Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models
Abstract
1. Introduction: The Clinical Need for In Vitro Neural Models
1.1. Epilepsy
1.1.1. Limitations of Animal Models for Epilepsy
1.1.2. Limitations of In Situ Methods
1.2. Personalised Disease Modelling
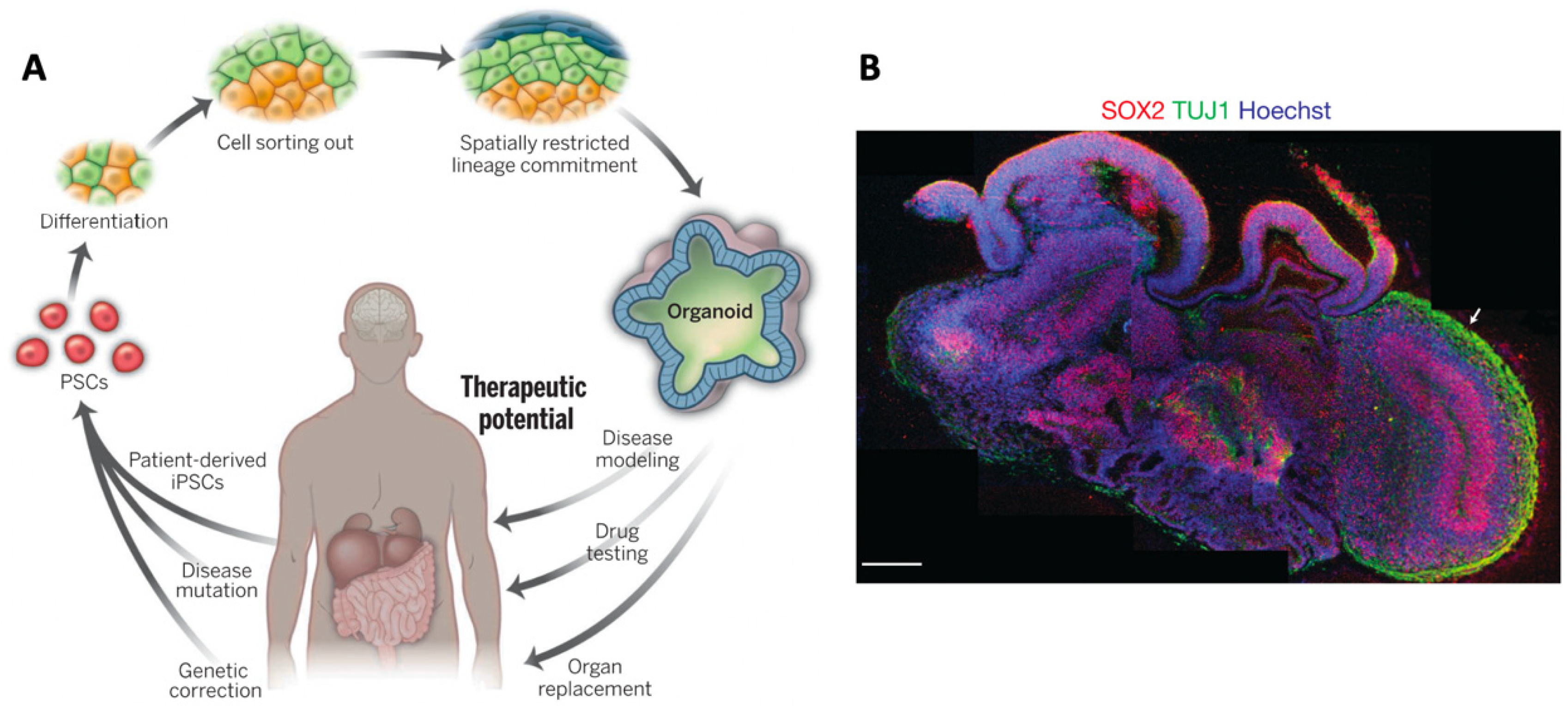
1.2.1. The Advent of Induced Pluripotency Stem Cells
1.2.2. Organoid Models
2. In Vitro Neural Models
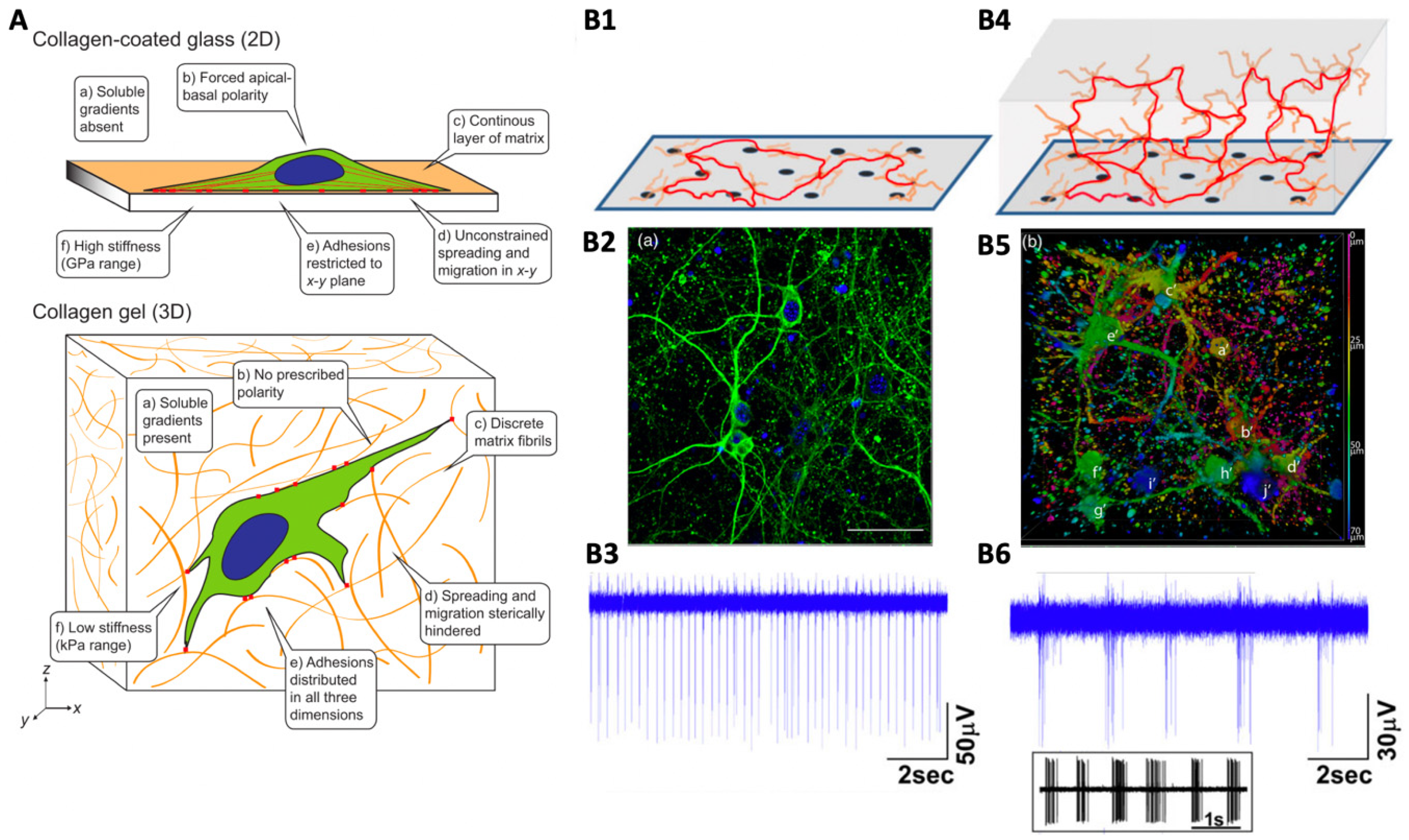
2.1. 2D versus 3D Neural Culture
2.2. Biomaterials Requirements for 3D Neuronal Culture
2.2.1. Mechanical Characteristics of the Brain
2.2.2. ECM Composition of the Brain
2.2.3. Microfluidic Devices: Drug Screening and Disease Modelling
3. Biomaterials for 3D Neuronal Culture Models
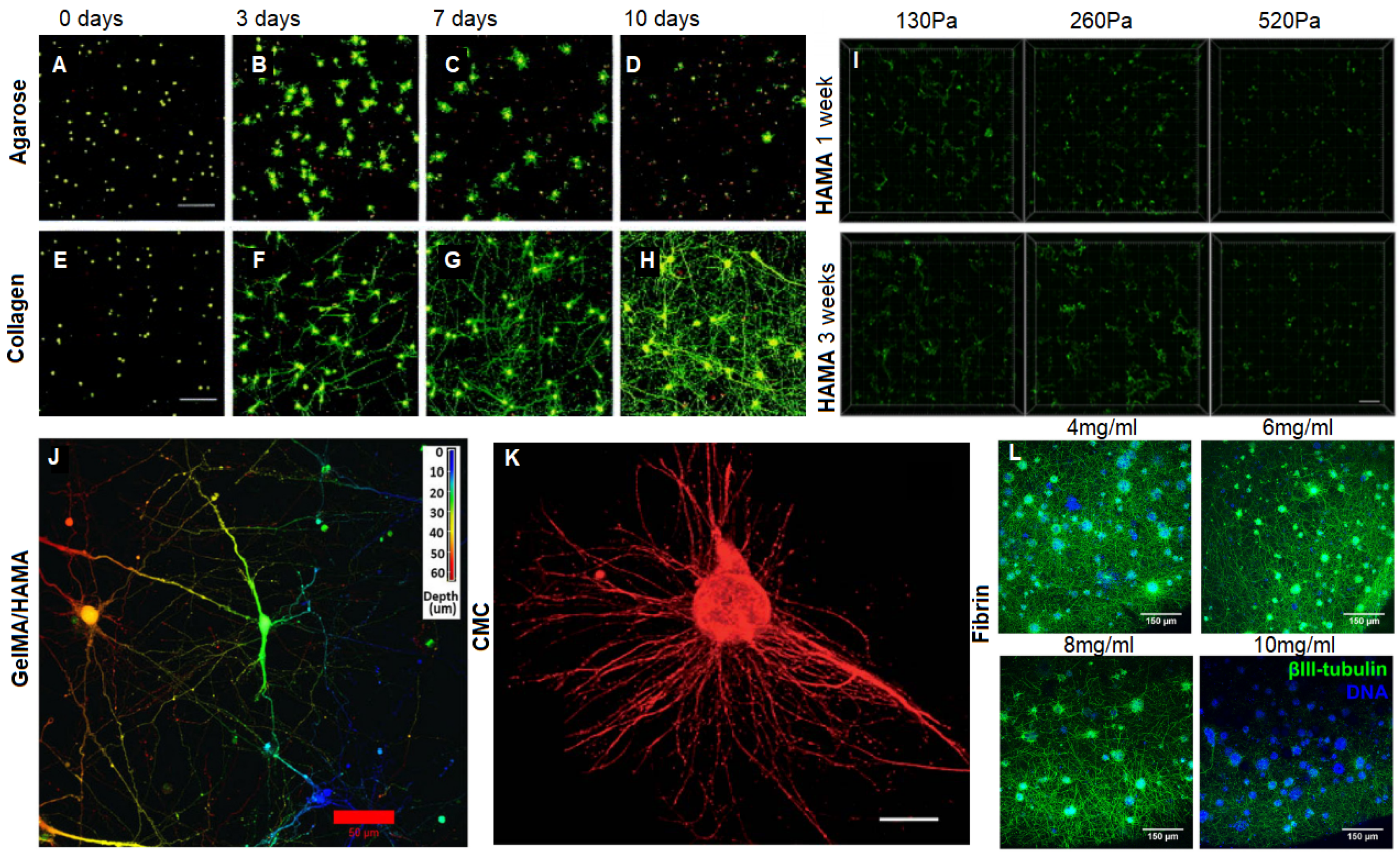
3.1. Collagen Based Materials
3.2. Hyaluronic Acid Based Materials
3.3. Other Hydrogels
3.3.1. GelMA
3.3.2. Agarose
3.3.3. Chitosan
3.3.4. Alginate
3.3.5. Matrigel
3.3.6. Fibrin
3.3.7. Poly(ethylene glycol) (PEG)
3.4. Disease Modelling in Bulk Gels
4. Strategies for Biofabricating Neural Models
4.1. Layered 3D Neural Models
4.2. Gradients in 3D Bulk Hydrogels
4.3. 3D Scaffolds
4.3.1. Colloidal 3D Scaffolds
4.3.2. Silk Scaffolds
4.3.3. Macroporous Scaffolds
4.3.4. Electrospun Scaffolds
4.4. 3D Printed Scaffolds
4.4.1. 3D Photopatterning
4.4.2. Extrusion Printed Scaffolds
4.4.3. Convergent Biofabrication
4.4.4. 3D Scaffold-Based Disease Models
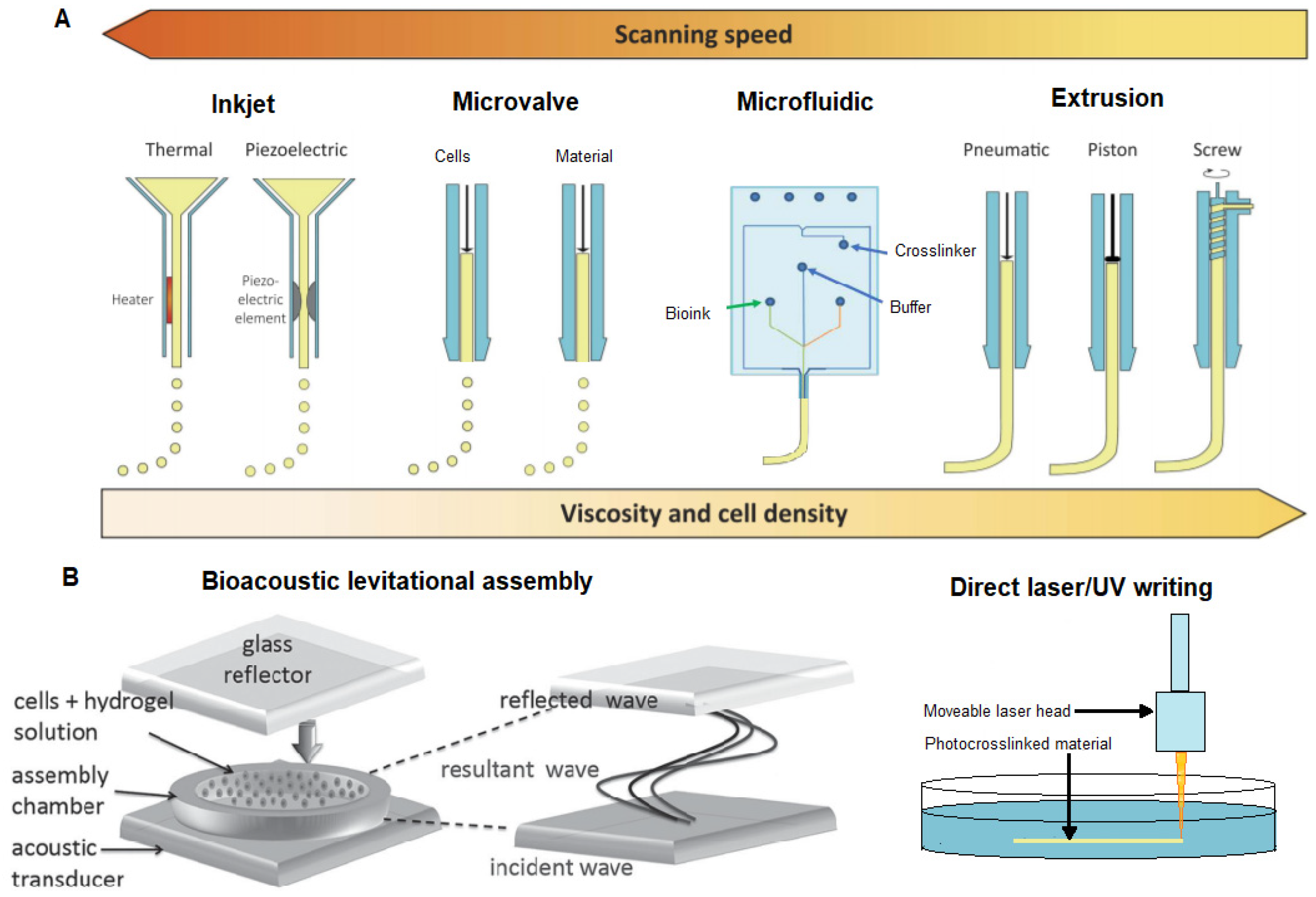
5. 3D Bioprinting
5.1. The Challenge of 3D Bioprinting of Soft Structures
5.2. Support Structure Methods
5.2.1. Sacrificial Support Scaffolds
5.2.2. Indirectly 3D Bioprinting
5.3. 3D Bioprinting of Neurons
5.3.1. Extrusion Based 3D Bioprinting of Neural Structures
5.3.2. Inkjet Bioprinting of Neurons
5.3.3. Other Methods of 3D Printing Neural Cells
5.3.4. 3D Bioprinting Summary
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Neurological Disorders: Public Health Challenges; World Health Organization: Geneva, Switzerland, 2006; p. 85. [Google Scholar]
- Centeno, M.; Tierney, T.M.; Perani, S.; Shamshiri, E.A.; StPier, K.; Wilkinson, C.; Konn, D.; Banks, T.; Vulliemoz, S.; Lemieux, L.; et al. Optimising EEG-fMRI for localisation of focal epilepsy in children. PLoS ONE 2016, 11, e0149048. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A. From maps to mechanisms through neuroimaging of schizophrenia. Nature 2010, 468, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. Br. Med. J. 2007, 334, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Pas, S.P. The rise of three-dimensional human brain cultures. Nature 2018, 553, 437–445. [Google Scholar]
- Gambardella, A.; Labate, A.; Mumoli, L.; Lopes-Cendes, I.; Cendes, F. Role of Pharmacogenomics in Antiepileptic Drug Therapy: Current Status and Future Perspectives. Curr. Pharm. Des. 2018, 23, 5760–5765. [Google Scholar] [CrossRef]
- Gomez-Ibañez, A.; McLachlan, R.S.; Mirsattari, S.M.; Diosy, D.C.; Burneo, J.G. Prognostic factors in patients with refractory idiopathic generalized epilepsy. Epilepsy Res. 2017, 130, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ito, G.; Okumura, A.; Kanemoto, K. Efficacy of a third or later antiepileptic drug regimen according to epilepsy syndrome among adult patients. Epilepsy Res. 2017, 136, 103–108. [Google Scholar] [CrossRef]
- Pohlen, M.S.; Jin, J.; Tobias, R.S.; Maheshwari, A. Pharmacoresistance with newer anti-epileptic drugs in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2017, 137, 56–60. [Google Scholar] [CrossRef]
- Rapacz, A.; Głuch-Lutwin, M.; Mordyl, B.; Filipek, B.; Abram, M.; Kamiński, K. Evaluation of anticonvulsant and analgesic activity of new hybrid compounds derived from N-phenyl-2-(2,5-dioxopyrrolidin-1-yl)-propanamides and –butanamides. Epilepsy Res. 2018, 143, 11–19. [Google Scholar] [CrossRef]
- Löscher, W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002, 50, 105–123. [Google Scholar] [CrossRef]
- Jeffery, A.F.; Churchward, M.A.; Mushahwar, V.K.; Todd, K.G.; Elias, A.L. Hyaluronic Acid-Based 3D Culture Model for In Vitro Testing of Electrode Biocompatibility. Biomacromolecules 2014, 15, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Turner, R. Uses, misuses, new uses and fundamental limitations of magnetic resonance imaging in cognitive science. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Maguire, E.A. Can we study 3D grid codes non-invasively in the human brain? Methodological considerations and fMRI findings. Neuroimage 2019, 186, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Barsby, T. Biofabrication of Three Dimensional Neural Microtissue Constructs from Human Induced Pluripotent Stem Cells. Ph.D. Thesis, University of Woolongong, Woolongong, Australia, 2018. [Google Scholar]
- Barry, C.; Schmitz, M.T.; Propson, N.E.; Hou, Z.; Zhang, J.; Nguyen, B.K.; Bolin, J.M.; Jiang, P.; McIntosh, B.E.; Probasco, M.D.; et al. Uniform neural tissue models produced on synthetic hydrogels using standard culture techniques. Exp. Biol. Med. 2017, 242, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Yamanaka, S. IPS cell technologies: Significance and applications to CNS regeneration and disease. Mol. Brain 2014, 7, 22. [Google Scholar] [CrossRef]
- Ma, W.; Fitzgerald, W.; Liu, Q.-Y.Y.; O’Shaughnessy, T.J.J.; Maric, D.; Lin, H.J.J.; Alkon, D.L.L.; Barker, J.L.L. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp. Neurol. 2004, 190, 276–288. [Google Scholar] [CrossRef]
- Walker, M.J.; Bourke, J.; Hutchison, K. Evidence for personalised medicine: Mechanisms, correlation, and new kinds of black box. Theor. Med. Bioeth. 2019, 40, 103–121. [Google Scholar] [CrossRef]
- Crook, J.M.; Wallace, G.; Tomaskovic-Crook, E. The potential of induced pluripotent stem cells in models of neurological disorders: Implications on future therapy. Expert Rev. Neurother. 2015, 15, 295–304. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Kelava, I.; Lancaster, M.A. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 2016, 420, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesisin a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, M.; Rey, F.; Fantini, V.; Pansarasa, O.; Di Giulio, A.M.; Carelli, S.; Cereda, C. From neuronal differentiation of iPSCs to 3D neuro-organoids: Modelling and therapy of neurodegenerative diseases. Int. J. Mol. Sci. 2018, 19, 3972. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Zhang, L.; Chen, J.; Zeng, Y.; Tang, Y.; Liu, Z. Advances in Cerebral Organoid Systems and their Application in Disease Modeling. Neuroscience 2019, 399, 28–38. [Google Scholar] [CrossRef]
- Yoon, K.J.; Nguyen, H.N.; Ursini, G.; Zhang, F.; Kim, N.S.; Wen, Z.; Makri, G.; Nauen, D.; Shin, J.H.; Park, Y.; et al. Modeling a genetic risk for schizophrenia in iPSCs and Mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 2014, 15, 79–91. [Google Scholar] [CrossRef]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, L.; Fang, A.; Li, P.; Wu, Q.; Wang, X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell 2017, 8, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Iefremova, V.; Manikakis, G.; Krefft, O.; Jabali, A.; Weynans, K.; Wilkens, R.; Marsoner, F.; Brändl, B.; Müller, F.J.; Koch, P.; et al. An Organoid-Based Model of Cortical Development Identifies Non-Cell-Autonomous Defects in Wnt Signaling Contributing to Miller-Dieker Syndrome. Cell Rep. 2017, 19, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449. [Google Scholar] [CrossRef]
- Allende, M.L.; Cook, E.K.; Larman, B.C.; Nugent, A.; Brady, J.M.; Golebiowski, D.; Sena-Esteves, M.; Tifft, C.J.; Proia, R.L. Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J. Lipid Res. 2018, 59, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Herai, R.H.; Nguyen Huu, V.A.; Wen, J.H.; Joshi-Barr, S.; et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef]
- Pamies, D.; Hartung, T.; Hogberg, H.T. Biological and medical applications of a brain-on-a-chip. Exp. Biol. Med. 2014, 239, 1096–1107. [Google Scholar] [CrossRef]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef]
- Stukel, J.M.; Willits, R.K. Mechanotransduction of Neural Cells Through Cell–Substrate Interactions. Tissue Eng. Part B Rev. 2015, 22, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bourke, J.L.; Coleman, H.A.; Pham, V.; Forsythe, J.S.; Parkington, H.C. Neuronal Electrophysiological Function and Control of Neurite Outgrowth on Electrospun Polymer Nanofibers Are Cell Type Dependent. Tissue Eng. Part A 2013, 20, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Homan, C.C.; Pederson, S.; To, T.H.; Tan, C.; Piltz, S.; Corbett, M.A.; Wolvetang, E.; Thomas, P.Q.; Jolly, L.A.; Gecz, J. PCDH19 regulation of neural progenitor cell differentiation suggests asynchrony of neurogenesis as a mechanism contributing to PCDH19 Girls Clustering Epilepsy. Neurobiol. Dis. 2018, 116, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, N.; Uchida, T.; Lossin, C.; Misumi, Y.; Okada, Y.; Akamatsu, W.; Imaizumi, Y.; Zhang, B.; Nabeshima, K.; Mori, M.X.; et al. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol. Brain 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Yang, Y.; Shi, Y.; Chen, J.; Gao, R.; Fan, Y.; Yao, H.; Liao, W.; Sun, X.F.; Gao, S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 2013, 22, 4241–4252. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Ning, B.; Liu, Z.; Chen, S.; Zhao, W. Drug discovery via human-derived stem cell organoids. Front. Pharmacol. 2016, 7, 334. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Masand, S.N.; Shreiber, D.I. Microfluidic Generation of Haptotactic Gradients through 3D Collagen Gels for Enhanced Neurite Growth. J. Neurotrauma 2011, 28, 2377–2387. [Google Scholar] [CrossRef]
- Holmes, D.B.; Heine, V.M. Streamlined 3D Cerebellar Differentiation Protocol with Optional 2D Modification. J. Vis. Exp. 2017, 130, e56888. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rösingh, L.N.; Molnár, K.; László, L.; Bellák, T.; Téglási, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Chwalek, K.; Stuntz, E.; Pouli, D.; Du, C.; Tang-Schomer, M.; Georgakoudi, I.; Black, L.D.; Kaplan, D.L. Fetal Brain Extracellular Matrix Boosts Neuronal Network Formation in 3D Bioengineered Model of Cortical Brain Tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Arulmoli, J.; Pathak, M.M.; McDonnell, L.P.; Nourse, J.L.; Tombola, F.; Earthman, J.C.; Flanagan, L.A. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep. 2015, 5, 8499. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cheng, K.; Kisaalita, W. Three Dimensional Neuronal Cell Cultures More Accurately Model Voltage Gated Calcium Channel Functionality in Freshly Dissected Nerve Tissue. PLoS ONE 2012, 7, e45074. [Google Scholar] [CrossRef]
- Bourke, J.L.; Quigley, A.F.; Duchi, S.; O’Connell, C.D.; Crook, J.M.; Wallace, G.G.; Cook, M.J.; Kapsa, R.M.I. Three-dimensional neural cultures produce networks that mimic native brain activity. J. Tissue Eng. Regen. Med. 2018, 12, 490–493. [Google Scholar] [CrossRef]
- Magill, P.J.; Bolam, J.P.; Bevan, M.D. Relationship of Activity in the Subthalamic Nucleus–Globus Pallidus Network to Cortical Electroencephalogram. J. Neurosci. 2000, 20, 820–833. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Packard, J.A.; Leach, J.B.; Powell, E.M. Three-Dimensional Environment Sustains Morphological Heterogeneity and Promotes Phenotypic Progression During Astrocyte Development. Tissue Eng. Part A 2016, 22, 885–898. [Google Scholar] [CrossRef]
- Irons, H.R.; Cullen, D.K.; Shapiro, N.P.; Lambert, N.A.; Lee, R.H.; LaPlaca, M.C. Three-dimensional neural constructs: A novel platform for neurophysiological investigation. J. Neural Eng. 2008, 5, 333–341. [Google Scholar] [CrossRef]
- Kofron, C.M.; Fong, V.J.; Hoffman-Kim, D. Neurite outgrowth at the interface of 2D and 3D growth environments. J. Neural Eng. 2009, 6, 016002. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Sommer, G.; Haybaeck, J.; Steinmann, P.; Holzapfel, G.A.; Kuhl, E. Rheological characterization of human brain tissue. Acta Biomater. 2017, 60, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kong, W.; Li, B.; Ni, Y.; Yuan, T.; Guo, L.; Lin, H.; Fan, H.; Fan, Y.; Zhang, X. Fabrication and characterization of collagen-based injectable and self-crosslinkable hydrogels for cell encapsulation. Colloids Surf. B Biointerfaces 2018, 167, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.; Campbell, M.K.; Xiao, X.; Zhong, Q.; Brunken, W.J.; Miner, J.H.; Greer, C.A.; Koleske, A.J. CNS Neurons Deposit Laminin α5 to Stabilize Synapses. Cell Rep. 2017, 21, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; Cheresh, D.A. Vitronectin and its receptors. Curr. Opin. Cell Biol. 1993, 5, 864–868. [Google Scholar] [CrossRef]
- Wu, X.; Reddy, D.S. Integrins as receptor targets for neurological disorders. Pharmacol. Ther. 2012, 134, 68–81. [Google Scholar] [CrossRef]
- Lilja, J.; Ivaska, J. Integrin activity in neuronal connectivity. J. Cell Sci. 2018, 131, jcs212803. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Enosawa, S.; Zhang, B.; Yuan, W.; Chiba, T.; Fujie, M.G. Evaluation of fetal tissue viscoelastic characteristics for robotic fetal surgery. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 797–802. [Google Scholar] [CrossRef]
- Zimmermann, D.R.; Dours-Zimmermann, M.T. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008, 130, 635–653. [Google Scholar] [CrossRef]
- Shao, X.; Gao, D.; Chen, Y.; Jin, F.; Hu, G.; Jiang, Y.; Liu, H. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal. Chim. Acta 2016, 934, 186–193. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Buonocore, J.; Park, J.; Welsh, C.J.; Li, J.; Han, A. A three-dimensional arrayed microfluidic blood-brain barrier model with integrated electrical sensor array. IEEE Trans. Biomed. Eng. 2018, 65, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Zhang, Q.; Kuang, S.; Cheung, C.W.J.; Chu, K.A.; He, Y.; Yang, M.; Zhao, X. Engineering three-dimensional microenvironments towards in vitro disease models of the central nervous system. Biofabrication 2019, 11, 032003. [Google Scholar] [CrossRef] [PubMed]
- Forró, C.; Thompson-Steckel, G.; Weaver, S.; Weydert, S.; Ihle, S.; Dermutz, H.; Aebersold, M.J.; Pilz, R.; Demkó, L.; Vörös, J. Modular microstructure design to build neuronal networks of defined functional connectivity. Biosens. Bioelectron. 2018, 122, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, A.; Pigareva, Y.; Kutyina, D.; Kolpakov, V.; Bukatin, A.; Mukhina, I.; Kazantsev, V.; Pimashkin, A. Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels. Sci. Rep. 2017, 7, 15625. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijdeven, R.; Ramstad, O.H.; Valderhaug, V.D.; Köllensperger, P.; Sandvig, A.; Sandvig, I.; Halaas, Ø. A novel lab-on-chip platform enabling axotomy and neuromodulation in a multi-nodal network. Biosens. Bioelectron. 2019, 140, 111329. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Healthc. Mater. 2018, 7, 1–29. [Google Scholar] [CrossRef]
- Wevers, N.R.; Van Vught, R.; Wilschut, K.J.; Nicolas, A.; Chiang, C.; Lanz, H.L.; Trietsch, S.J.; Joore, J.; Vulto, P. High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci. Rep. 2016, 6, 38856. [Google Scholar] [CrossRef]
- Liu, J.; Sternberg, A.R.; Ghiasvand, S.; Berdichevsky, Y. Epilepsy-on-a-chip system for antiepileptic drug discovery. IEEE Trans. Biomed. Eng. 2019, 66, 1231–1241. [Google Scholar] [CrossRef]
- Huang, G.; Wang, L.; Wang, S.; Han, Y.; Wu, J.; Zhang, Q.; Xu, F.; Lu, T.J. Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication 2012, 4, 042001. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; Du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Stenger, D.A.; Shaffer, K.M.; Ma, W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001, 304, 189–193. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R. Roles of Extracellular Matrix in Neural Development. Annu. Rev. Physiol. 2003, 45, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.A.; Kim, J.W.; Kim, J.; Lee, S.; Kim, S.; Suh, W.H.; Kim, H.S.; Kwon, S.; Kim, S.J.; Suh, Y.H. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS ONE 2011, 6, e18738. [Google Scholar]
- Feng, J.F.; Liu, J.; Zhang, X.Z.; Zhang, L.; Jiang, J.Y.; Nolta, J.; Zhao, M. Brief report: Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Silva, J.P.; Rambo, C.R.; Barra, G.M.O.; Dourado, F.; Gama, F.M. Neuronal cells behavior on polypyrrole coated bacterial nanocellulose three-dimensional (3D) scaffolds. J. Biomater. Sci. Polym. Ed. 2013, 24, 1368–1377. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A.; Rahighi, R. Rolled graphene oxide foams as three-dimensional scaffolds for growth of neural fibers using electrical stimulation of stem cells. Carbon N. Y. 2016, 97, 71–77. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Burdick, J.A. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules 2011, 12, 2344–2350. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Stenger, D.A.; Shaffer, K.M.; Maric, D.; Barker, J.L.; Ma, W. Primary neural precursor cell expansion, differentiation and cytosolic Ca2+ response in three-dimensional collagen gel. J. Neurosci. Methods 2000, 102, 187–195. [Google Scholar] [CrossRef]
- Labour, M.N.; Vigier, S.; Lerner, D.; Marcilhac, A.; Belamie, E. 3D compartmented model to study the neurite-related toxicity of Aβ aggregates included in collagen gels of adaptable porosity. Acta Biomater. 2016, 37, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Swindle-Reilly, K.E.; Papke, J.B.; Kutosky, H.P.; Throm, A.; Hammer, J.A.; Harkins, A.B.; Willits, R.K. The impact of laminin on 3D neurite extension in collagen gels. J. Neural Eng. 2012, 9, 046007. [Google Scholar] [CrossRef] [PubMed]
- Krewson, C.E.; Chung, S.W.; Dai, W.; Mark Saltzman, W. Cell aggregation and neurite growth in gels of extracellular matrix molecules. Biotechnol. Bioeng. 1994, 43, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Milman, B.D.; Vanscoy, J.E.; Seidlits, S.K.; Grill, R.J.; Schmidt, C.E. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 2011, 8, 046033. [Google Scholar] [CrossRef] [PubMed]
- Tarus, D.; Hamard, L.; Caraguel, F.; Wion, D.; Szarpak-Jankowska, A.; Van Der Sanden, B.; Auzély-Velty, R. Design of Hyaluronic Acid Hydrogels to Promote Neurite Outgrowth in Three Dimensions. ACS Appl. Mater. Interfaces 2016, 8, 25051–25059. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Liang, J.; Bierman, R.D.; Sohrabi, A.; Karam, J.; Holley, S.M.; Cepeda, C.; Walthers, C.M. Peptide-modified, hyaluronic acid-based hydrogels as a 3D culture platform for neural stem/progenitor cell engineering. J. Biomed. Mater. Res. Part A 2019, 107, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.; Ramachandran, M.; Moharil, P.; Harumalani, M.; Jaiswal, A.K. Biomaterials and cells for cardiac tissue engineering: Current choices. Mater. Sci. Eng. C 2017, 79, 950–957. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, R.; Duan, B.; Jiang, P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B 2017, 5, 3870–3878. [Google Scholar] [CrossRef]
- Broguiere, N.; Isenmann, L.; Zenobi-Wong, M. Novel enzymatically cross-linked hyaluronan hydrogels support the formation of 3D neuronal networks. Biomaterials 2016, 99, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef]
- Al Rifai, N.; Hasan, A.; Kobeissy, F.; Gazalah, H.; Charara, J. Culture of PC12 neuronal cells in GelMA hydrogel for brain tissue engineering. In Proceedings of the 2015 International Conference on Advances in Biomedical Engineering, ICABME 2015, Beirut, Lebanon, 16–18 September 2015; pp. 254–257. [Google Scholar]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2018, 10, 17742–17755. [Google Scholar] [CrossRef]
- Magariños, A.M.; Pedron, S.; Creixell, M.; Kilinc, M.; Tabansky, I.; Pfaff, D.W.; Harley, B.A.C. The Feasibility of Encapsulated Embryonic Medullary Reticular Cells to Grow and Differentiate into Neurons in Functionalized Gelatin-Based Hydrogels. Front. Mater. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dillon, G.P.; Bellamkonda, R.V. A Laminin and Nerve Growth Factor-Laden Three-Dimensional Scaffold for Enhanced Neurite Extension. Tissue Eng. 1999, 5, 291–304. [Google Scholar] [CrossRef]
- Valmikinathan, C.M.; Mukhatyar, V.J.; Jain, A.; Karumbaiah, L.; Dasari, M.; Bellamkonda, R. V Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter 2012, 8, 1964–1976. [Google Scholar] [CrossRef]
- Palazzolo, G.; Broguiere, N.; Cenciarelli, O.; Dermutz, H.; Zenobi-Wong, M. Ultrasoft Alginate Hydrogels Support Long-Term Three-Dimensional Functional Neuronal Networks. Tissue Eng. Part A 2015, 21, 2177–2185. [Google Scholar] [CrossRef]
- Hayashi, Y.; Furue, M.K. Biological Effects of Culture Substrates on Human Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 5380560. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.R.; Quelhas, P.; Oliveira, M.J.; Pêgo, A.P.; Amaral, I.F. Three-dimensional culture of single embryonic stem-derived neural/stem progenitor cells in fibrin hydrogels: Neuronal network formation and matrix remodelling. J. Tissue Eng. Regen. Med. 2017, 11, 3494–3507. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 2007, 28, 5518–5525. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Monteiro, G.A.; Firestein, B.L.; Shreiber, D.I. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol. Bioeng. 2009, 102, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Molnar, P.; Gregory, C.; Das, M.; Boland, T.; Hickman, J.J. Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3D collagen hydrogel. Biomaterials 2009, 30, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Robel, S. Astroglial scarring and seizures: A cell biological perspective on epilepsy. Neuroscientist 2017, 23, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Gupta, P.K.; Diaz-Arrastia, R. Epilepsy after Traumatic Brain Injury; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; ISBN 9781466584914. [Google Scholar]
- Zhang, D.; Pekkanen-Mattila, M.; Shahsavani, M.; Falk, A.; Teixeira, A.I.; Herland, A. A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials 2014, 35, 1420–1428. [Google Scholar] [CrossRef]
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells – Vascular interactions. Biomaterials 2019, 198, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M.; Lee, M.W. Indirect fabrication of collagen scaffold based on inkjet printing technique. Rapid Prototyp. J. 2006, 12, 229–237. [Google Scholar] [CrossRef]
- Farrukh, A.; Ortega, F.; Fan, W.; Marichal, N.; Paez, J.I.; Berninger, B.; del Campo, A.; Salierno, M.J. Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis. Stem Cell Rep. 2017, 9, 1432–1440. [Google Scholar] [CrossRef]
- Tong, Y.W.; Shoichet, M.S. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials 2001, 22, 1029–1034. [Google Scholar] [CrossRef]
- Pautot, S.; Wyart, C.; Isacoff, E.Y. Colloid-guided assembly of oriented 3D neuronal networks. Nat. Methods 2008, 5, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Frega, M.; Tedesco, M.; Massobrio, P.; Pesce, M.; Martinoia, S. Network dynamics of 3D engineered neuronal cultures: A new experimental model for in-vitro electrophysiology. Sci. Rep. 2014, 4, 5489. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Dellacasa, E.; Raiteri, R.; Martinoia, S.; Pastorino, L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Benchérif, S.; Mooney, D.J.; Renaud, P. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A.; et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.K.; Feng, Z.-Q.; Tuck, S.J.; Corey, J.M. Electrospinning Fundamentals: Optimizing Solution and Apparatus Parameters. J. Vis. Exp. 2011, 47, e2494. [Google Scholar] [CrossRef] [PubMed]
- Kador, K.E.; Montero, R.B.; Venugopalan, P.; Hertz, J.; Zindell, A.N.; Valenzuela, D.A.; Uddin, M.S.; Lavik, E.B.; Muller, K.J.; Andreopoulos, F.M.; et al. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials 2013, 34, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014, 11, 066009. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef]
- Jakobsson, A.; Ottosson, M.; Zalis, M.C.; O’carroll, D.; Johansson, U.E.; Johansson, F. Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1563–1573. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing structural integrity of hydrogels by using highly organised melt electrospun fibre constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Schaefer, N.; Janzen, D.; Bakirci, E.; Hrynevich, A.; Dalton, P.D.; Villmann, C. 3D Electrophysiological Measurements on Cells Embedded within Fiber-Reinforced Matrigel. Adv. Healthc. Mater. 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Hanson Shepherd, J.N.; Parker, S.T.; Shepherd, R.F.; Gillette, M.U.; Lewis, J.A.; Nuzzo, R.G. 3D microperiodic hydrogel scaffolds for robust neuronal cultures. Adv. Funct. Mater. 2011, 21, 47–54. [Google Scholar] [CrossRef]
- Soman, P.; Tobe, B.T.D.; Lee, J.W.; Winquist, A.M.; Singec, I.; Vecchio, K.S.; Snyder, E.Y.; Chen, S. Three-dimensional scaffolding to investigate neuronal derivatives of human embryonic stem cells. Biomed. Microdevices 2012, 14, 829–838. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Ning, L.; Schreyer, D.J.; Chen, X. Bio-fabrication of peptide-modified alginate scaffolds: Printability, mechanical stability and neurite outgrowth assessments. Bioprinting 2019, 14, e00045. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- Lin, M.; Firoozi, N.; Tsai, C.T.; Wallace, M.B.; Kang, Y. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomater. 2019, 83, 119–129. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, F.; Huang, G.; Lu, T.J.; Xing, W. Single neuron capture and axonal development in three-dimensional microscale hydrogels. Lab Chip 2012, 12, 4724–4731. [Google Scholar] [CrossRef]
- Klein, F.; Striebel, T.; Fischer, J.; Jiang, Z.; Franz, C.M.; Von Freymann, G.; Wegener, M.; Bastmeyer, M. Elastic fully three-dimensional microstructure scaffolds for cell force measurements. Adv. Mater. 2010, 22, 868–871. [Google Scholar] [CrossRef]
- Turunen, S.; Joki, T.; Hiltunen, M.L.; Ihalainen, T.O.; Narkilahti, S.; Kellomäki, M. Direct Laser Writing of Tubular Microtowers for 3D Culture of Human Pluripotent Stem Cell-Derived Neuronal Cells. ACS Appl. Mater. Interfaces 2017, 9, 25717–25730. [Google Scholar] [CrossRef]
- Melissinaki, V.; Gill, A.A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.W.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011, 3, 045005. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Liu, X.; Yue, Z.; Wallace, G.G. Three-dimensional neuronal cell culture: In pursuit of novel treatments for neurodegenerative disease. MRS Commun. 2017, 7, 320–331. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D neural tissue models: From spheroids to bioprinting. Biomaterials 2017, 154, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.E.; Galvan, S.; Dini, D. Models and tissue mimics for brain shift simulations. Biomech. Model. Mechanobiol. 2018, 17, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Metter, R.B.; Burdick, J.A. Electrospun fibrous scaffolds with multiscale and photopatterned porosity. Macromol. Biosci. 2010, 10, 265–270. [Google Scholar] [CrossRef]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a Highly Aligned Neural Scaffold via a Table Top Stereolithography 3D Printing and Electrospinning. Tissue Eng. Part A 2016, 23, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ilkhanizadeh, S.; Teixeira, A.I.; Hermanson, O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 2007, 28, 3936–3943. [Google Scholar] [CrossRef]
- Weng, B.; Liu, X.; Shepherd, R.; Wallace, G.G. Inkjet printed polypyrrole/collagen scaffold: A combination of spatial control and electrical stimulation of PC12 cells. Synth. Met. 2012, 162, 1375–1380. [Google Scholar] [CrossRef]
- Ikeda, K.; Bekkers, J.M. Autapses. Curr. Biol. 2006, 16, R308. [Google Scholar] [CrossRef]
- McKenzie, A.T.; Wang, M.; Hauberg, M.E.; Fullard, J.F.; Kozlenkov, A.; Keenan, A.; Hurd, Y.L.; Dracheva, S.; Casaccia, P.; Roussos, P.; et al. Brain Cell Type Specific Gene Expression and Co-expression Network Architectures. Sci. Rep. 2018, 8, 8868. [Google Scholar] [CrossRef]
- Sigaard, R.K.; Kjær, M.; Pakkenberg, B. Development of the Cell Population in the Brain White Matter of Young Children. Cereb. Cortex 2016, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Naidich, T.P.; Nimchinsky, E.A. Cerebral Cortex. In Imaging of the Brain; Elsevier: Amsterdam, The Netherland, 2012; pp. 9–38. [Google Scholar]
- Thomas, M.; Willerth, S.M. 3-D Bioprinting of Neural Tissue for Applications in Cell Therapy and Drug Screening. Front. Bioeng. Biotechnol. 2017, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.S.; Bhattacharjee, T.; Niemi, S.R.; Balachandar, S.; Baldwin, N.; Ellison, S.T.; Taylor, C.R.; Sawyer, W.G.; Angelini, T.E. Three-dimensional printing with sacrificial materials for soft matter manufacturing. MRS Bull. 2017, 42, 571–577. [Google Scholar] [CrossRef]
- Lee, S.J.; Esworthy, T.; Stake, S.; Miao, S.; Zuo, Y.Y.; Harris, B.T.; Zhang, L.G. Advances in 3D Bioprinting for Neural Tissue Engineering. Adv. Biosyst. 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Aradhya, S.V.; Frei, M.; Hybertsen, M.S.; Venkataraman, L. Van der Waals interactions at metal/organic interfaces at the single-molecule level. Nat. Mater. 2012, 11, 872–876. [Google Scholar] [CrossRef]
- Pischedda, F.; Montani, C.; Obergasteiger, J.; Frapporti, G.; Corti, C.; Rosato Siri, M.; Volta, M.; Piccoli, G. Cryopreservation of Primary Mouse Neurons: The Benefit of Neurostore Cryoprotective Medium. Front. Cell. Neurosci. 2018, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Quasthoff, K.; Ferrea, S.; Fleischer, W.; Theiss, S.; Schnitzler, A.; Dihné, M.; Walter, J. Freshly frozen E18 rat cortical cells can generate functional neural networks after standard cryopreservation and thawing procedures. Cytotechnology 2015, 67, 419–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Day, A.G.; Bhangra, K.S.; Murray-Dunning, C.; Stevanato, L.; Phillips, J.B. The Effect of Hypothermic and Cryogenic Preservation on Engineered Neural Tissue. Tissue Eng. Part C Methods 2017, 23, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gryka, M.C.; Comi, T.J.; Forsyth, R.A.; Hadley, P.M.; Deb, S.; Bhargava, R. Controlled dissolution of freeform 3D printed carbohydrate glass scaffolds in hydrogels using a hydrophobic spray coating. Addit. Manuf. 2019, 26, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.D.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Lin, H.H.; Hsu, S. hui 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, J.A.; Yoon, W.H.; Kim, J.; Jung, S. Drop-on-demand inkjet-based cell printing with 30-μm nozzle diameter for cell-level accuracy. Biomicrofluidics 2016, 10, 064110. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Kador, K.E.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; Grogan, S.P.; Dorthé, E.W.; D’Lima, D.D. Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng. - Part A 2016, 22, 286–294. [Google Scholar] [CrossRef]
- Tse, C.; Whiteley, R.; Yu, T.; Stringer, J.; MacNeil, S.; Haycock, J.W.; Smith, P.J. Inkjet printing Schwann cells and neuronal analogue NG108-15 cells. Biofabrication 2016, 8. [Google Scholar] [CrossRef]
- Lorber, B.; Hsiao, W.K.; Hutchings, I.M.; Martin, K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2014, 6, 015001. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, C.; Chen, P.; Güven, S.; Demirtaş, T.T.; Nieland, T.J.F.; Padilla, F.; Demirci, U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Adv. Mater. 2016, 28, 161–167. [Google Scholar] [CrossRef]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.H.; Fischer, K.; Polio, S.; Park, J.K.; Yoo, S.S. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 2009, 20, 798–803. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef]
- de la Vega, L.; Rosas Gómez, D.A.; Abelseth, E.; Abelseth, L.; Allisson da Silva, V.; Willerth, S. 3D Bioprinting Human Induced Pluripotent Stem Cell-Derived Neural Tissues Using a Novel Lab-on-a-Printer Technology. Appl. Sci. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Zhu, W.; Harris, B.T.; Zhang, L.G. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4185–4188. [Google Scholar]
- Greer, J.E.; Povlishock, J.T.; Jacobs, K.M. Electrophysiological abnormalities in both axotomized and nonaxotomized pyramidal neurons following mild traumatic brain injury. J. Neurosci. 2012, 32, 6682–6687. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, V.K.; Polio, S.; Fischer, K.; Lee, J.H.; Park, J.K.; Yoo, S.S. Three-dimensional cell-hydrogel printer using electromechanical microvalve for tissue engineering. In Proceedings of the TRANSDUCERS 2009–2009 International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009; pp. 2230–2233. [Google Scholar]
- Haring, A.P.; Thompson, E.G.; Tong, Y.; Laheri, S.; Cesewski, E.; Sontheimer, H.; Johnson, B.N. Process- And bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 2019, 11, 025009. [Google Scholar] [CrossRef] [PubMed]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser bioprinting of human induced pluripotent stem cells—The effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef] [PubMed]


| Biomaterial | Conc. (w/v) | Mechanical Properties | Cell Type | Culture Details | Network Formation? | Functional? | Ref |
|---|---|---|---|---|---|---|---|
| Agarose | 0.5% | NA | Day-18 rat cortical neurons | 14 days | No | NA | [84] |
| Agarose | 1.0% | NA | DRGs | 6 days | No | NA | [110] |
| Alginate | 0.25% | 180 Pa(G’) | Rat hippocampal NSC | 7 days | No | NA | [115] |
| Alginate + PLGA microspheres | 1.0% | NA | Rat hippocampal NSC | 8 days | No | NA | [116] |
| Alginate | 0.1% | 10 Pa (G’) | Day-17 rat cortical neurons | 4 weeks | Yes | Yes 1,2, network multiple sites calcium flux-network | [112] |
| CMC | 0.5% | 104 ± 13.46 Pa(G’) | Rat hippocampal cortical neurons, DRGs and NSCs | 14 days | No | NA | [111] |
| Collagen type I | 0.04% | NA | Day-18 rat cortical neurons | 10 days | Yes | NA | [84] |
| Collagentype I | 0.3% | NA | NPCs | 30 days | No | Yes individual neurons only 1 | [93] |
| Collagen type I | 0.2% | 57–377 Pa (G’) | Chick dorsal root ganglions | 5 days | NA | NA | [117] |
| Collagen type I | 0.04% | 10 Pa (G*) | Rat dorsal root ganglions | 24 h | NA | NA | [95] |
| Collagen type I | 0.04% | NA | Day 13 rat cortical and subcortical neurons | 24 days | Yes | Yes, neuron whole cell patch clamp | [20] |
| Collagen type I | 0.04% | NA | Embryonic day-18 rat hippocampal neurons | 35 days | Yes | Yes, networkmultiple sites connected MEA | [58] |
| Collagen type I | 0.1% | NA | Embryonic day-18 rat hippocampal neurons | 21 days | Yes | Yes, neuron whole cell patch clamp | [118] |
| HA-SH + peptides | 4–6% | 188 ± 42 Pa (G’) | H9 human embryonic stem cells | 10 weeks | No | No, patch clamp recording cells immature | [100] |
| HA-SH+RGD | 3% | 400 Pa (G’) | Embryonic mouse hippocampal NPC | 21 days | NA | NA | [99] |
| HAMA (+peptides) | 1% | 130 Pa (E) | NPCs from normal and Rett syndrome patient derived hiPSCs | 3 weeks | Yes | Yes, neuron whole cell patch clamp | [40] |
| HAMA | 0.5% | 200 Pa (Ec) | mixed glial cells | 14 days | No | NA | [13] |
| HAMA | 0.5% | 510–1410 P (Ec) | NPCs from normal and Down syndrome patient derived hiPSCs | 28 days | No | NA | [103] |
| HAMA | 1.5% | 3000 Pa (Ec) | Rat ventral midbrain NPCs | 14 days | No | NA | [102] |
| HA-TG/Lys-Gln | 0.5% | 100 Pa (G’) | Embryonic rat cortical neurons | 2 months | Yes | Yes 1, network multiple sites calcium flux-network | [104] |
| GelMA/HAMA | (3.5/0.5%) | 1100 Pa (Ec) | E12.5 hindbrain cell | 15 days | Yes | NA | [109] |
| Matrigel | 0.05% | NA | Rat embryonic cortical neurons and astrocytes | 60 days | NA | Yes, neuron whole cell patch clamp | [61] |
| GelMA | 4% and 8% | NA | PC12 cells | 7 days | NA | Maybe, healed mouse brain injury | [107] |
| GelMA | 3% | 680 Pa(G’) | iPSC derived NPC | 7 days | NA | Maybe, restored functional recovery after SCI | [108] |
| Fibrin | 0.4–1% | 400–1200 Pa (G’) | Mouse embryo derived NPSC | 14 days | Yes | NA | [114] |
| PEG +RGD+ MMP degrading peptides | 4.4% | NA | iPSC derived NPC, EC and microglial cells | 21 days | NA | NA | [18] |
| Print Type | Cell Type | Bioink | Cell Morphology | Cell Viability | Mechanical Properties | Functionally Active | Ref |
|---|---|---|---|---|---|---|---|
| Extrusion | Primary rat cortical neurons | gellan gum-RGD | Dendrite extension, interconnecting networks. | 74 ± 2% | NA | NA | [186] |
| Extrusion | Murine NPCs | PCL diol & poly (D, L-lactide) diol | Globular, no dendrite extension | proliferation | 680 Pa (E) | Yes (zebra fish brain injury model) 1 | [184] |
| Extrusion | Human NPCs | alginate, agarose, carboxymethyl-chitosan. | Limited dendrite extension | 90% (day 7) | 7.5 kPa (Ec), 4.75 kPa (E) | Yes, neuron, calcium flux assay 2 | [182] |
| Extrusion | iPSCs | “ | “ | Not reported | “ | Yes, calcium flux assay 2 | [183] |
| Extrusion 3 | Schwann cells | Composite of alginate, RGD and YIGSR | Globular | 95% (Day 0), 95% (Day 7) | 40–14 kPa (day 0–15) (E) | NA | [145] |
| Extrusion 3 | Schwann cells | Composite of fibrin, HA and Factor XIII | Bipolar, aligned with strands | 98% | NA | NA | [201] |
| Extrusion | Schwann, neuronal (rodent) glioma (human) | Pluronic F-127, gelatin, HA | Globular | metabolic activity inc. | 6.7 kPa (G’) | NA | [200] |
| Extrusion | sNPC and OPCs | 50% Matrigel | Axon propagation | >75% (day 4) | ~55 kPa (E) | Yes, calcium flux assay 2 | [185] |
| Thermal inkjet | rat neurons | Liquid media | Dendrite extension | 72.4% | NA | Yes, neuron, patch clamp | [189] |
| Thermal inkjet | Rat neurons | Liquid media | NA | NA | 2.92 MPa (substrate) (E) | NA | [189] |
| Thermal Inkjet | Rat retinal ganglion cells | Liquid media containing BDNF & CNGF | Neurite extension | NS w.r.t. controls | NA | Yes, patch clamp | [190] |
| Peizo inkjet | Rat glial, retinal ganglion cells | 2D Liquid media (DMEM) | Dendrite extension | 69 ± 5% 69 ± 12% | NA | NA | [192] |
| Peizo inkjet | NG108-15,Human fibroblasts, Porcine Schwann cells | 2D Liquid media (DMEM) +10% feotal calf serum | Neurite extension | 86–96%, 82–92%, 89–92% | NA | NA | [191] |
| Bio-acoustic levitation | Neural progenitors (from human ESC) | Fibrin gel | Some neurite extension | ‘majority’ | 474 Pa (G’) | NA | [193] |
| Microvalve | Rat embryonic neurons | Layered collagen (0.2%) | NA | 78.6% | NA | NA | [199] |
| Microvalve | Embryonic rat neurons and astrocytes | Layered collagen (0.112%) | Neurite extension, 3D network | 78.6%, 78.7% | NA | NA | [194] |
| Microvalve | Murine neural stem cells | Layered collagen (0.116%), integrated VEGF- fibrin gel | Neurite extension | 92.89 ± 2.32% | NA | NA | [195] |
| Microfludic | iPSC derived NPCs | Fibrin, chitosan, alginate | Neurite extension, 3D networks | >81% | NA | NA | [196] |
| Direct laser writing | Chick spinal cord cells | Media | some neurite extension | Not quantified | NA | NA | [202] |
| Direct laser writing | hiPSCs | 15% hyaluronic acid in media | NA | 82 ± 1% | NA | NA | [203] |
| UV laser writing | Mouse NPCs | 10% GelMA, 10% GelMA + graphene nano platelets | neurite extension | Not quantified | 30 kPa (GelMA) (EC) | NA | [197] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antill-O’Brien, N.; Bourke, J.; O’Connell, C.D. Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models. Materials 2019, 12, 3218. https://doi.org/10.3390/ma12193218
Antill-O’Brien N, Bourke J, O’Connell CD. Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models. Materials. 2019; 12(19):3218. https://doi.org/10.3390/ma12193218
Chicago/Turabian StyleAntill-O’Brien, Natasha, Justin Bourke, and Cathal D. O’Connell. 2019. "Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models" Materials 12, no. 19: 3218. https://doi.org/10.3390/ma12193218
APA StyleAntill-O’Brien, N., Bourke, J., & O’Connell, C. D. (2019). Layer-By-Layer: The Case for 3D Bioprinting Neurons to Create Patient-Specific Epilepsy Models. Materials, 12(19), 3218. https://doi.org/10.3390/ma12193218





