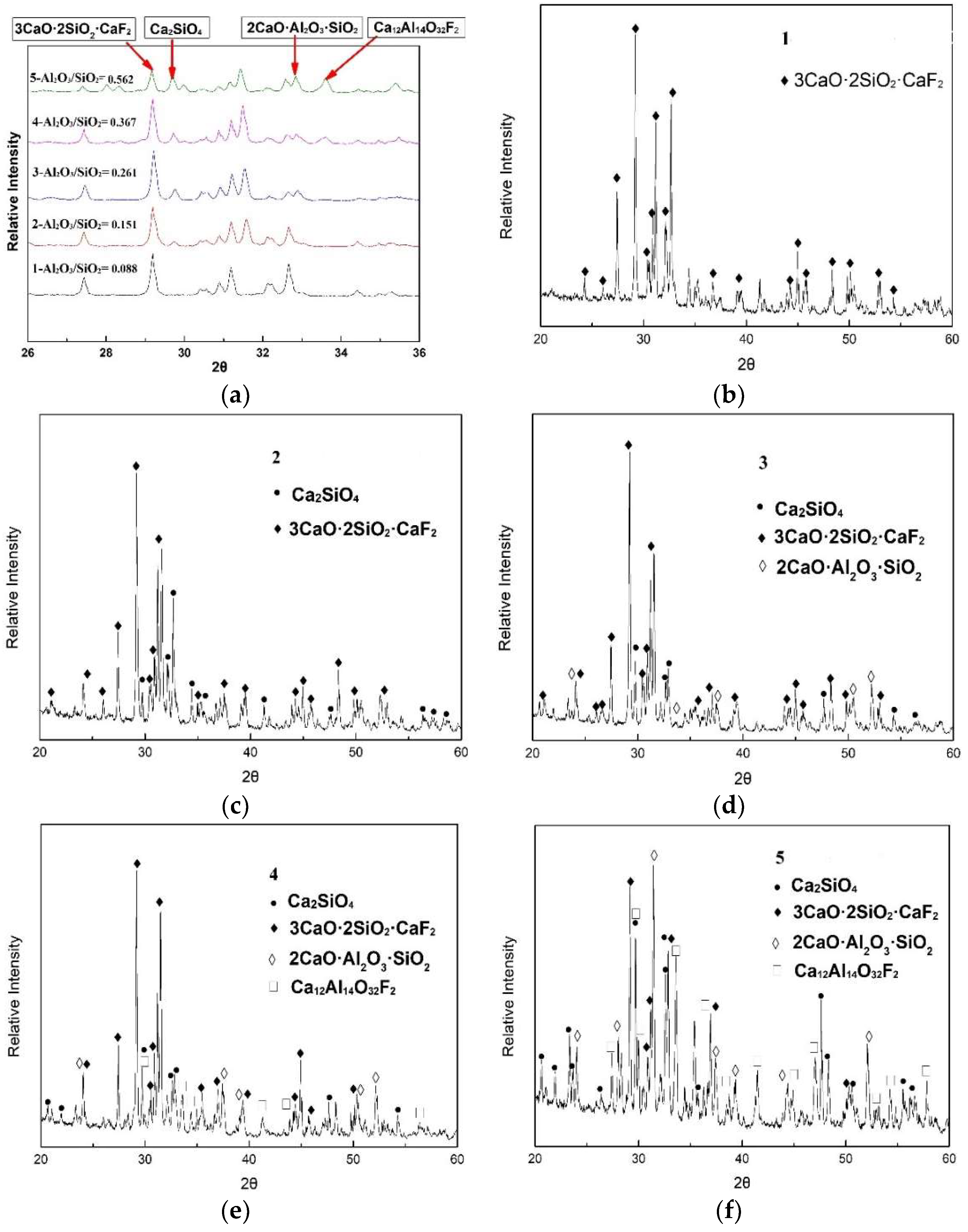
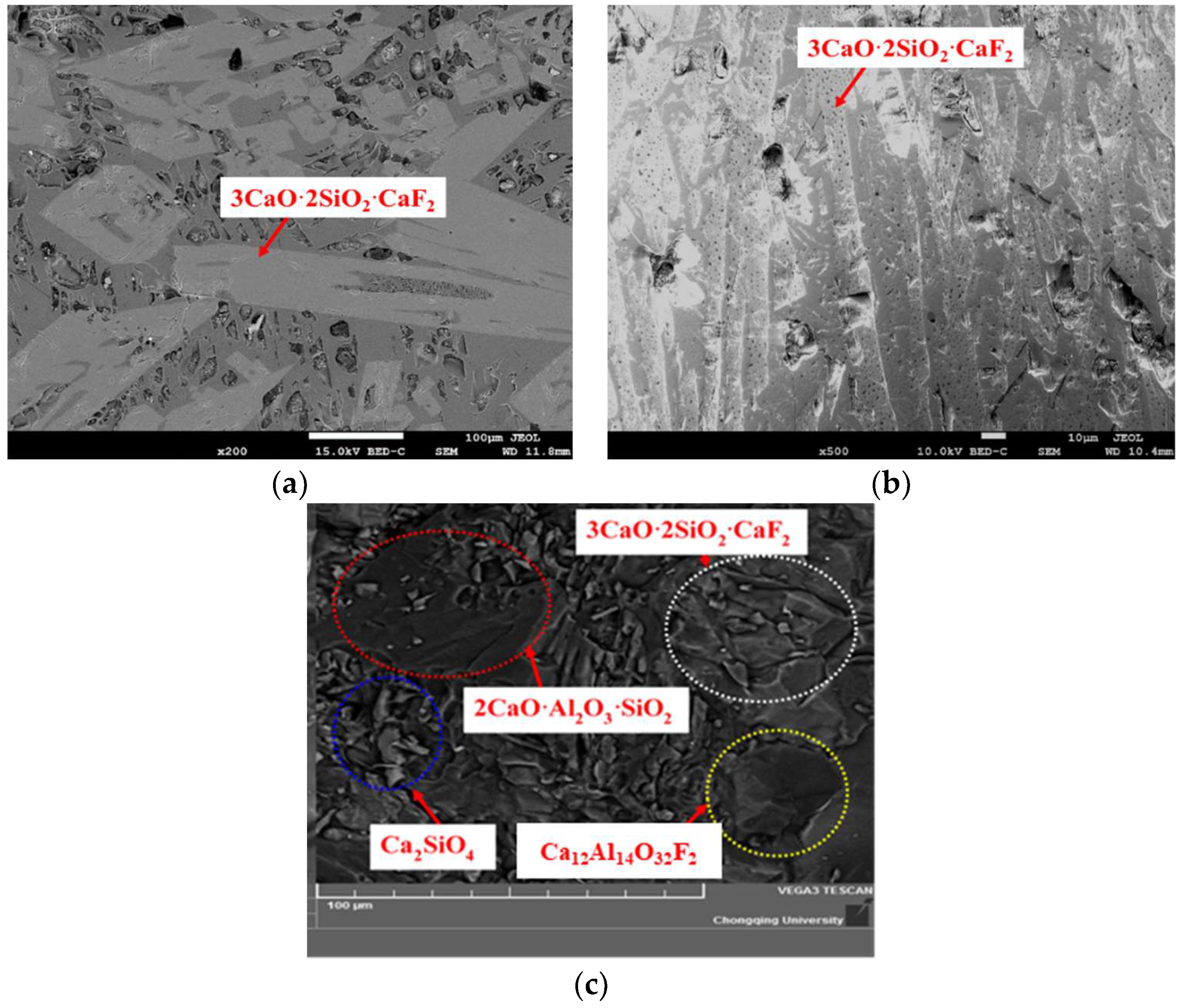
3.1. Crystallization Analysis of CaO-SiO2-Based Mold Fluxes
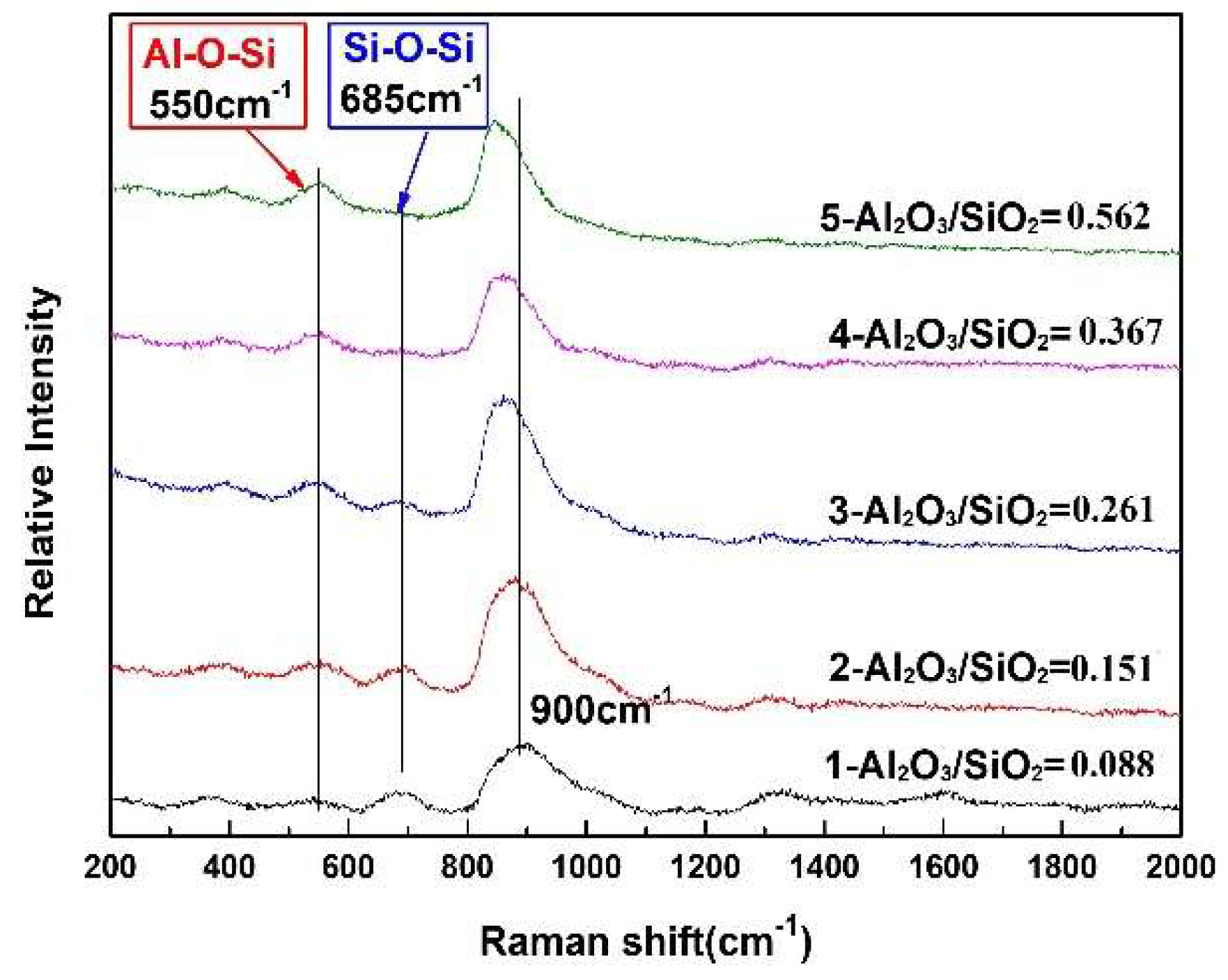
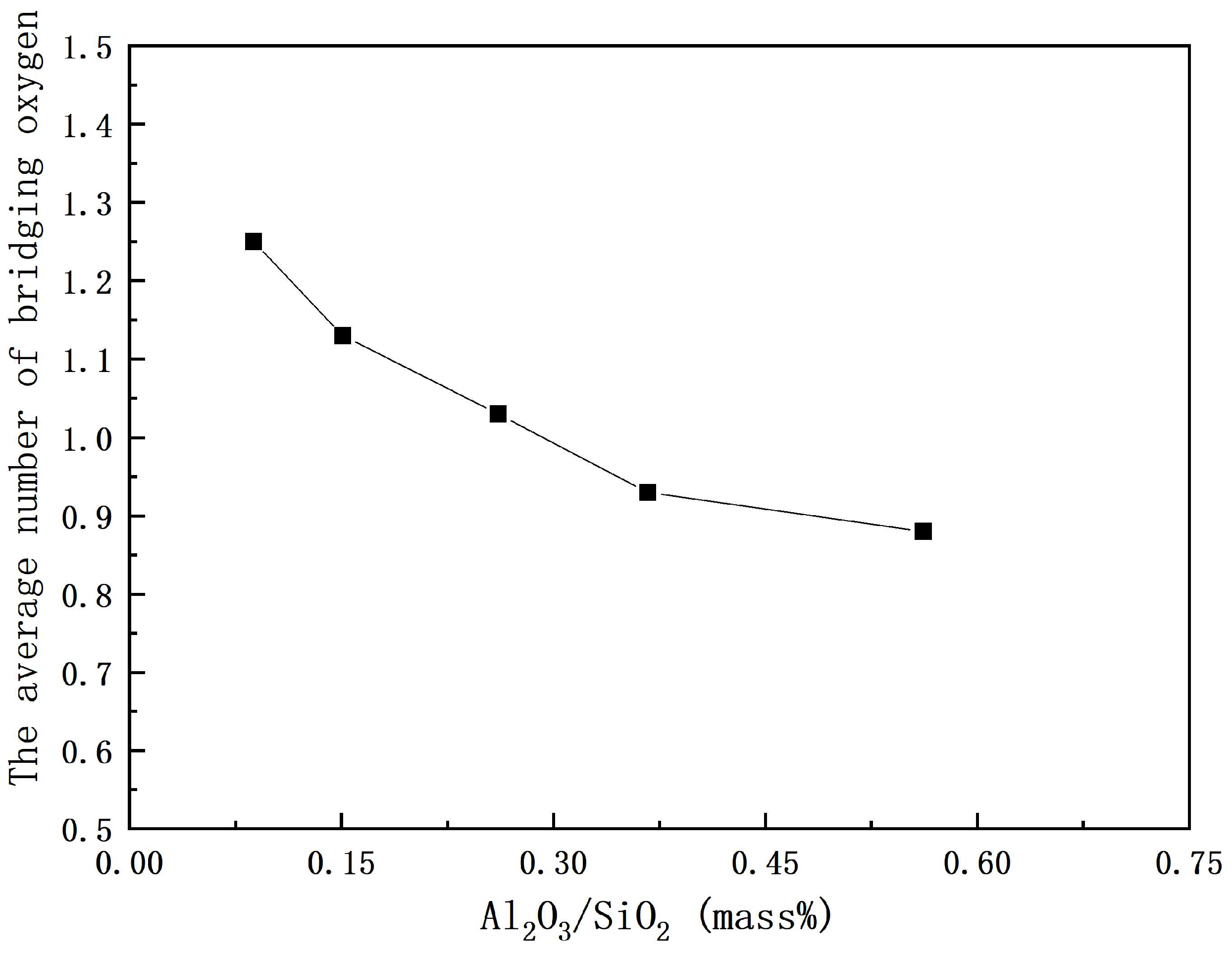
3.2. Structural Analysis of CaO-SiO2-Based Mold Fluxes
4. Conclusions
- (1)
- The crystallization temperature and the crystallization products have been changed. The crystallization greatly increases from 1152 °C to 1354 °C with the Al2O3/SiO2 ratio changing from 0.147 to 0.258, and then it increases slowly. The crystalline phases are increased from two kinds (Ca4Si2O7F2 and Ca2SiO4) to four kinds (Ca4Si2O7F2, Ca2SiO4, 2CaO·Al2O3·SiO2 and Ca12Al14O32F2). The crystallization ability of cuspidine decreases, but the other species show the opposite trend.
- (2)
- Two types of bridge oxygen linkages, i.e., Al–O–Si and Si–O–Si, are formed in CaO-SiO2-based mold fluxes. The polymerization degree of the network and the average number of bridging oxygens decrease. The relatively strong Si–O–Si linkage gradually decreases and the relatively weak Al–O–Si bond gradually increases, which cause the weaker link of the molten fluxes.
- (3)
- The gradual increase of the weaker Al–O and the decrease in the amount of the stronger Si–O bond, which causes the relatively weaker connections of the network of the mold flux, give rise to the lower energy barrier for ions transferring from bulk glass to the glass-crystal interface during crystallization. Consequently, the crystallization ability increases.
- (4)
- The increase in the Al–O–Si linkage in molten slag would increase the similarity between the molten slag and crystals containing both Si and Al, which would induce the precipitation of 2CaO·Al2O3·SiO2 crystal containing both Si and Al from the CaO-SiO2-CaF2 system. The gradual decrease in the amount of Q1 in the CaO-SiO2-CaF2-based mold flux would decrease the similarity between the molten slag and cuspidine and reduce the nucleation and growth of cuspidine.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sohn, S.S.; Choi, K.; Kwak, J.H.; Kim, N.J.; Lee, S. Novel ferrite–austenite duplex lightweight steel with 77% ductility by transformation induced plasticity and twinning induced plasticity mechanisms. Acta Mater. 2014, 78, 181–189. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Ben-Shan, Y.U. Key technology for skeleton structure of automobile body with steel and aluminum intergration and latest development. Mach. Des. Manuf. 2012, 1, 251–253. [Google Scholar]
- Kim, M.-S.; Lee, S.-W.; Cho, J.-W.; Park, M.-S.; Lee, H.-G.; Kang, Y.-B. A Reaction Between High Mn-High Al Steel and CaO-SiO2-Type Molten Mold Flux: Part I. Composition Evolution in Molten Mold Flux. Metall. Mater. Trans. B 2013, 44, 299–308. [Google Scholar] [CrossRef]
- Li, J.; Yan, B.; Shu, Q.; Chou, K. Structure and crystallization kinetics of glassy CaO-Al2O3-SiO2-CaF2-Na2O mold fluxes with varying basicity. Metall. Mater. Trans. B 2015, 46, 2458–2469. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Xie, S.; Zhang, K.; Sohn, I. Effect of basicity and B2O3 on the viscosity and structure of fluorine-free mold flux. J. Non-Cryst. Solids 2017, 460, 113–118. [Google Scholar] [CrossRef]
- Fukuyama, H.; Tabata, H.; Nagata, K. Determination of gibbs energy of formation of cuspidine (3CaO·2SiO2·CaF2 ) from the electromotive force method using CaF2 as the solid electrolyte. Metall. Mater. Trans. B 2003, 34, 307–311. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Wen, G.H.; Liao, J.L.; Sridhar, S. Observations of crystallization in mold slags with varying Al2O3/SiO2 ratio. Steel Res. Int. 2010, 81, 516–528. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Chou, K. Crystallization Kinetics and Structure of Mold Fluxes with SiO2 Being Substituted by TiO2 for Casting of Titanium-Stabilized Stainless Steel. Metall. Mater. Trans. B 2013, 44, 606–613. [Google Scholar] [CrossRef]
- Li, J.; Shu, Q.; Chou, K. Structural Study of Glassy CaO-SiO2-CaF2-TiO2 Slags by Raman Spectroscopy and MAS-NMR. ISIJ Int. 2014, 54, 721–727. [Google Scholar] [CrossRef]
- Cui, S.P.; Liu, L.L.; Chen, J.; Wang, Y.L.; Wang, J.F.; Wang, H.; Dong, S.J. Influence of SiO2/Al2O3 Content on Structure and Hydration Activity of Granulated Blast Furnace Slag. Key Eng. Mater. 2015, 633, 240–244. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Sridhar, S.; Wang, X.; Zhang, Z. Effect of Al2O3/SiO2 Ratio on the Viscosity and Structure of Slags. ISIJ Int. 2012, 52, 753–758. [Google Scholar] [CrossRef]
- Seo, M.-D.; Shi, C.-B.; Cho, J.-W.; Kim, S.-H. Crystallization Behaviors of CaO-SiO2-Al2O3-Na2O-CaF2-(Li2O-B2O3) Mold Fluxes. Metall. Mater. Trans. B 2014, 45, 1874–1886. [Google Scholar] [CrossRef]
- Li, J.; Shu, Q.; Chou, K. Phase Relations in CaO-SiO2-Al2O3-15 mass pct CaF2 System at 1523 K (1250 °C). Metall. Mater. Trans. B 2014, 45, 1593–1599. [Google Scholar] [CrossRef]
- Guo, J.; Seo, M.-D.; Shi, C.-B.; Cho, J.-W.; Kim, S.-H. Control of Crystal Morphology for Mold Flux During High-Aluminum AHSS Continuous Casting Process. Metall. Mater. Trans. B 2016, 47, 2211–2221. [Google Scholar] [CrossRef]
- Cho, J.-W.; Blazek, K.; Frazee, M.; Yin, H.; Park, J.H.; Moon, S.-W. Assessment of CaO-Al2O3 Based Mold Flux System for High Aluminum TRIP Casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef]
- Chah, K.; Boizot, B.; Reynard, B.; Ghaleb, D.; Petite, G. Micro-Raman and EPR studies of β-radiation damages in aluminosilicate glass. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2002, 191, 337–341. [Google Scholar] [CrossRef]
- Sharma, S.K.; Simons, B.; Yoder, H.S. Raman study of anorthite, calcium Tschermak’s pyroxene, and gehlenite in crystalline and glassy states. Am. Mineral. 1983, 68, 1113–1125. [Google Scholar]
- Mysen, B.O.; Virgo, D.; Seifert, F.A. Relationships between properties and structure of aluminosilicate melts. Am. Mineral. 1985, 70, 88–105. [Google Scholar]
- Lu, K.; Mahapatra, M.K. Network structure and thermal stability study of high temperature seal glass. J. Appl. Phys. 2008, 104, 074910–074919. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–97. [Google Scholar]
- Mysen, B.O.; Finger, L.W.; Virgo, D.; Seifert, F.A. Curve-fitting of Raman spectra of silicate glasses. Am. Mineral. 1982, 67, 686–695. [Google Scholar]
- Wang, Z.; Shu, Q.; Chou, K. Study on Structure Characteristics of B2O3 and TiO2-bearing F-Free Mold Flux by Raman Spectroscopy. High Temp. Mater. Process. 2013, 32, 265–273. [Google Scholar] [CrossRef]
- Frantza, J.D.; Mysen, B.O. Raman spectra and structure of BaO-SiO2 SrO-SiO2 and CaO-SiO2 melts to 1600 °C. Chem. Geol. 1995, 121, 155–176. [Google Scholar] [CrossRef]
- Alberto, H.V.; Ayres de Campos, N.; Mysen, B.O. The structural role of titanium in silicate glasses: A Raman study of the system CaO-SiO2-TiO2. Phys. Chem. Glasses 1995, 36, 114–122. [Google Scholar]
- Smith, K.A.; Kirkpatrick, R.J.; Oldfield, E.; Henderson, D.M. High-resolution silicon-29 nuclear magetic resonance spectroscopic study of rock-forming silicates. Am. Mineral. 1983, 68, 1206–1215. [Google Scholar]
- Saburi, S.; Kawahara, A.; Henmi, C.; Kusachi, I.; Kihara, K. The refinement of the crystal structure of cuspidine. Mineral. J. 1977, 8, 286–298. [Google Scholar] [CrossRef]


| Sample No. | Composition (mass%/mole%) | |||||
|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | CaF2 | Na2O | Al2O3/SiO2 | |
| 1 | 38.0/41.9 | 34.0/35.1 | 5.0/3.1 | 15.0/11.9 | 8.0/8.0 | 0.147/0.088 |
| 2 | 38.0/42.5 | 31.0/32.4 | 8.0/4.9 | 15.0/12.1 | 8.0/8.1 | 0.258/0.151 |
| 3 | 38.0/43.3 | 27.0/28.7 | 12.0/7.5 | 15.0/12.3 | 8.0/8.2 | 0.444/0.261 |
| 4 | 38.0/43.9 | 24.0/25.9 | 15.0/9.5 | 15.0/12.4 | 8.0/8.3 | 0.625/0.367 |
| 5 | 38.0/44.7 | 20.0/21.9 | 19.0/12.3 | 15.0/12.6 | 8.0/8.5 | 0.950/0.562 |
| Sample No. | Q0 | Q1 | Q2 | Q3 | Ni |
|---|---|---|---|---|---|
| 1 | 27.10 | 37.56 | 18.46 | 16.88 | 1.25 |
| 2 | 32.63 | 35.94 | 17.70 | 13.73 | 1.13 |
| 3 | 37.03 | 34.43 | 17.26 | 11.28 | 1.03 |
| 4 | 41.62 | 34.16 | 13.46 | 10.77 | 0.93 |
| 5 | 44.46 | 33.67 | 11.86 | 10.01 | 0.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Leng, M.; Chen, Y.; Chen, Z.; Li, J. Crystallization Products and Structural Characterization of CaO-SiO2-Based Mold Fluxes with Varying Al2O3/SiO2 Ratios. Materials 2019, 12, 206. https://doi.org/10.3390/ma12020206
Gao Y, Leng M, Chen Y, Chen Z, Li J. Crystallization Products and Structural Characterization of CaO-SiO2-Based Mold Fluxes with Varying Al2O3/SiO2 Ratios. Materials. 2019; 12(2):206. https://doi.org/10.3390/ma12020206
Chicago/Turabian StyleGao, Yuxiang, Mei Leng, Yangfan Chen, Zhichao Chen, and Jiangling Li. 2019. "Crystallization Products and Structural Characterization of CaO-SiO2-Based Mold Fluxes with Varying Al2O3/SiO2 Ratios" Materials 12, no. 2: 206. https://doi.org/10.3390/ma12020206
APA StyleGao, Y., Leng, M., Chen, Y., Chen, Z., & Li, J. (2019). Crystallization Products and Structural Characterization of CaO-SiO2-Based Mold Fluxes with Varying Al2O3/SiO2 Ratios. Materials, 12(2), 206. https://doi.org/10.3390/ma12020206




