Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing
Abstract
1. Introduction
2. The Motorised Prosthetic Limb
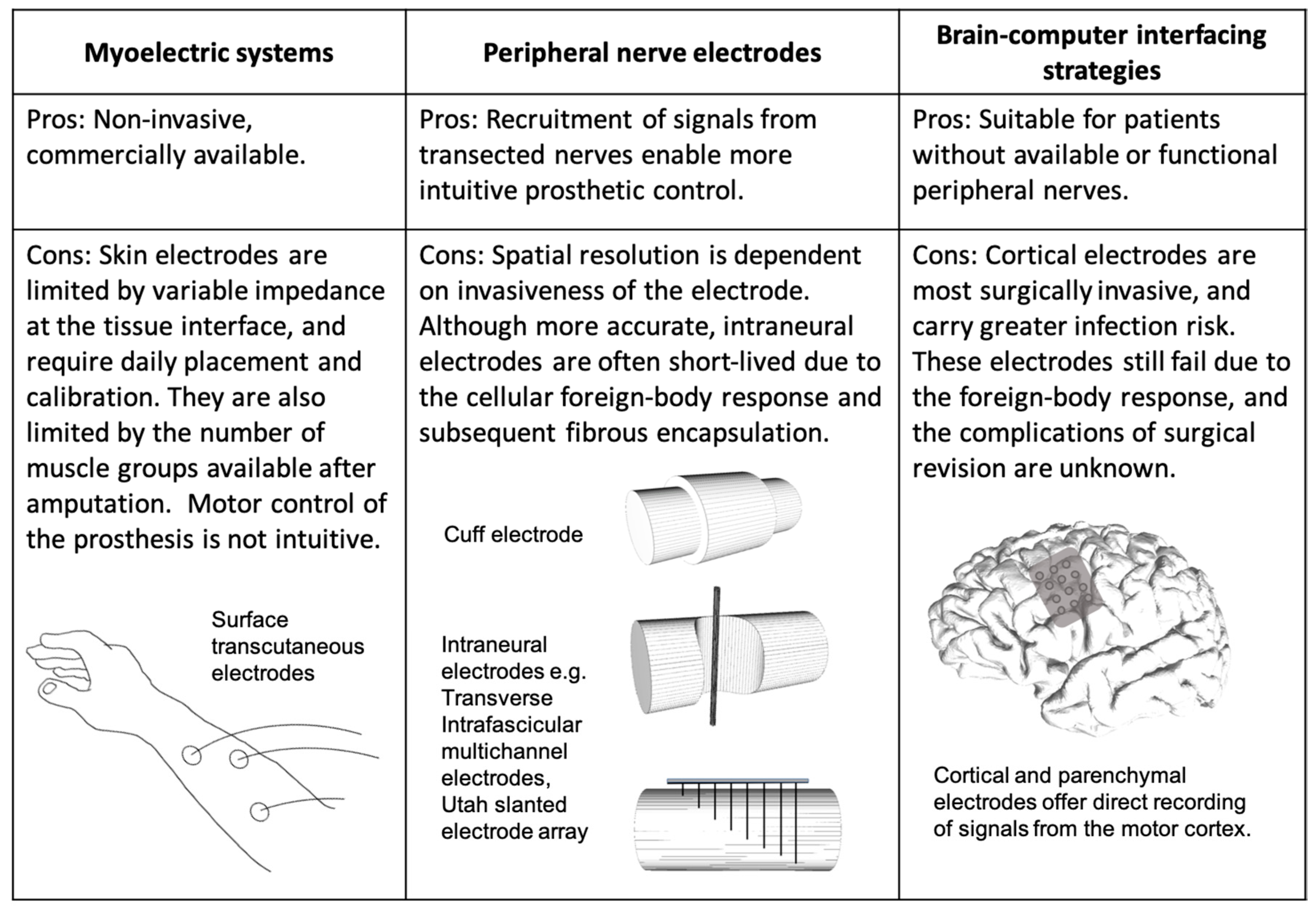
2.1. The Myoelectric Prosthesis
2.2. Peripheral Nerve Electrodes
2.3. Brain–Computer Interfaces
3. The Challenges of Tissue-Electronic Interfacing
4. Grafting Skeletal Muscle onto Residual Nerves
5. Tissue Engineering in the Neuroprosthetic Interface
6. Bioprinting and 3D Printing
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inkellis, E.; Low, E.E.; Langhammer, C.; Morshed, S. Incidence and Characterization of Major Upper-Extremity Amputations in the National Trauma Data Bank. JBJS Open Access 2018, 3, e0038. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Khare, M.; Singh, A.; Zamboni, P. Prospect of brain-machine interface in motor disabilities: The future support for multiple sclerosis patient to improve quality of life. Ann. Med. Health Sci. Res. 2014, 4, 305–312. [Google Scholar] [PubMed]
- Zuniga, J.; Katsavelis, D.; Peck, J.; Stollberg, J.; Petrykowski, M.; Carson, A.; Fernández, C. Cyborg beast: A low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes 2015, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kate Ten, J.; Smit, G.; Breedveld, P. 3D-printed upper limb prostheses: A review. Disabil. Rehabil. Assist. Technol. 2017, 12, 300–314. [Google Scholar] [CrossRef]
- van der Niet, O.; Bongers, R.M.; van der Sluis, C.K. Functionality of i-LIMB and i-LIMB pulse hands: Case report. J. Rehabil. Res. Dev. 2013, 50, 1123–1128. [Google Scholar] [CrossRef]
- Resnik, L.; Meucci, M.R.; Lieberman-Klinger, S.; Fantini, C.; Kelty, D.L.; Disla, R.; Sasson, N. Advanced upper limb prosthetic devices: Implications for upper limb prosthetic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 710–717. [Google Scholar] [CrossRef]
- O’Keeffe, B. Prosthetic rehabilitation of the upper limb amputee. Indian J. Plast. Surg. 2011, 44, 246–252. [Google Scholar] [CrossRef]
- Kejlaa, G.H. Consumer concerns and the functional value of prostheses to upper limb amputees. Prosthet. Orthot. Int. 1993, 17, 157–163. [Google Scholar]
- Marniemi, J.; Parkki, M.G. Radiochemical assay of glutathione S-epoxide transferase and its enhancement by phenobarbital in rat liver In Vivo. Biochem. Pharm. 1975, 24, 1569–1572. [Google Scholar] [CrossRef]
- Østlie, K.; Lesjø, I.M.; Franklin, R.J.; Garfelt, B.; Skjeldal, O.H.; Magnus, P. Prosthesis use in adult acquired major upper-limb amputees: Patterns of wear, prosthetic skills and the actual use of prostheses in activities of daily life. Disabil. Rehabil. Assist. Technol. 2012, 7, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.J.; Olson, J.L. The evolution of functional hand replacement: From iron prostheses to hand transplantation. Plast. Surg. 2014, 22, 44–51. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Nalpantidis, L.; Andersen, R.S.; Schou, C.; Bøgh, S.; Krüger, V.; Madsen, O. Robot skills for manufacturing: From concept to industrial deployment. Robot. Comput.-Integr. Manuf. 2016, 37, 282–291. [Google Scholar] [CrossRef]
- Resnik, L.; Klinger, S.L.; Etter, K. The DEKA Arm: Its features, functionality, and evolution during the Veterans Affairs Study to optimize the DEKA Arm. Prosthet. Orthot. Int. 2014, 38, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Johannes, M.S.; Bigelow, J.D.; Burck, J.M.; Harshbarger, S.D.; Kozlowski, M.V.; Van Doren, T. An Overview of the Developmental Process for the Modular Prosthetic Limb. John Hopkins APL Tech. Dig. 2011, 30, 207–216. [Google Scholar]
- Zhou, H.; Mohammadi, A.; Oetomo, D.; Alici, G. A Novel Monolithic Soft Robotic Thumb for an Anthropomorphic Prosthetic Hand. IEEE Robot. Autom. Lett. 2019, 4, 602–609. [Google Scholar] [CrossRef]
- Luchetti, M.; Cutti, A.G.; Verni, G.; Sacchetti, R.; Rossi, N. Impact of Michelangelo prosthetic hand: Findings from a crossover longitudinal study. J. Rehabil. Res. Dev. 2015, 52, 605–618. [Google Scholar] [CrossRef]
- Cipriani, C.; Controzzi, M.; Carrozza, M.C. The SmartHand transradial prosthesis. J. Neuroeng. Rehabil. 2011, 8, 29. [Google Scholar] [CrossRef]
- Castellini, C.; van der Smagt, P. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245–253. [Google Scholar]
- Cheesborough, J.E.; Smith, L.H.; Kuiken, T.A.; Dumanian, G.A. Targeted muscle reinnervation and advanced prosthetic arms. Semin. Plast. Surg. 2015, 29, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Loeb, G.E. Taking control of prosthetic arms. JAMA 2009, 301, 670–671. [Google Scholar] [CrossRef]
- Pasquina, P.F.; Evangelista, M.; Carvalho, A.J.; Lockhart, J.; Griffin, S.; Nanos, G.; McKay, P.; Hansen, M.; Ipsen, D.; Vandersea, J.; et al. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J. Neurosci. Methods 2015, 244, 85–93. [Google Scholar] [CrossRef]
- Christie, B.P.; Freeberg, M.; Memberg, W.D.; Pinault, G.J.C.; Hoyen, H.A.; Tyler, D.J.; Triolo, R.J. Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J. Neuroeng. Rehabil. 2017, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Silberstein, S.D.; Reed, K.L.; Deer, T.R.; Slavin, K.V.; Huh, B.; Sharan, A.D.; Narouze, S.; Mogilner, A.Y.; Trentman, T.L.; et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2015, 35, 344–358. [Google Scholar] [CrossRef]
- Booth, J.; Connelly, L.; Dickson, S.; Duncan, F.; Lawrence, M. The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: A systematic review. Neurourol. Urodyn. 2018, 37, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.M.; Dhillon, G.S.; Jensen, W.; Yoshida, K.; Horch, K.W. Acute peripheral nerve recording characteristics of polymer-based longitudinal intrafascicular electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef]
- Branner, A.; Stein, R.B.; Normann, R.A. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J. Neurophysiol. 2001, 85, 1585–1594. [Google Scholar] [CrossRef]
- Navarro, X.; Lago, N.; Vivo, M.; Yoshida, K.; Koch, K.P.; Poppendieck, W.; Micera, S. Neurobiological evaluation of thin-film longitudinal intrafascicular electrodes as a peripheral nerve interface. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; pp. 643–649. [Google Scholar]
- Kundu, A.; Harreby, K.R.; Yoshida, K.; Boretius, T.; Stieglitz, T.; Jensen, W. Stimulation selectivity of the “thin-film longitudinal intrafascicular electrode” (tfLIFE) and the ‘transverse intrafascicular multi-channel electrode’ (TIME) in the large nerve animal model. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 400–410. [Google Scholar] [CrossRef]
- Davis, T.S.; Wark, H.A.C.; Hutchinson, D.T.; Warren, D.J.; O’Neill, K.; Scheinblum, T.; Clark, G.A.; Normann, R.A.; Greger, B. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 2016, 13, 036001. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Stein, R.B.; Fernandez, E.; Aoyagi, Y.; Normann, R.A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 2004, 51, 146–157. [Google Scholar] [CrossRef]
- Christensen, M.B.; Pearce, S.M.; Ledbetter, N.M.; Warren, D.J.; Clark, G.A.; Tresco, P.A. The foreign body response to the Utah Slant Electrode Array in the cat sciatic nerve. Acta Biomater. 2014, 10, 4650–4660. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.H.; Zoratti, M.J.; Langhals, N.B.; Purcell, E.K. Regenerative Electrode Interfaces for Neural Prostheses. Tissue Eng. Part B Rev. 2016, 22, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.H.; Anand, S.; Tran, M.; Kanneganti, A.; Vasudevan, S.; Seifert, J.L.; Cheng, J.; Keefer, E.W.; Romero-Ortega, M.I. Chronic sensory-motor activity in behaving animals using regenerative multi-electrode interfaces. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1973–1976. [Google Scholar]
- Musick, K.M.; Rigosa, J.; Narasimhan, S.; Wurth, S.; Capogrosso, M.; Chew, D.J.; Fawcett, J.W.; Micera, S.; Lacour, S.P. Chronic multichannel neural recordings from soft regenerative microchannel electrodes during gait. Sci. Rep. 2015, 5, 14363. [Google Scholar] [CrossRef] [PubMed]
- Burle, B.; Spieser, L.; Roger, C.; Casini, L.; Hasbroucq, T.; Vidal, F. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. Int. J. Psychophysiol. 2015, 97, 210–220. [Google Scholar] [CrossRef]
- Birbaumer, N.; Hinterberger, T.; Kübler, A.; Neumann, N. The thought-translation device (TTD): Neurobehavioral mechanisms and clinical outcome. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 120–123. [Google Scholar] [CrossRef]
- Neuper, C.; Müller-Putz, G.R.; Scherer, R.; Pfurtscheller, G. Motor imagery and EEG-based control of spelling devices and neuroprostheses. Prog. Brain Res. 2006, 159, 393–409. [Google Scholar]
- Pfurtscheller, G.; Müller-Putz, G.R.; Pfurtscheller, J.; Rupp, R. EEG-based asynchronous BCI controls functional electrical stimulation in a tetraplegic patient. EURASIP J. Appl. Signal Process. 2005, 2005, 3152–3155. [Google Scholar] [CrossRef]
- Palmini, A.; Gambardella, A.; Andermann, F.; Dubeau, F.; Da Costa, J.C.; Tampieri, D.; Gloor, P.; Quesney, F.; Andermann, E.; Paglioli-Neto, E.; et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann. Neurol. 1995, 37, 476–487. [Google Scholar] [CrossRef]
- Yang, T.; Hakimian, S.; Schwartz, T.H. Intraoperative ElectroCorticoGraphy (ECog): Indications, techniques, and utility in epilepsy surgery. Epileptic Disord. 2014, 16, 271–279. [Google Scholar] [PubMed]
- Yanagisawa, T.; Hirata, M.; Saitoh, Y.; Kishima, H.; Matsushita, K.; Goto, T.; Fukuma, R.; Yokoi, H.; Kamitani, Y.; Yoshimine, T. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann. Neurol. 2012, 71, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Sato, K.; Watanabe, H.; Nishimura, Y.; Isa, T.; Kato, R.; Nakamura, T.; Yokoi, H. Brain-machine interface to control a prosthetic arm with monkey ECoGs during periodic movements. Front. Neurosci. Front. 2014, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Hotson, G.; McMullen, D.P.; Fifer, M.S.; Johannes, M.S.; Katyal, K.D.; Para, M.P.; Armiger, R.; Anderson, W.S.; Thakor, N.V.; Wester, B.A.; et al. Individual finger control of a modular prosthetic limb using high-density electrocorticography in a human subject. J. Neural Eng. 2016, 13, 026017. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, C.S.; Oh, S.J.; Al-Kofahi, Y.A.; Lim, Y.J.; Smith, K.L.; Turner, J.N.; De, S.; Roysam, B.; Shain, W.; Kim, S.J. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 2006, 3, 196–207. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; Van Der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Klinger, S.L.; Etter, K. User and clinician perspectives on DEKA arm: Results of VA study to optimize DEKA arm. J. Rehabil. Res. Dev. 2014, 51, 27–38. [Google Scholar] [CrossRef]
- Oxley, T.J.; Opie, N.L.; Rind, G.S.; Liyanage, K.; John, S.E.; Ronayne, S.; McDonald, A.J.; Dornom, A.; Lovell, T.J.H.; Mitchell, P.J.; et al. An ovine model of cerebral catheter venography for implantation of an endovascular neural interface. J. Neurosurg. 2018, 128, 1020–1027. [Google Scholar] [CrossRef]
- Oxley, T.J.; Opie, N.L.; E John, S.; Rind, G.S.; Ronayne, S.M.; Wheeler, T.L.; Judy, J.W.; McDonald, A.J.; Dornom, A.; Lovell, T.J.H.; et al. Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat. Biotechnol. 2016, 34, 320–327. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. 2007, 83, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Henson, P.M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J. Immunol. Am. Assoc. Immunol. 1971, 107, 1535–1546. [Google Scholar]
- Ratner, B.D. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J. Control. Release 2002, 78, 211–218. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Pachter, J.S.; de Vries, H.E.; Fabry, Z. The Blood-Brain Barrier and Its Role in Immune Privilege in the Central Nervous System. J. Neuropathol. Exp. Neurol. 2003, 62, 593–604. [Google Scholar] [CrossRef]
- Griffith, R.W.; Humphrey, D.R. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci. Lett. 2006, 406, 81–86. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. Activated Macrophages and the Blood–Brain Barrier: Inflammation after CNS Injury Leads to Increases in Putative Inhibitory Molecules. Exp. Neurol. 1997, 148, 587–603. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Blomstedt, G.C. Infections in neurosurgery: A retrospective study of 1143 patients and 1517 operations. Acta Neurochir. 1985, 78, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Helton, K.L.; Ratner, B.D.; Wisniewski, N.A. Biomechanics of the Sensor-Tissue Interface—Effects of Motion, Pressure, and Design on Sensor Performance and the Foreign Body Response—Part I: Theoretical Framework. J. Diabetes Sci. Technol. 2011, 5, 632–646. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation Versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 1148, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Cağavi, F.; Akalan, N.; Celik, H.; Gür, D.; Güçiz, B. Effect of hydrophilic coating on microorganism colonization in silicone tubing. Acta Neurochir. 2004, 146, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.W.; Ortiz, V.; Gonzalo, C.P.; Moulton, S.E.; Wang, X.; Martin, L.L.; Michalczky, A.; Howlett, P.C. Lubricin Antiadhesive Coatings Exhibit Size-Selective Transport Properties that Inhibit Biofouling of Electrode Surfaces with Minimal Loss in Electrochemical Activity. Adv. Mater. Interfaces 2018, 5, 1701296. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Hatzipantelis, K.P.; Natsis, K.; Albani, M. Effect of acute limb ischaemia on neuromuscular function in rats. Eur. J. Surg. 2001, 167, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, J.; Teräväinen, H. Ultrastructure of striated muscle of the rat after temporary ischemia. Acta Neuropathol. 1977, 37, 237–245. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Hopkins, P.M. Skeletal muscle physiology. Contin. Educ. Anaesth. Crit. Care Pain. 2006, 6, 1–6. [Google Scholar] [CrossRef]
- Close, R.I. Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 1972, 52, 129–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, W.; Yue, Z.; Too, C.O.; Wallace, G.G. Buckled, Stretchable Polypyrrole Electrodes for Battery Applications. Adv. Mater. 2011, 23, 3580–35844. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.; Gentle, I.; Lu, G.; Cheng, H.M. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Hansen, T.S.; West, K.; Hassager, O.; Larsen, N.B. Highly Stretchable and Conductive Polymer Material Made from Poly(3,4-ethylenedioxythiophene) and Polyurethane Elastomers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Park, J.; Choi, S.; Janardhan, A.H.; Lee, S.-Y.; Raut, S.; Soares, J.; Shin, K.; Yang, S.; Lee, C.; Kang, K.W.; et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 2016, 8, 344ra86. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Guvanasen, G.S.; Xi, L.; Tuthill, C.; Nichols, T.R.; DeWeerth, S.P. A PDMS-based integrated stretchable microelectrode array (isMEA) for neural and muscular surface interfacing. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lobodzinski, S.S.; Laks, M. New material for implantable cardiac leads. J. Electrocardiol. 2009, 42, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Langhals, N.B.; Woo, S.L.; Moon, J.D.; Larson, J.V.; Leach, M.K.; Cederna, P.S.; Urbanchek, M.G. Electrically stimulated signals from a long-term Regenerative Peripheral Nerve Interface. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1989–1992. [Google Scholar]
- Ursu, D.C.; Urbanchek, M.G.; Nedic, A.; Cederna, P.S.; Gillespie, R.B. In Vivo characterization of regenerative peripheral nerve interface function. J. Neural Eng. 2016, 13, 026012. [Google Scholar] [CrossRef]
- Irwin, Z.T.; Schroeder, K.E.; Vu, P.P.; Tat, D.M.; Bullard, A.J.; Woo, S.L.; Sando, I.C.; Urbanchek, M.G.; Cederna, P.S.; Chestek, C.A. Chronic recording of hand prosthesis control signals via a regenerative peripheral nerve interface in a rhesus macaque. J. Neural Eng. 2016, 13, 046007. [Google Scholar] [CrossRef]
- Vu, P.P.; Irwin, Z.T.; Bullard, A.J.; Ambani, S.W.; Sando, I.C.; Urbanchek, M.G.; Cederna, P.S.; Chestek, C.A. Closed-Loop Continuous Hand Control via Chronic Recording of Regenerative Peripheral Nerve Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 515–526. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film*. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Ludwig, K.A.; Marzullo, T.C.; Martin, D.C.; Kipke, D.R. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly(3,4-ethylenedioxythiophene) Nanotubes. Adv. Mater. 2009, 21, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Wiler, J.A.; Anderson, D.J.; Kipke, D.R.; Martin, D.C. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010, 6, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lim, K.S.; Henderson, W.C.; Hassarati, R.T.; Martens, P.J.; Lovell, N.H.; Poole-Warren, L.A. Living electrodes: Tissue engineering the neural interface. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6957–6960. [Google Scholar]
- Urbanchek, M.G.; Kung, T.A.; Frost, C.M.; Martin, D.C.; Larkin, L.M.; Wollstein, A.; Cederna, P.S. Development of a Regenerative Peripheral Nerve Interface for Control of a Neuroprosthetic Limb. Biomed. Res. Int. 2016, 2016, 5726730. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Cooke, M.E.; Weber, M.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D. The adoption of three-dimensional additive manufacturing from biomedical material design to 3d organ printing. Appl. Sci. 2019, 9, 811. [Google Scholar]
- Kim, J.H.; Seol, Y.-J.; Ko, I.K.; Kang, H.-W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, J.; Choong, P.F.M. Three-dimensional printed calcaneal prosthesis following total calcanectomy. Int. J. Surg. Case Rep. 2015, 10, 83–87. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kanga, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip. R. Soc. Chem. 2016, 16, 1720–1742. [Google Scholar]
- Smith, L.J.; Li, P.; Holland, M.R.; Ekser, B. FABRICA: A Bioreactor Platform for Printing, Perfusing, Observing, & Stimulating 3D Tissues. Sci. Rep. 2018, 8, 7561. [Google Scholar] [PubMed]
- Kamei, K.-I.; Mashimo, Y.; Koyama, Y.; Fockenberg, C.; Nakashima, M.; Nakajima, M.; Nakajima, M.; Li, J.; Chen, Y. 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed. Microdevices 2015, 17, 6801. [Google Scholar] [CrossRef] [PubMed]
- Erkal, J.L.; Selimovic, A.; Gross, B.C.; Lockwood, S.Y.; Walton, E.L.; McNamara, S.; Martin, R.S.; Spence, D.M. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab Chip. R. Soc. Chem. 2014, 14, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.Y.; Lim, H.N.; Mahdi, M.A.; Wahid, M.H.; Huang, N.M. Three-Dimensional Printed Electrode and Its Novel Applications in Electronic Devices. Sci. Rep. 2018, 8, 7399. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Cao, Q.; Puthongkham, P.; Lee, S.T.; Ganesana, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Electrodes for Neurotransmitter Detection. Angew. Chem. Int. Ed. Engl. 2018, 57, 14255–14259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, C.; Gorkin, R., III; Beirne, S.; Shu, K.; Wallace, G.G. Three dimensional (3D) printed electrodes for interdigitated supercapacitors. Electrochem. Commun. 2014, 41, 20–23. [Google Scholar] [CrossRef]
- Ngan, C.G.Y.; O’Connell, C.D.; Blanchard, R.; Boyd-Moss, M.; Williams, R.J.; Bourke, J.; Quigley, A.; McKelvie, P.; Kapsa, R.M.I.; Choong, P.F.M. Optimising the biocompatibility of 3D printed photopolymer constructs in vitro and In Vivo. Biomed. Mater. 2019, 14, 035007. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngan, C.G.Y.; Kapsa, R.M.I.; Choong, P.F.M. Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing. Materials 2019, 12, 1927. https://doi.org/10.3390/ma12121927
Ngan CGY, Kapsa RMI, Choong PFM. Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing. Materials. 2019; 12(12):1927. https://doi.org/10.3390/ma12121927
Chicago/Turabian StyleNgan, Catherine G.Y., Rob M.I. Kapsa, and Peter F.M. Choong. 2019. "Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing" Materials 12, no. 12: 1927. https://doi.org/10.3390/ma12121927
APA StyleNgan, C. G. Y., Kapsa, R. M. I., & Choong, P. F. M. (2019). Strategies for neural control of prosthetic limbs: from electrode interfacing to 3D printing. Materials, 12(12), 1927. https://doi.org/10.3390/ma12121927





