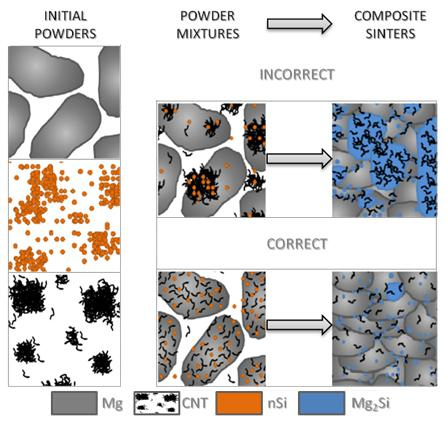
Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Powder Mixtures
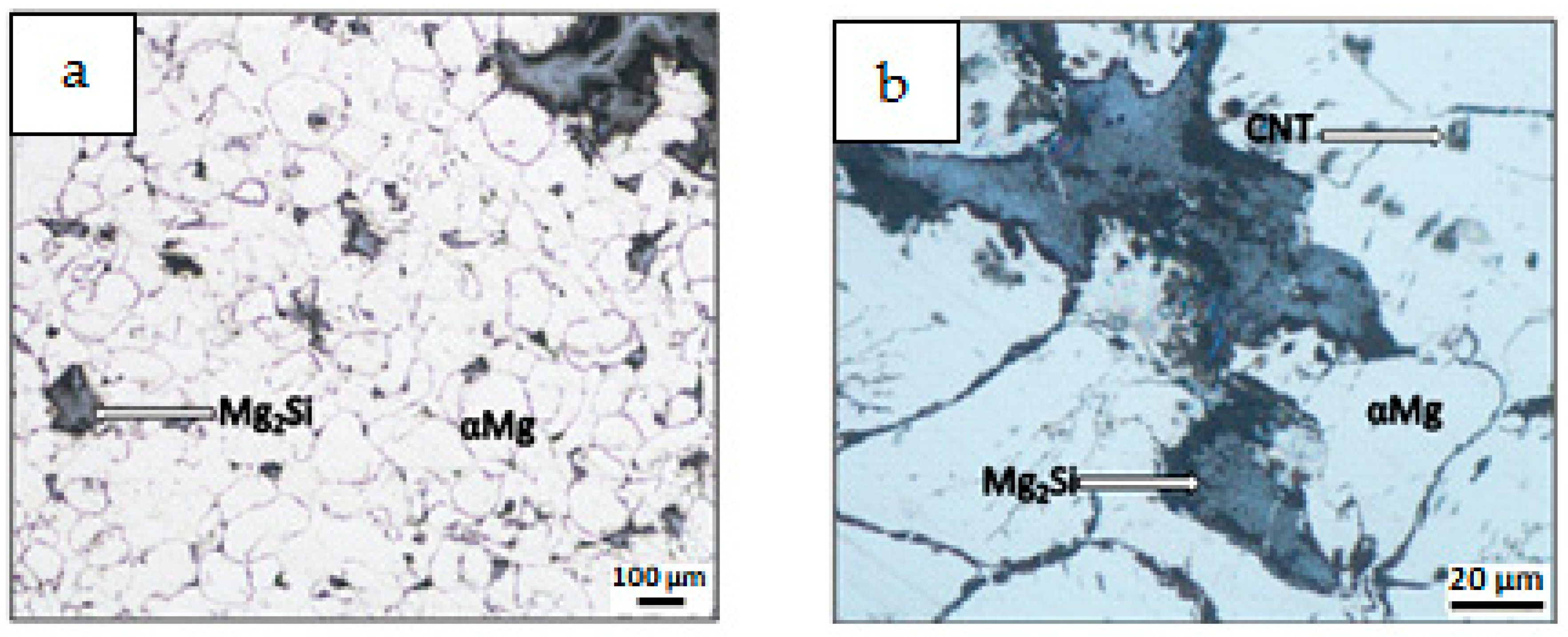
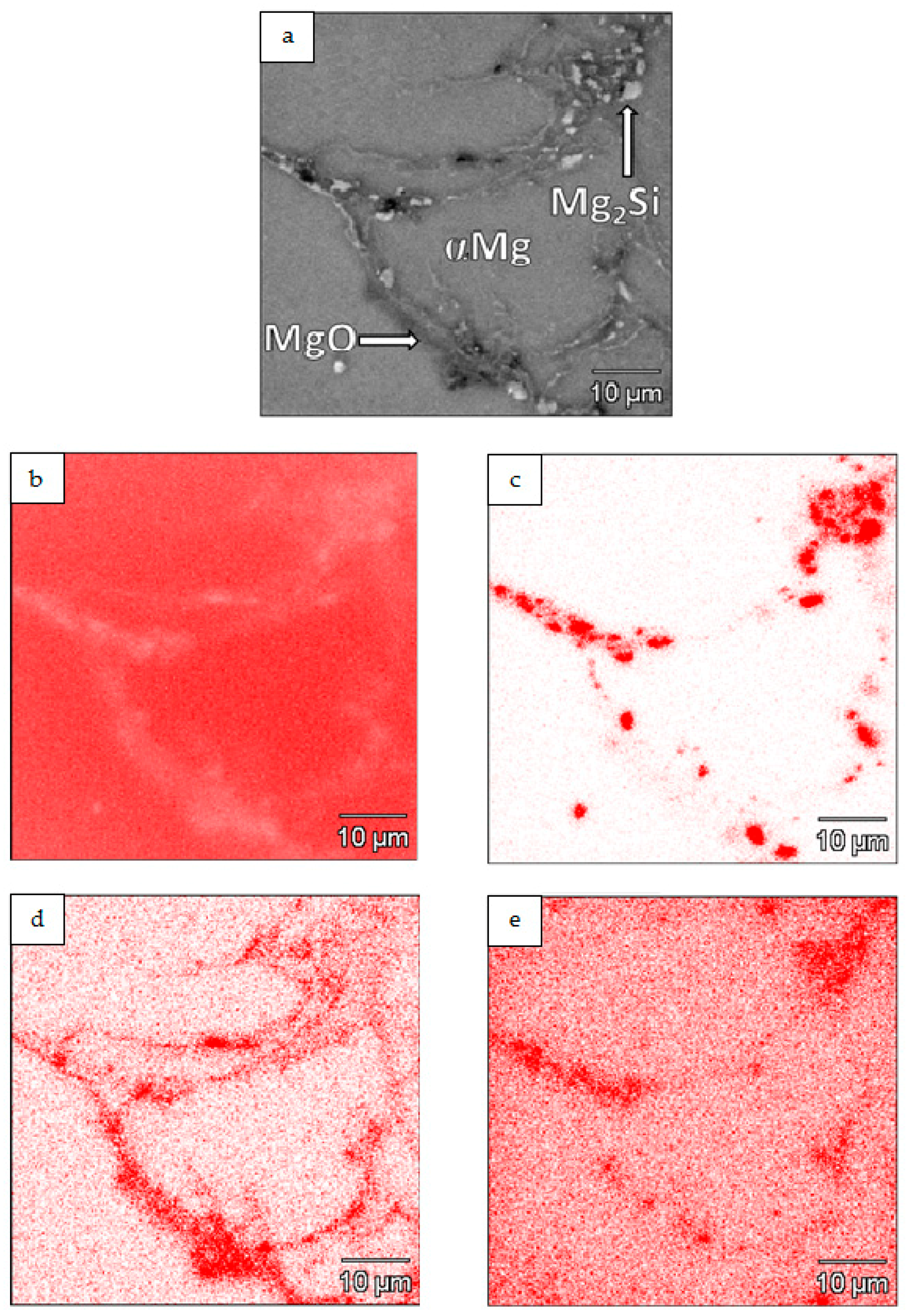
3.2. Characterization of Composite Sinters
4. Conclusions
- The fabrication of the microsized powder, nanopowder and nanotube mixture was successfully tested for the Mg-nSi-CNT system, and the ultrasonic method for de-agglomerating the nanocomponents in liquid base suspension proved to be useful if the appropriate order of technological procedures has been preserved.
- The final ternary powder mixture consisted of a microsized powder coated with two nanocomponents, and the components were uniformly distributed when the nanocomponents were first de-agglomerated separately, and then mixed together and deposited at the microsized powder surface.
- Differences in the microstructure of the Mg-Mg2Si-CNT composite depending on the initial morphology of the powder mixture were observed. The most noticeable changes were in the size of the Mg2Si particles, where the values could be very high (a few dozen micrometers in diameter), when nSi re-agglomeration occurred. Simultaneously, the size of the CNT agglomerates, detected in the composite, often surrounded by the Mg2Si phase, was also greater.
- The procedure for preparing the Mg-nSi-CNT mixture, which was proposed as the most effective, ensured the lowest porosity and the highest hardness of the Mg-Mg2Si-CNT composite obtained by sintering under pressure.
Author Contributions
Funding
Conflicts of Interest
References
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- De Volder, F.L.M.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 399, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Begtrup, G.E.; Ray, K.G.; Kessler, B.M.; Yuzvinsky, T.D.; Garcia, H.; Zettl, A. Extreme thermal stability of carbon nanotubes. Phys. Status Solidi 2007, 11, 3960–3963. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.; Kukovecz, A.; Konya, Z.; Vilarinho, P. Studies on the thermal decomposition ofmultiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Kozioł, M.; Jesionek, M.; Szperlich, P. Addition of a small amount of multiwalled carbon nanotubes and flaked grapheme to epoxy resin. J. Reinf. Plast. Comp. 2017, 36, 640–654. [Google Scholar] [CrossRef]
- Rutkowski, P.; Klimczyk, P.; Jaworska, L.; Stobierski, L.; Dubiel, A. Thermal properties of pressure sintered alumina–grapheme. J. Therm. Anal. Calorim. 2015, 122, 105–114. [Google Scholar] [CrossRef]
- Sobczak, N.; Sobczak, J.J.; Kudyba, A.; Homa, M.; Bruzda, G.; Grobelny, M.; Kalisz, M.; Strobl, K.; Singhal, R.; Monville, M. Wetting transparency of graphene deposited on copper in contact with liquid tin. Transact. Foundry Res. Inst. 2014, 54, 3–11. [Google Scholar]
- Wijnhoven, S.; Peijnenburg, W.; Herberts, C.; Hagens, W.; Oomen, A.; Heugens, E.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano–silver—A review of available data and knowledge gaps in human. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano–silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef]
- Latko–Durałek, P.; Dydek, K.; Sobczak, M.; Boczkowska, A. Processing and characterization of thermoplastic nanocomposite fibers of hot melt copolyamide and carbon nanotubes. J. Thermoplast. Compos. 2018, 31, 1–17. [Google Scholar] [CrossRef]
- Gupta, A.K.; Harsha, S.P. Analysis of mechanical properties of carbon nanotube reinforced polymer composites using continuum mechanics approach. Procedia Mater. Sci. 2014, 6, 18–25. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Miki, S.; Soga, T.; Itoh, I.; Todoroki, H.; Hosono, T.; Sakaki, K.; Hayashi, T.; Kim, Y.A.; Endo, M.; et al. Multi-walled carbon nanotube-reinforced magnesium alloy composites. Scr. Mater. 2008, 58, 267–270. [Google Scholar] [CrossRef]
- Han, G.; Wang, Z.; Liu, K.; Li, S.; Du, X.; Du, W. Synthesis of CNT reinforced AZ31 magnesium alloy composites with uniformly distributed CNTs. Mater. Sci. Eng. A 2015, 628, 350–357. [Google Scholar] [CrossRef]
- Hekner, B.; Myalski, J.; Pawlik, T.; Sopicka–Lizer, M. Effect of Carbon in Fabrication Al–SiC Nanocomposites for Tribological Application. Materials 2017, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Myalska, H.; Swadźba, R.; Rozmus, R.; Moskal, G.; Wiedermann, J.; Szymański, K. STEM analysis of WC–Co coatings modified by nano–sized TiC and nano–sized WC addition. Surf. Coat. Tech. 2017, 318, 279–287. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Liao, W.; Guo, W.; Li, W.; Jiang, H.; Ding, W. Microstructure and mechanical properties of the carbon nanotubes reinforced AZ91D magnesium matrix composites processed by cyclic extrusion and compression. Mater. Sci. Eng. A 2017, 689, 427–434. [Google Scholar] [CrossRef]
- Kostecki, M.; Grybczyk, M.; Klimczyk, P.; Cygan, T.; Woźniak, J.; Wejrzanowski, T.; Jaworska, L.; Morgiel, J.; Olszyna, A. Structural and mechanical aspects of multilayer graphene addition in alumina matrix composites–validation of computer simulation model. J. Eur. Ceram. Soc. 2016, 36, 4171–4179. [Google Scholar] [CrossRef]
- Stankiewicz, N.; Lelusz, M. Nanotechnologia w budownictwie–przegląd zastosowań. Civ. Environ. Eng. 2014, 5, 101–112. [Google Scholar]
- Li, H.; Cheng, L.; Sun, X.; Li, Y.; Li, B.; Liang, C.; Wang, H.; Fan, J. Fabrication and properties of magnesium matrix composite reinforced by urchin–like carbon nanotube–alumina in situ composite structure. J. Alloy Compd. 2018, 746, 320–327. [Google Scholar] [CrossRef]
- Li, Q.; Viereckl, A.; Rottmair, C.A.; Singer, R.F. Improved processing of carbon nanotube/magnesium alloy composites. Compos. Sci. Technol. 2009, 69, 1193–1199. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuda, H.; Umeda, J.; Imai, H.; Fugetsu, B. Microstructural and mechanical behavior of multi–walled carbon nanotubes reinforced Al–Mg–Si alloy composites in aging treatment. Carbon 2014, 72, 15–21. [Google Scholar] [CrossRef]
- Kondoh, H.; Fukuda, H.; Umeda, J.; Imai, H.; Fugetsu, B.; Endo, M. Microstructural and mechanical analysis of carbon nanotube reinforced magnesium alloy powder composites. Mater. Sci. Eng. A 2010, 527, 4103–4108. [Google Scholar] [CrossRef]
- Murugan, S.; Nguyen, Q.B.; Gupta, M. Synthesis of Magnesium Based Nano–composites. IntechOpen 2019, 1–19. [Google Scholar]
- Malaki, M.; Xu, W.; Ashish, K.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced Metal Matrix Nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Olszówka–Myalska, A.; McDonald, S.A.; Withers, P.J.; Myalska, H.; Moskal, G. Microstructure of in situ Mg metal matrix composites based on silica nanoparticles. Solid State Phenom. 2012, 191, 189–198. [Google Scholar] [CrossRef]
- Olszówka–Myalska, A. Sintered in situ magnesium matrix composites. Mechanik 2016, 89, 504–505. [Google Scholar] [CrossRef]
- Umeda, J.; Kondoh, K.; Kawakami, M.; Imai, H. Powder metallurgy magnesium composite with magnesium silicide in using rice husk silica particles. Powder Technol. 2009, 189, 399–403. [Google Scholar] [CrossRef]
- Nazer, N.S.; Denys, R.V.; Andersen, H.F.; Arnberg, L.; Yarty, V.A. Nanostructured magnesium silicide Mg2Si and its electrochemical performance as an anode of a lithium ion battery. J. Alloy Compd. 2017, 718, 478–491. [Google Scholar] [CrossRef]
- Nieroda, P.; Zybała, R.; Wojciechowski, K.T. Właściwości termoelektryczne Mg2Si otrzymywanego techniką SPS. Ceram. Mater. 2012, 64, 490–493. [Google Scholar]
- Zhang, S.; Chen, T.; Cheng, F.; Li, P. A Comparative Characterization of the Microstructures and Tensile Properties of As–Cast and Thixoforged in situ AM60B–10 vol% Mg2Sip Composite and Thixoforged AM60B. Metals 2015, 5, 457–470. [Google Scholar] [CrossRef]
- Chegini, M.; Shaeri, M.H.; Taghiabadi, R.; Chegini, S.; Djavanroodi, F. The Correlation of Microstructure and Mechanical Properties of In–Situ Al–Mg2Si Cast Composite Processed by Equal Channel Angular Pressing. Materials 2019, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Zainon, F.; Rafezi Ahmad, K.R.; Daud, R. The effects of Mg2Si(p) on microstructure and mechanical properties of AA332 composite. Adv. Mat. Res. 2016, 5, 55–66. [Google Scholar] [CrossRef]
- Ellendt, N.; Uhlenwinkel, V.; Stelling, O.; Irretier, A.; Kessler, O. Spray Forming of Mg2Si Rich Aluminum Alloys. Mater. Sci. Forum 2007, 534, 437–440. [Google Scholar] [CrossRef]
- Sun, Y.; Johnson, D.R.; Trumble, K.P.; Priya, P.; Krane, M.J. Krane1 Effect of Mg2Si Phase on Extrusion of AA6005 Aluminum Alloy. Light Metals 2014, 429–433. [Google Scholar]
- Prusova, E.; Deevb, V.; Rakhubab, E. Aluminum Matrix In–Situ Composites Reinforced with Mg2Si and Al3Ti. Mater. Today Proc. 2019, 11, 386–391. [Google Scholar] [CrossRef]
- Olszówka–Myalska, A.; Myalska, H. Method For Initial Consolidation of Nanograin Powders with Micrograin Powders; Nanograin Powders with Submicrograin Powders in Order to Obtain the In Situ Type Composites. Polish Patent Application No. P.225001, 22 December 2014. [Google Scholar]
- Kwon, H.; Estili, M.; Takagi, K.; Miyazaki, T.; Kawasaki, A. Combination of hot extrusion and spark plasma sintering for producing carbon nanotube reinforced aluminum matrix composites. Carbon 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Chen, B.; Kondoh, K.; Imai, H.; Umeda, J.; Takahashi, M. Simultaneously enhancing strength and ductility of carbon nanotube/aluminum composites by improving bonding conditions. Scr. Mater. 2016, 113, 158–162. [Google Scholar] [CrossRef]
- Chen, B.; Kondoh, K. Sintering behaviors of carbon nanotubes–aluminum composite powders. Metals 2016, 6, 213. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhao, K.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Fabrication of CNT/Al composites with low damage to CNTs by a novel solution–assisted wet mixing combined with powder metallurgy processing. Mater. Design 2016, 97, 424–430. [Google Scholar] [CrossRef]
- Seo, H.Y.; Jiang, L.R.; Kang, C.G.; Jin, C.K. A hot extrusion process without sintering by applying MWCNTs/Al6061 composites. Metals 2018, 8, 184. [Google Scholar] [CrossRef]
- Seo, H.Y.; Jiang, L.R.; Kang, C.G.; Jin, C.K. Effect of compression process of MWCNT–reinforced AL6061 powder on densification characteristics and its mechanical properties. Metals 2017, 7, 437. [Google Scholar] [CrossRef]
- Wang, J.T.; Figueiredo, R.B.; Langdon, T.G. Mechanical properties and microstructures of Severely Plastic Deformed pure titanium by mechanical milling and spark plasma sintering. Mater. Sci. Forum 2010, 667, 559–564. [Google Scholar]
- Lakshmikanthan, P.; Prabu, B. Mechanical and tribological behavior of aluminium Al6061–coconut shell ash composite obtained using stir casting pellet method. J. Balk. Tribol. Assoc. 2016, 22, 4008–4018. [Google Scholar]
- Sujith, S.V.; Mahapatra, M.M.; Mulik, R. An investigation into fabrication and characterization of direct reaction synthesized Al–7079–TiC in situ metal matrix composites. Arch. Civ. Mech. Eng. 2018, 19, 63–78. [Google Scholar] [CrossRef]
- Olejnik, E.; Szymański, Ł.; Tokarski, T.; Tumidajewicz, M. TiC—Based local composite reinforcement obtained in–situ in ductile iron based castings with use of rode preform. Mater. Lett. 2018, 222, 192–195. [Google Scholar] [CrossRef]
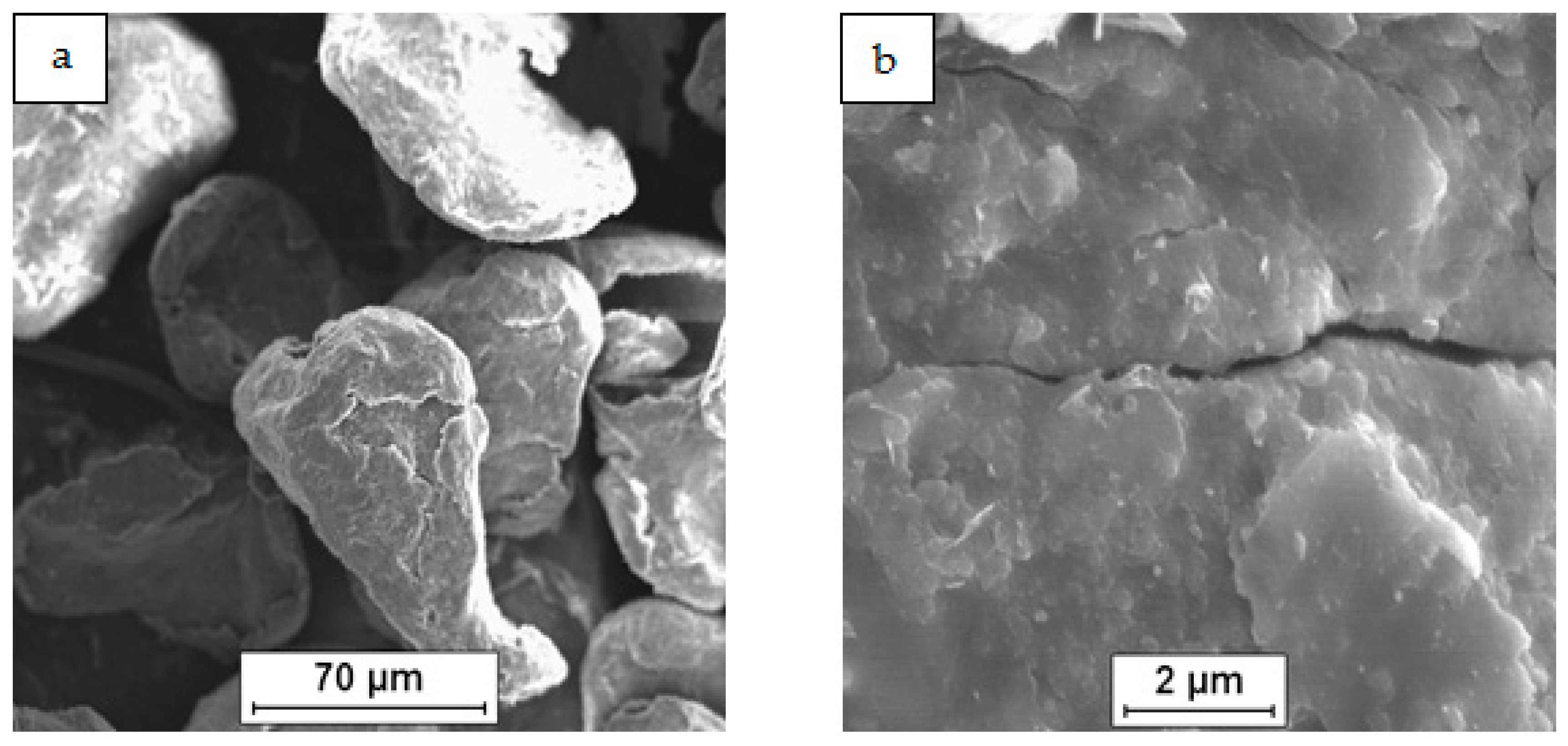
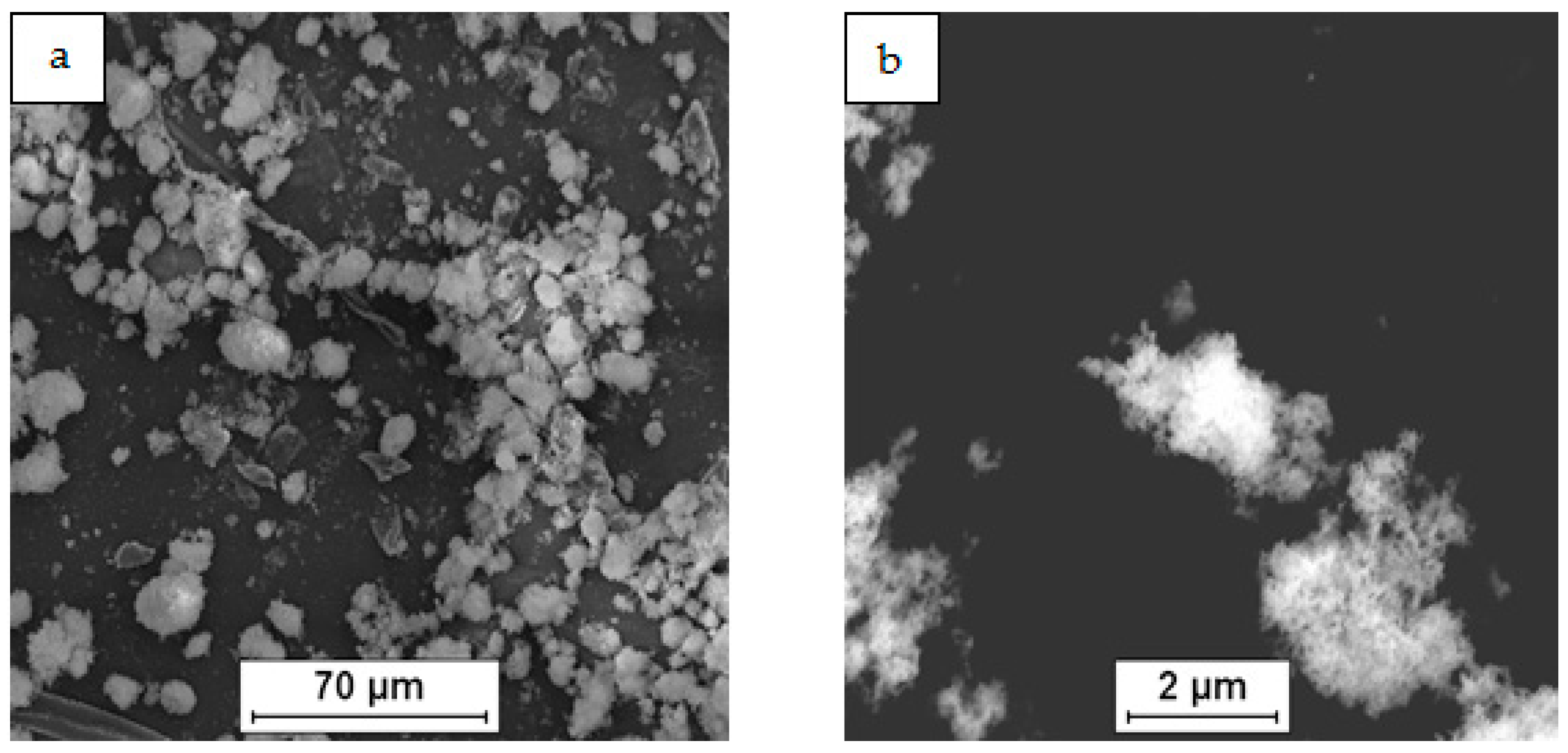
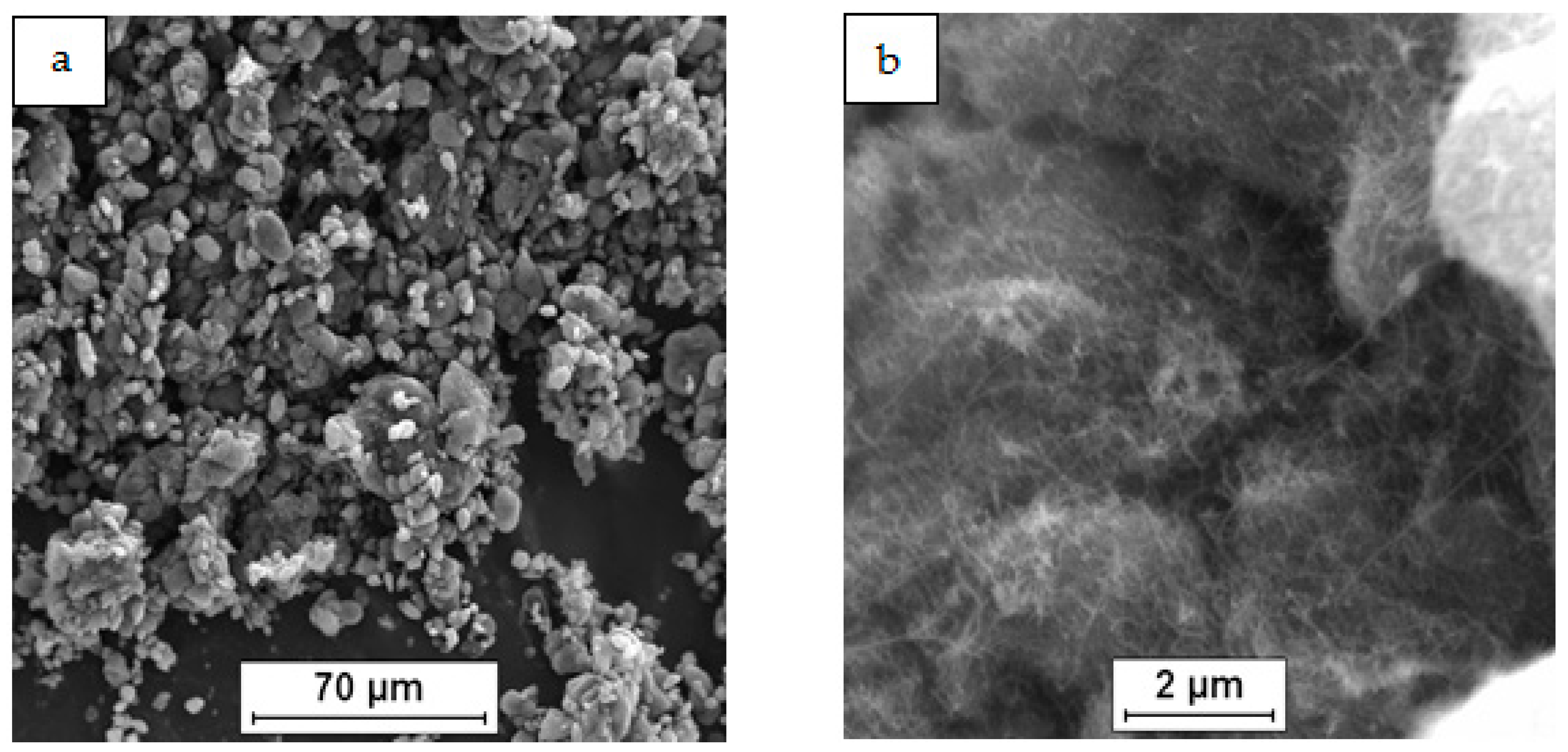

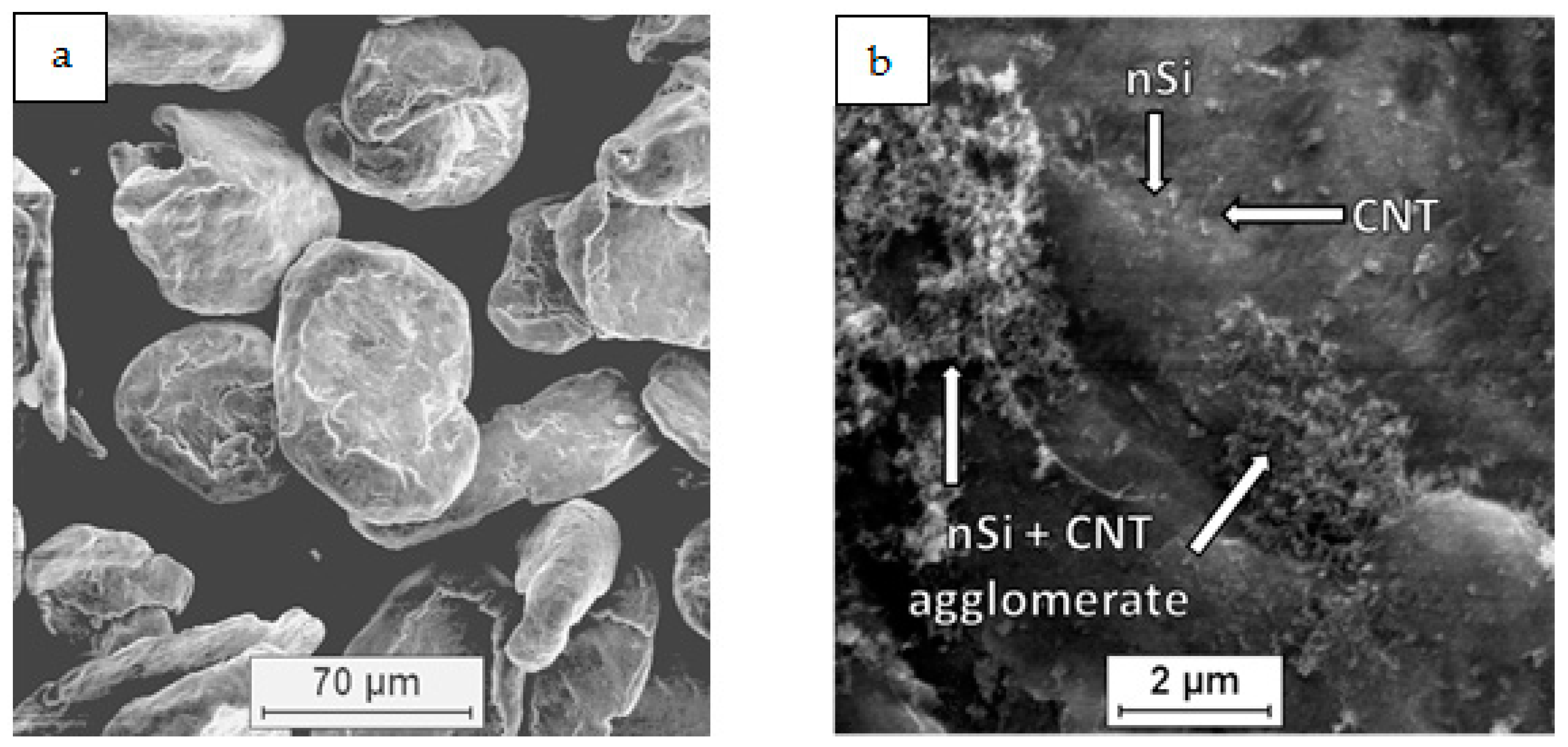
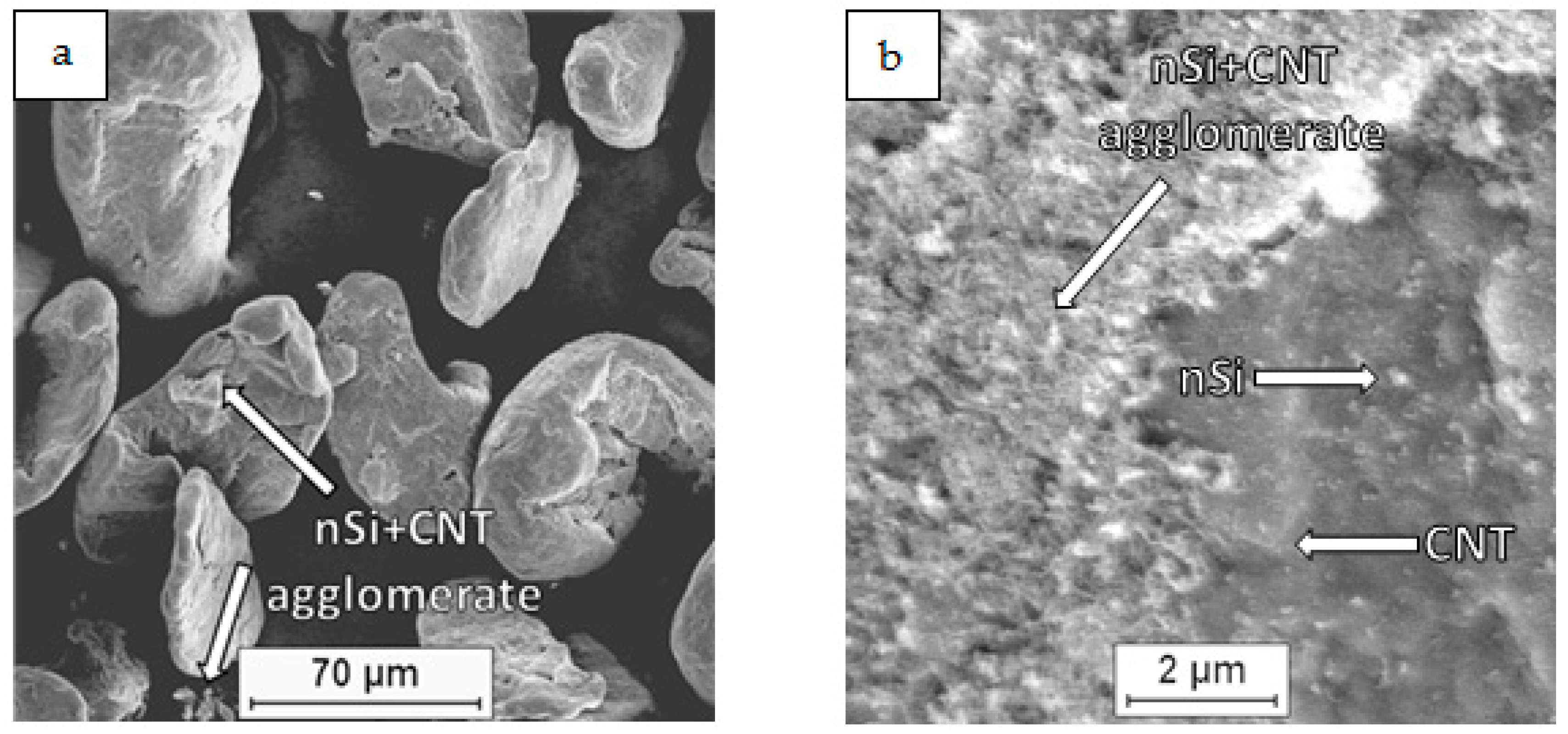

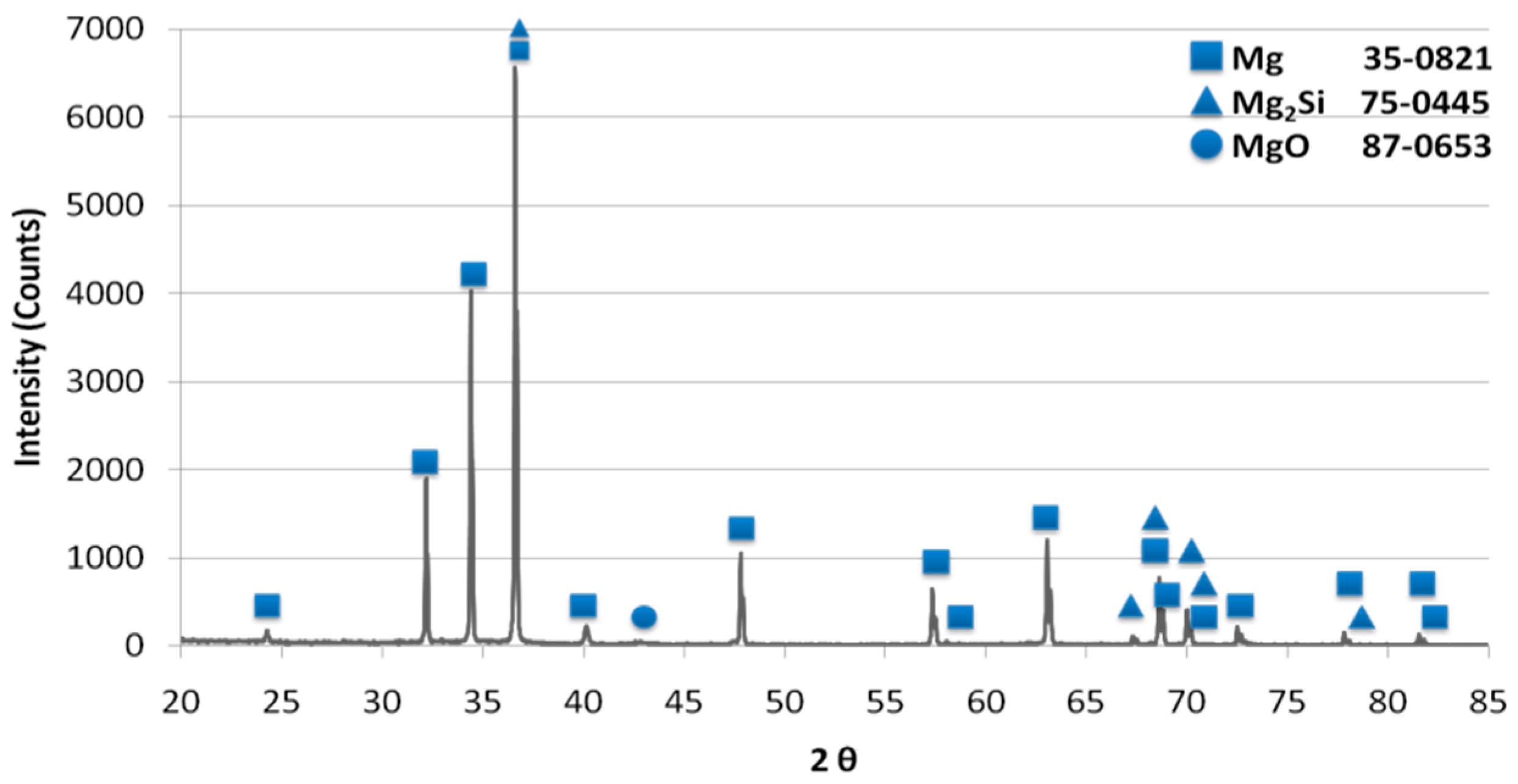

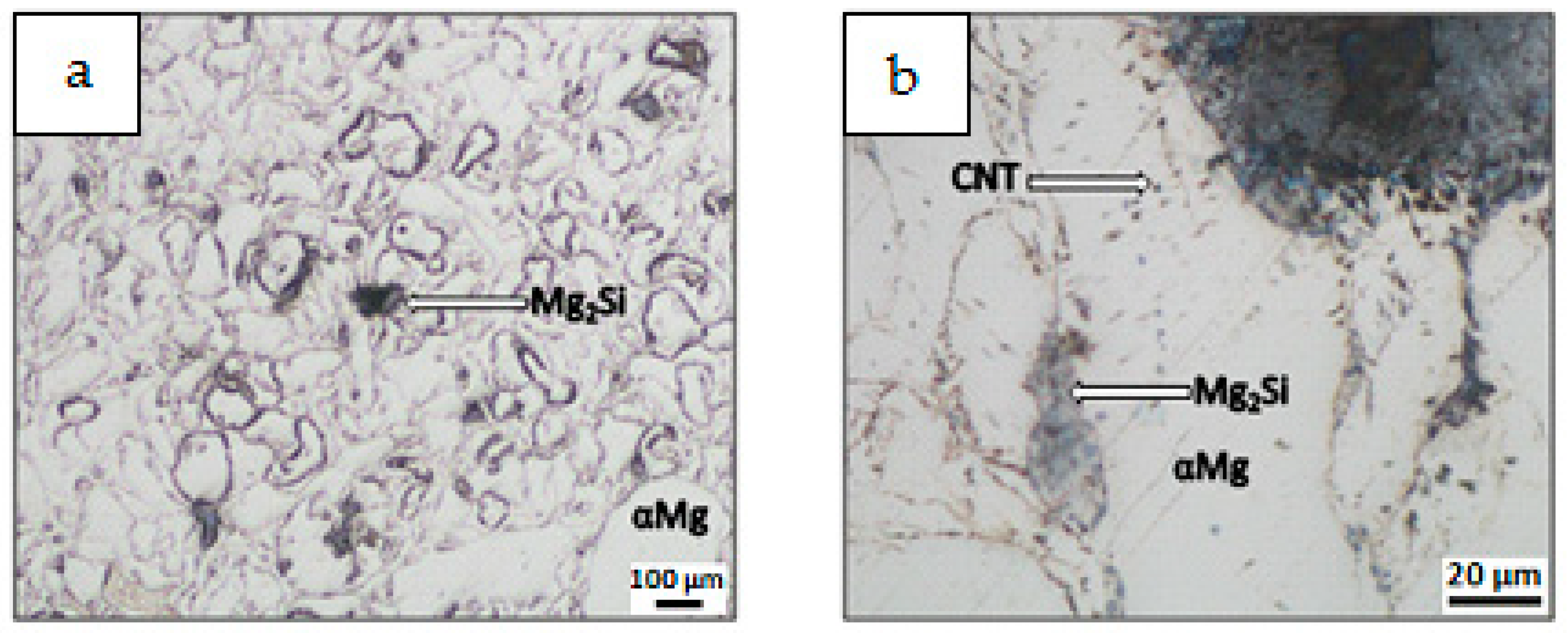

| Mixture Signature | Mixture Composition [vol.%] | Mixing Procedure | Sintering Conditions |
|---|---|---|---|
| Mg | 100 Mg | Reference sample | 580 °C, 15 MPa, vacuum |
| Mg+(nSiD + CNTD) | 97% Mg; 2% nSi; 1% CNT | - separate de-agglomeration nSi and CNT - preparation of (nSi)D + (CNT)D suspension - addition of Mg to (nSi)D + (CNT)D suspension | |
| Mg + (nSi + CNT)D | - common de-agglomeration of nSi and CNT - addition of Mg+(nSi+CNT)D suspension | ||
| (Mg + nSiD) + CNTD | - separate de-agglomeration of nSi and CNT - preparation of (nSi)D + Mg suspension - addition of (nSi)D to Mg + (CNT)D suspension | ||
| (Mg + CNTD) + nSiD | - separate de-agglomeration of nSi and CNT - preparation of (CNT)D + Mg suspension - addition of (CNT)D to Mg + (nSi)D suspension |
| Material | Hardness, HV 0.2 | Open Porosity, % |
|---|---|---|
| Mg | 41.98 ± 2.8 | 1.22 |
| Mg + (nSiD + CNTD) | 46.5 ± 3.0 | 0.71 |
| Mg + (nSi + CNT)D | 44.5 ± 2.7 | 1.05 |
| (Mg + nSiD) + CNTD | 43.6 ± 4.7 | 0.88 |
| (Mg + CNTD) + nSiD | 44.6 ± 3.9 | 0.76 |
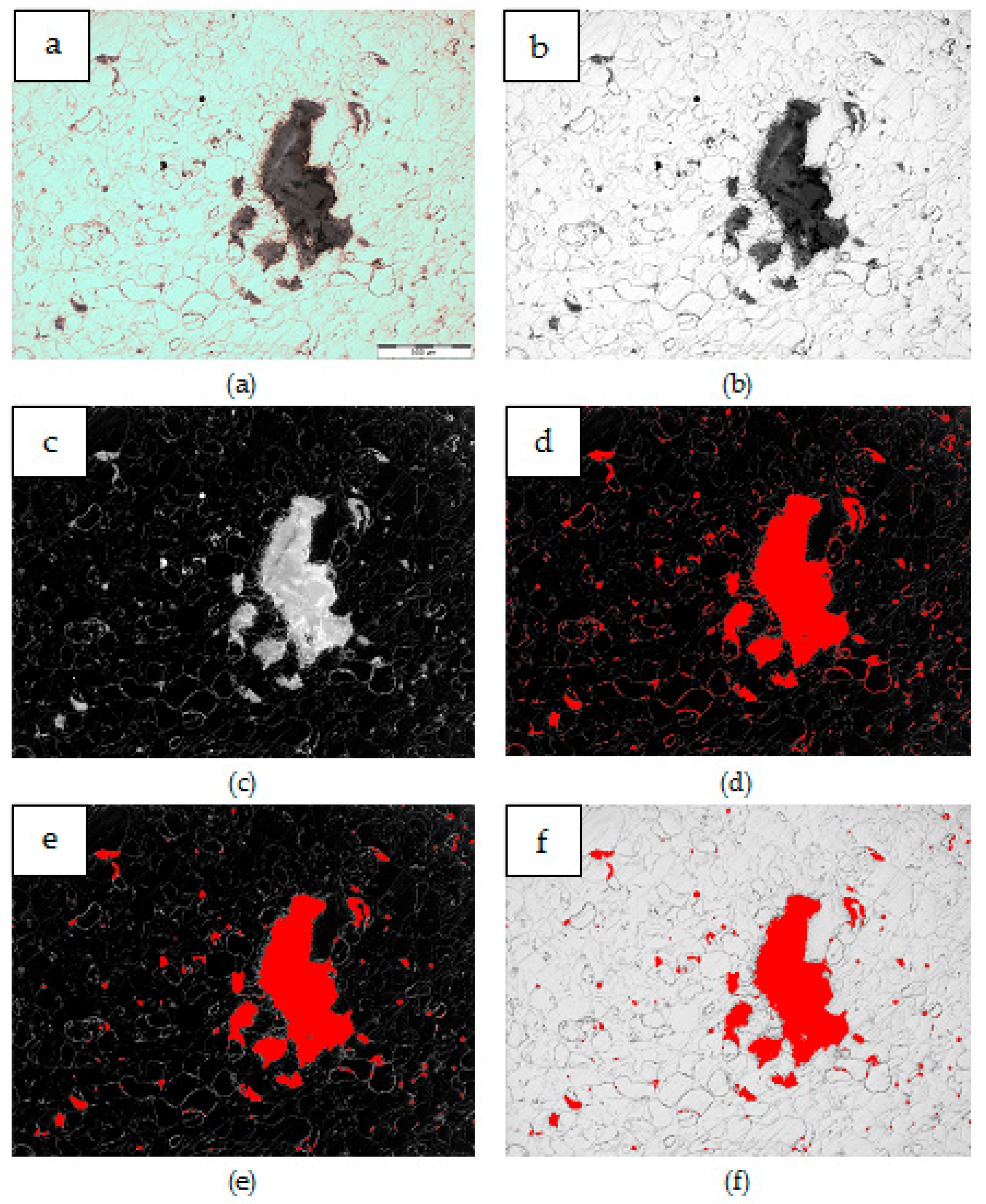
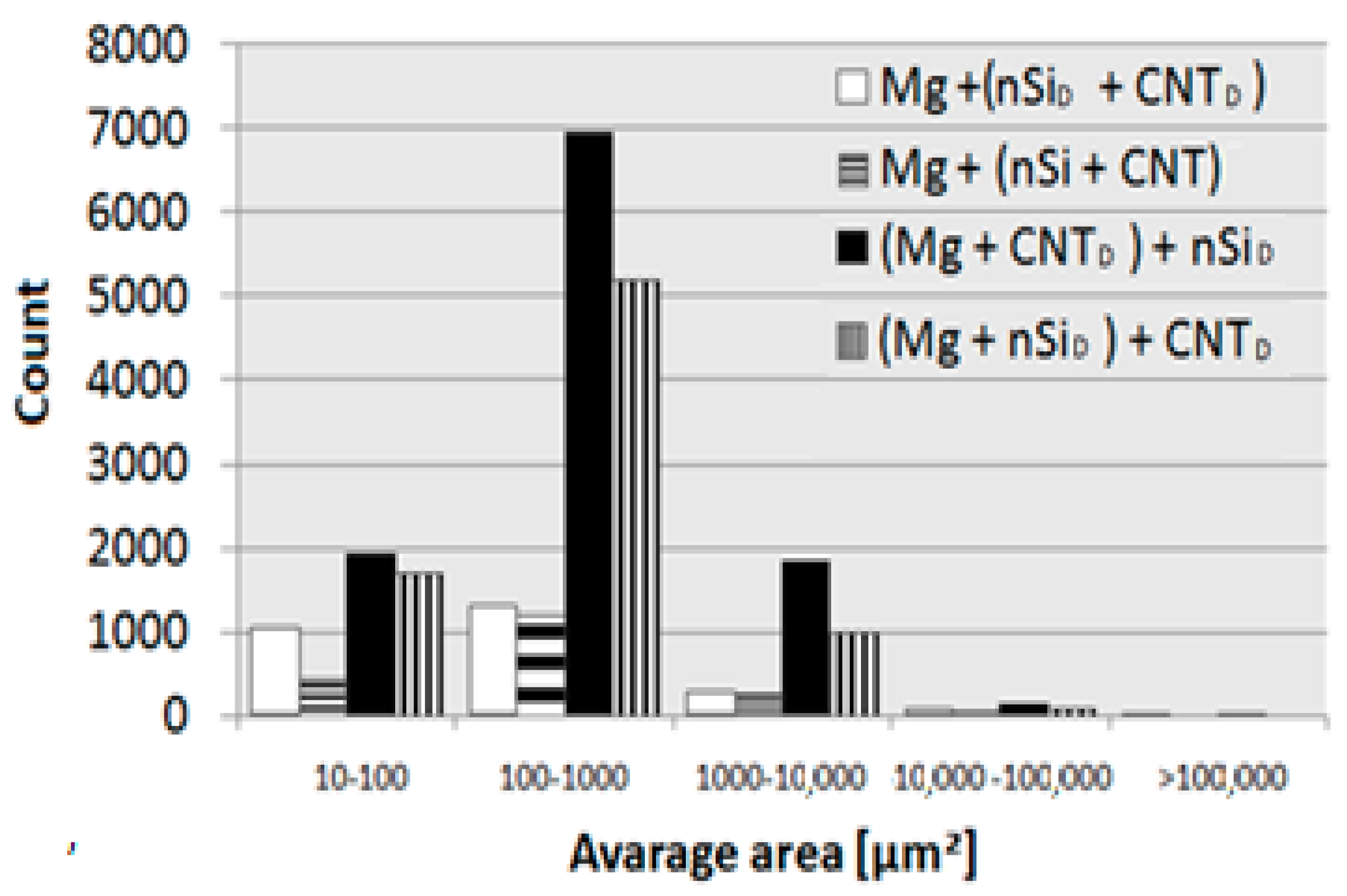
| Material | Area fraction, AA [%] | Area [µm2] | Count | Count |
|---|---|---|---|---|
| Mg + (nSiD + CNTD) | 5.38 ± 2.66 | 10–100 | 747 | 2367 |
| 100–1000 | 1295 | |||
| 1000–10,000 | 272 | |||
| 10,000–100,000 | 49 | |||
| > 100,000 | 4 | |||
| Mg + (nSi + CNT)D | 6.69 ± 4.11 | 10–100 | 441 | 1950 |
| 100–1000 | 1198 | |||
| 1000–10,000 | 246 | |||
| 10,000–10,0000 | 52 | |||
| > 10,0000 | 13 | |||
| (Mg + nSiD) + CNTD | 18.75 ± 9.88 | 10–100 | 1708 | 8018 |
| 100–1000 | 5208 | |||
| 1000–10,000 | 994 | |||
| 10,000–10,0000 | 97 | |||
| > 100,000 | 11 | |||
| (Mg + CNTD) + nSiD | 17.50 ± 2.59 | 10–100 | 1917 | 10814 |
| 100–1000 | 6935 | |||
| 1000–10,000 | 1833 | |||
| 10,000–100,000 | 122 | |||
| > 100,000 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszówka-Myalska, A.; Wrześniowski, P.; Myalska, H.; Godzierz, M.; Kuc, D. Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters. Materials 2019, 12, 3242. https://doi.org/10.3390/ma12193242
Olszówka-Myalska A, Wrześniowski P, Myalska H, Godzierz M, Kuc D. Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters. Materials. 2019; 12(19):3242. https://doi.org/10.3390/ma12193242
Chicago/Turabian StyleOlszówka-Myalska, Anita, Patryk Wrześniowski, Hanna Myalska, Marcin Godzierz, and Dariusz Kuc. 2019. "Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters" Materials 12, no. 19: 3242. https://doi.org/10.3390/ma12193242
APA StyleOlszówka-Myalska, A., Wrześniowski, P., Myalska, H., Godzierz, M., & Kuc, D. (2019). Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters. Materials, 12(19), 3242. https://doi.org/10.3390/ma12193242