Mechanical and Corrosion Resistance Enhancement of Closed-Cell Aluminum Foams through Nano-Electrodeposited Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Solution
2.2. Morphology Investigation
2.3. Properties Investigation
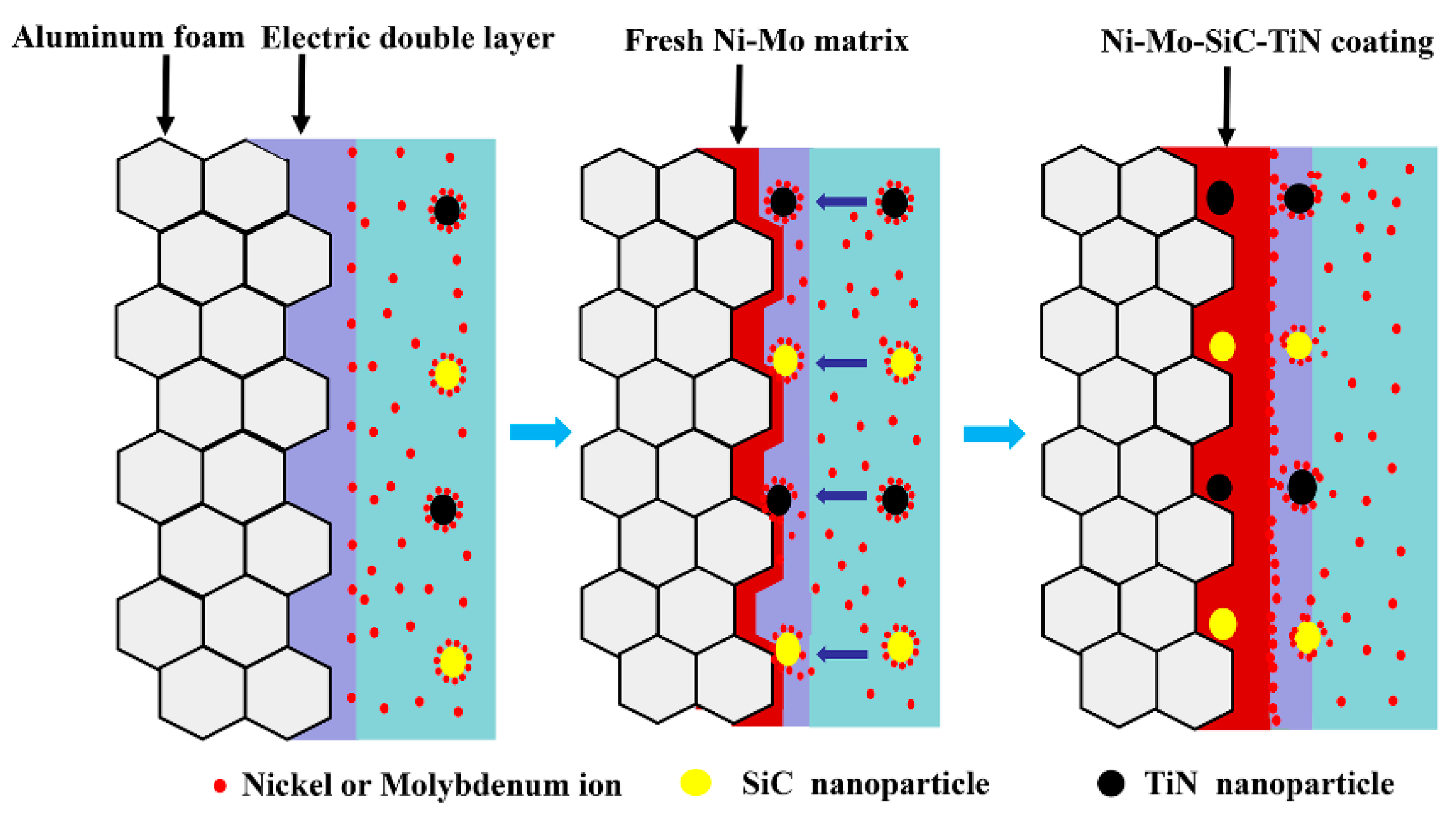
3. Theoretical Models
4. Results and Discussion
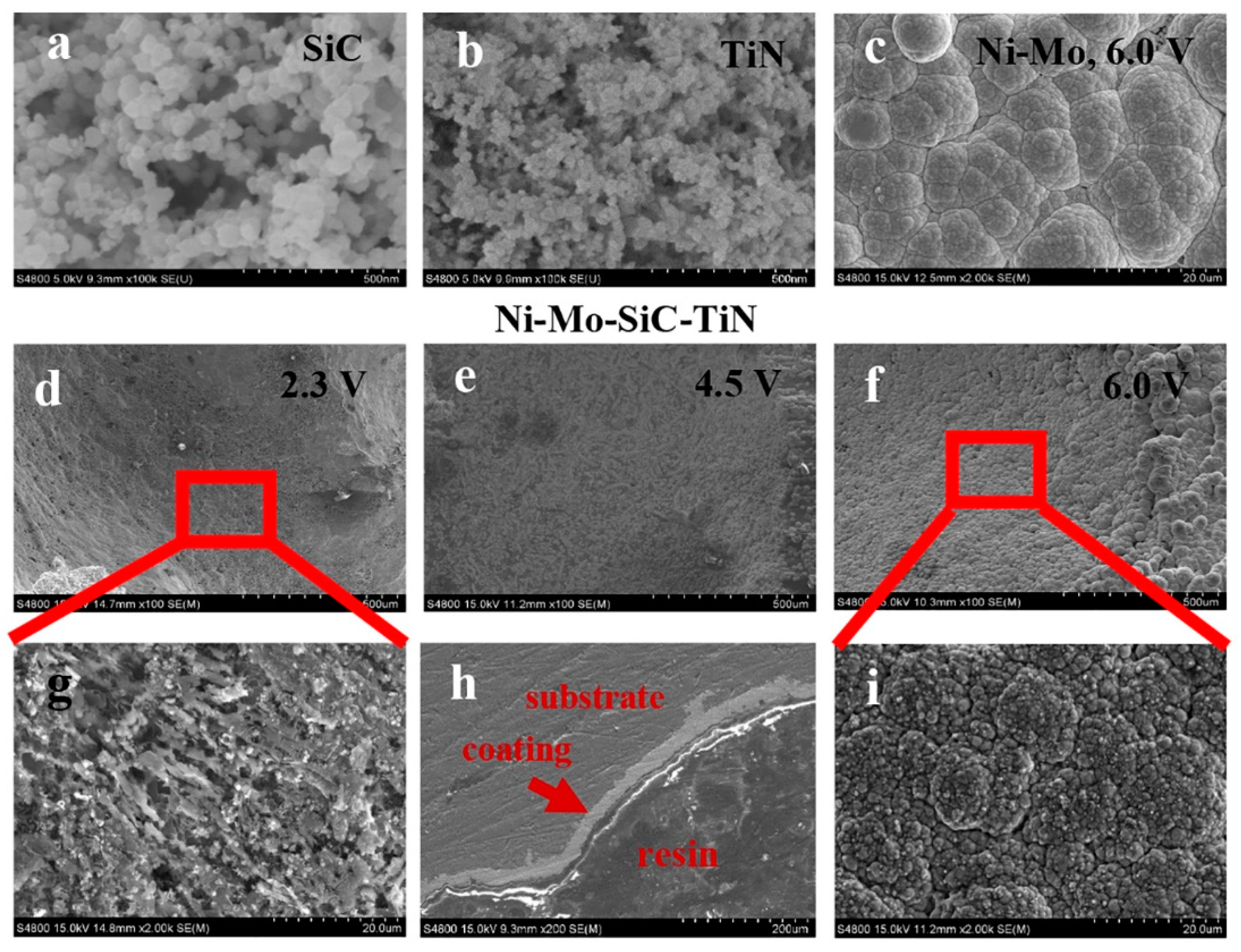
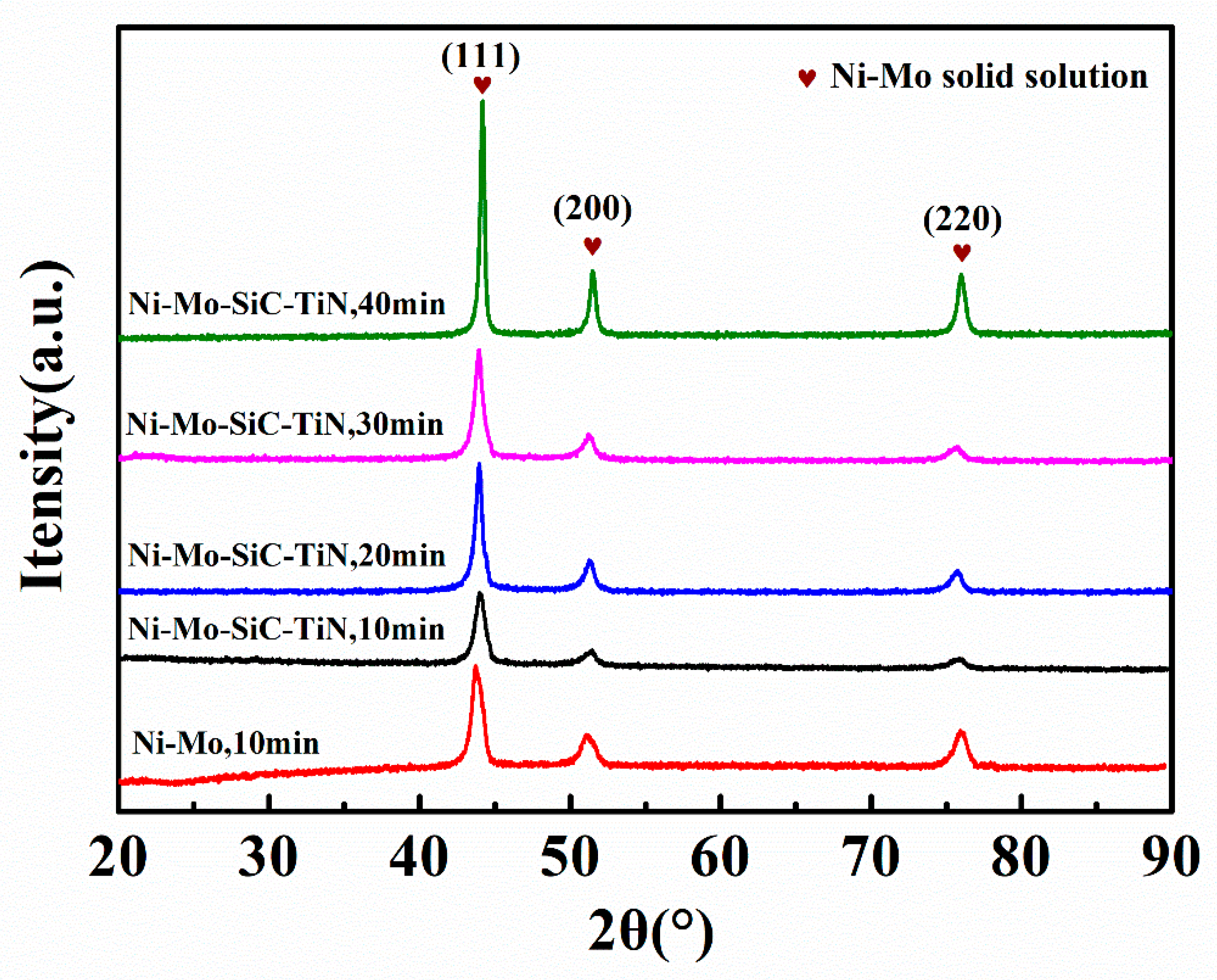
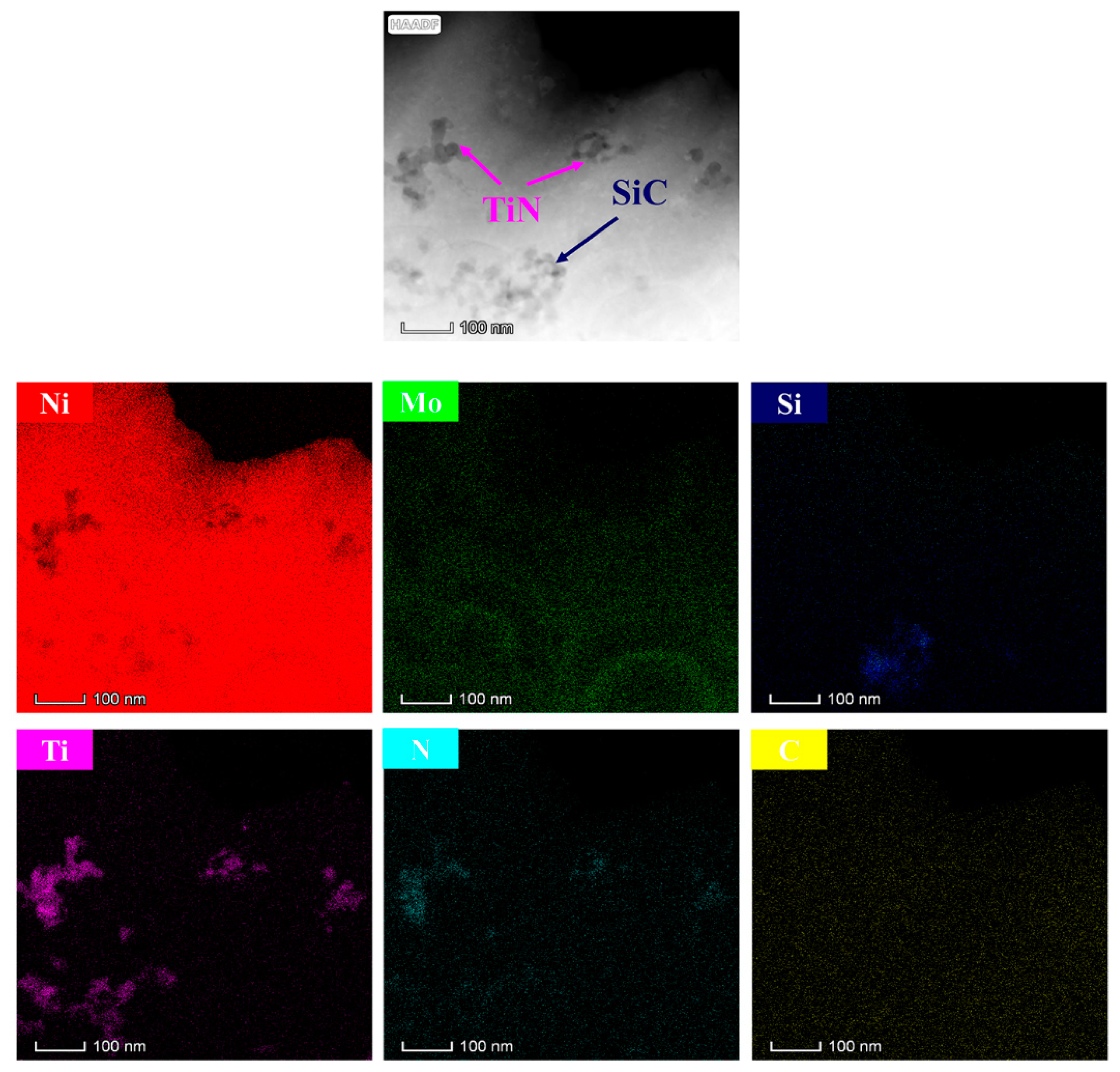
4.1. Coating Characterization
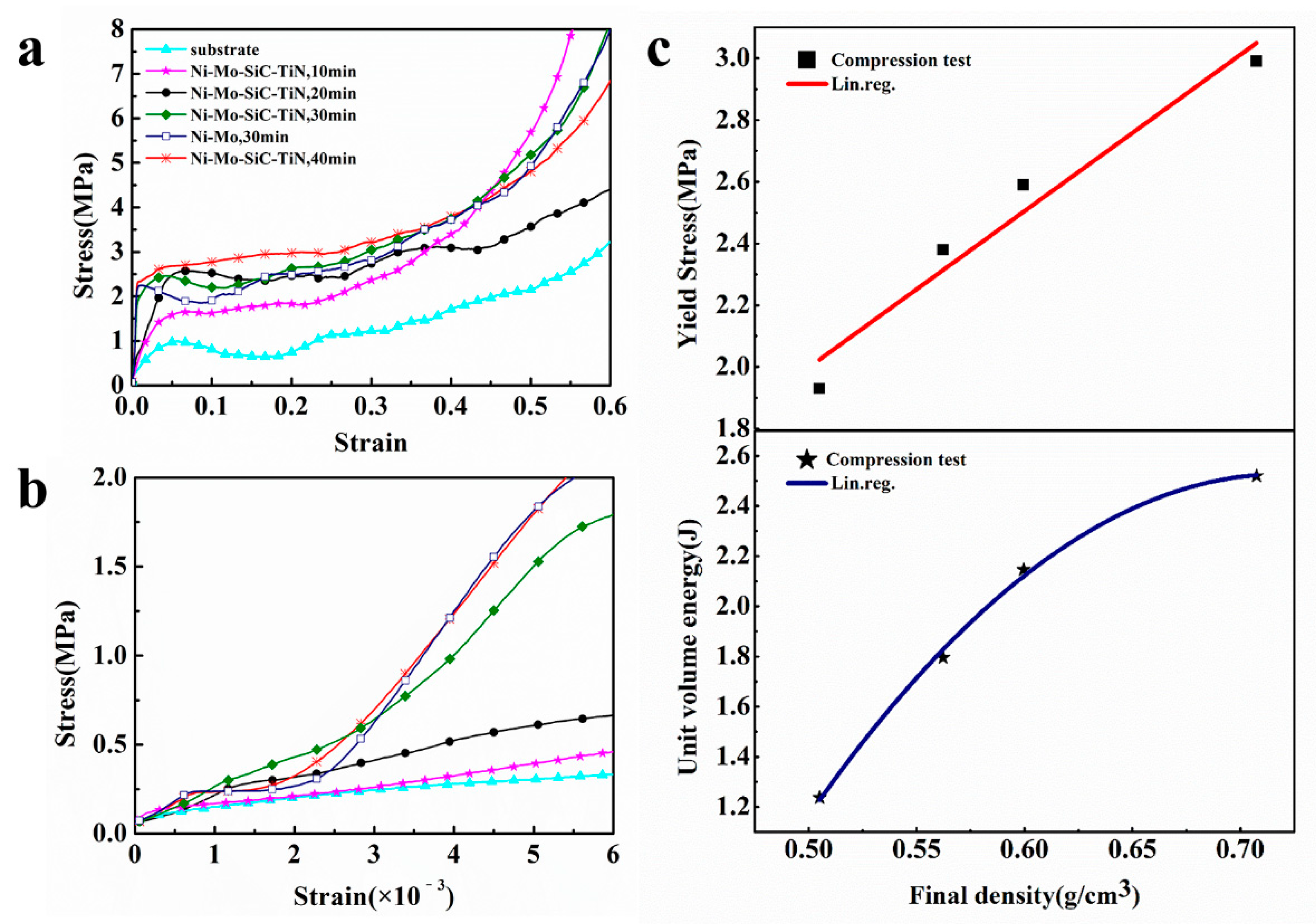
4.2. Mechanical Behavior
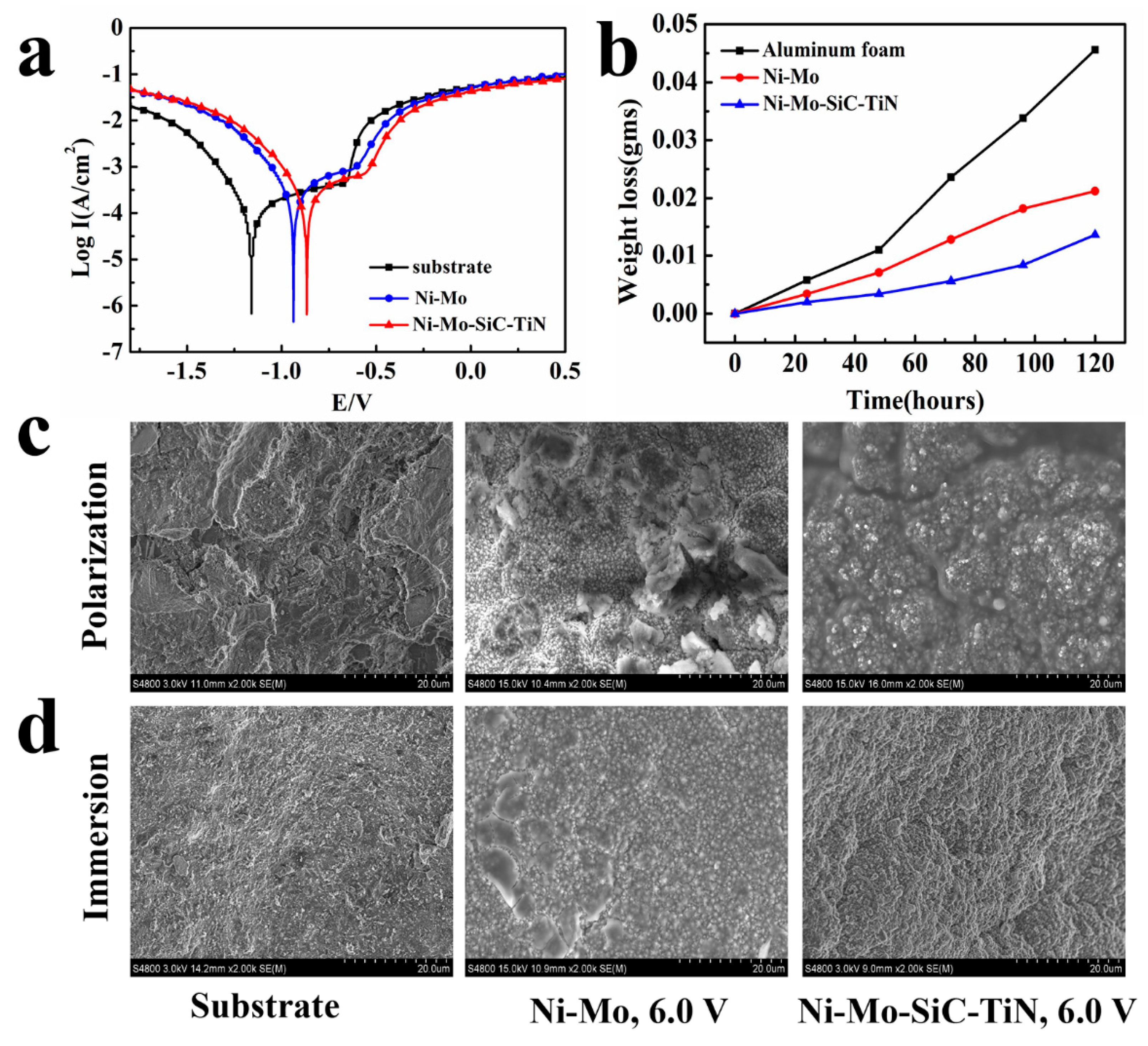
4.3. Corrosion Resistance
5. Conclusions
- A uniform and dense duplex nanoparticles-reinforced Ni–Mo coating with a thickness of 25 μm was obtained by electroplating on the aluminum foam surface for 10 min at 6.0 V. The bond between the substrate and the coating was good.
- The duplex nanoparticles reinforced Ni–Mo coating had a structure of FCC. The crystallite size of the Ni–Mo coatings was decreased from 13.31 nm to 12.14 nm after adding the duplex nanoparticles. The results indicate that increasing the electrodeposition time can effectively enlarge the crystallite size.
- After the aluminum foams were coated with a duplex nanoparticles-reinforced Ni–Mo coating, there was a significant improve in the mechanical properties of the aluminum foams. When the electrodeposition time was 40 min, the Wv of the aluminum foam increased from 0.852 J to 2.520 J, and the σs increased from 1.06 MPa to 2.99 MPa. The addition of nanoparticles made a limited improvement to the mechanical properties.
- The duplex nanoparticles-reinforced Ni–Mo coating was found to have better corrosion resistance. Compared to the aluminum foams, the self-corrosion potential, the pitting potential, and the potential for primary passivation were positively shifted by 294 mV, 99 mV, and 301 mV, respectively. The corrosion rate of the aluminum foam covered with a Ni–Mo coating was reduced by 51.9%. After adding nanoparticles, the corrosion rate was reduced by 72.5%. The nanoparticles obviously improved the corrosion resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Banhart, J.; Baumeister, J.; Weber, M. Damping properties of aluminum foams. Mater. Sci. Eng. A 1996, 205, 221–228. [Google Scholar] [CrossRef]
- Uzun, A.; Karakoc, H.; Gokmen, U.; Cinici, H. Investigation of mechanical properties of tubular aluminum foams. Int. J. Mater. Res. 2017, 107, 996. [Google Scholar] [CrossRef]
- Gong, L.; Kyriakides, S.; Jang, W.Y. Compressive response of open-cell foams. Part I: Morphology and elastic properties. Int. J. Solids Struct. 2005, 42, 1355–1379. [Google Scholar] [CrossRef]
- Song, H.W.; Fan, Z.J.; Yu, G.; Wang, Q.C.; Tobota, A. Partition energy absorption of axially crushed aluminum foam-filled hat sections. Int. J. Solids Struct. 2005, 42, 2575–2600. [Google Scholar] [CrossRef]
- Wang, W.; Burgueño, R.; Hong, J.W.; Lee, I. Nano-deposition on 3-d open-cell aluminum foam materials for improved energy absorption capacity. Mater. Sci. Eng. 2013, 572, 75–82. [Google Scholar] [CrossRef]
- Kim, A.; Hasan, M.A.; Nahm, S.H. Evaluation of compressive mechanical properties of Al-foams using electrical conductivity. Compos. Struct. 2005, 71, 191–198. [Google Scholar] [CrossRef]
- Rajendran, R.; Sai, K.P.; Chandrasekar, B.; Gokhale, A.; Basu, S. Preliminary investigation of aluminium foam as an energy absorber for nuclear transportation cask. Mater. Des. 2008, 29, 1732–1739. [Google Scholar] [CrossRef]
- Liang, L.S.; Yao, G.C.; Wang, L.; Ma, J.; Hua, Z.S. Sound absorption of perforated closed-cell aluminum foam. Chin. J. Nonferrous Met. 2010, 20, 2372–2376. [Google Scholar]
- Zhang, C.J.; Feng, Y.; Zhang, X.B. Mechanical properties and energy absorption properties of aluminum foamfilled square tubes. Trans. Nonferrous Met. Soc. China 2010, 20, 1380–1386. [Google Scholar] [CrossRef]
- Liu, H.; Yao, G.C.; Cao, Z.K.; Hua, Z.K.; Shi, J.C. Properties of aluminum foams with electrodeposited Ni coatings. Chin. J. Nonferrous Met. 2012, 22, 2572–2577. [Google Scholar]
- Marchi, C.S.; Mortensen, A. Deformation of open-cell aluminum foam. Acta Mater. 2001, 49, 3959–3969. [Google Scholar] [CrossRef][Green Version]
- Jung, A.; Natter, H.; Diebels, S.; Lach, E.; Hempelmann, R. Nanonickel Coated Aluminum Foam for Enhanced Impact Energy Absorption. Adv. Eng. Mater. 2011, 13, 23–28. [Google Scholar] [CrossRef]
- Jung, A.; Lach, E.; Diebels, S. New hybrid foam materials for impact protection. Int. J. Impact Eng. 2014, 64, 30–38. [Google Scholar] [CrossRef]
- Yi, F.; Zhu, Z.; Zu, F.; Hu, S.; Yi, P. Strain rate effects on the compressive property and the energy-absorbing capacity of aluminum alloy foams. Mater. Charact. 2001, 47, 417–422. [Google Scholar] [CrossRef]
- Evans, A.G.; Hutchinson, J.W.; Ashby, M.F. Multifunctionality of cellular metal systems. Prog. Mater. Sci. 1998, 43, 171–221. [Google Scholar] [CrossRef]
- Gibson, L. Mechanical behavior of metallic foams. Annu. Rev. Mater. Sci. 2000, 30, 191–227. [Google Scholar] [CrossRef]
- Baumeister, J.; Banhart, J. Weber M. Aluminium foams for transport industry. Mater. Des. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Barchi, L.; Bardi, U.; Caporali, S.; Fantini, M.; Scrivani, A.; Scrivani, A. Electroplated bright aluminium coatings for anticorrosion and decorative purposes. Prog. Org. Coat. 2010, 68, 120–125. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.D.; Wang, J.; Sun, B.D. High temperature corrosion behavior of microcrystalline aluminide coatings by electro-pulse deposition. Trans. Nonferrous Met. Soc. China 2008, 18, 13–17. [Google Scholar]
- Lu, J.; Do, I.; Drzal, L.T.; Worden, R.M.; Lee, I. Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2008, 2, 1825–1832. [Google Scholar] [CrossRef]
- Boonyongmaneerat, Y.; Schuh, C.A.; Dunand, D.C. Mechanical properties of reticulated aluminum foams with electrodeposited Ni–W coatings. Scr. Mater. 2008, 59, 336–339. [Google Scholar] [CrossRef]
- Li, Z.D.; Huang, Y.J.; Wang, X.F.; Wang, X.F.; Wang, D.; Han, F.S. Enhancement of open cell aluminum foams through thermal evaporating Zn film. Mater. Lett. 2016, 172, 120–124. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.Y.; Jothi, S.; Diao, W.; Yu, S. Increased Corrosion Resistance of Closed-Cell Aluminum Foams by Electroless Ni-P Coatings. Mater. Trans. 2011, 52, 2282–2284. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Winiarski, J.; Szczygiel, B.; Szczygiel, I. Electrodeposition and characterization of Ni–Mo–ZrO2 composite coatings. Appl. Surf. Sci. 2016, 369, 224–231. [Google Scholar] [CrossRef]
- Alizadeh, M.; Cheshmpish, A. Electrodeposition of Ni-Mo/Al2O3 nano-composite coatings at various deposition current densities. Appl. Surf. Sci. 2019, 466, 433–440. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Indyka, P. Kinetics of Ni–Mo electrodeposition from Ni-rich citrate baths. Thin Solid Film 2012, 520, 2046–2051. [Google Scholar] [CrossRef]
- Chang, C.S.; Hou, K.H.; Ger, M.D.; Chung, C.K.; Lin, J.F. Effects of annealing temperature on microstructure, surface roughness, mechanical and tribological properties of Ni–P and Ni–P/SiC films. Surf. Coat. Technol. 2016, 288, 135–143. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, F.Q.; Wu, X.Q.; Zhao, W.D.; Chen, X. A novel plating apparatus for electrodeposition of Ni-SiC composite coatings using circulating-solution co-deposition technique. J. Alloy Compd. 2017, 699, 366–377. [Google Scholar] [CrossRef]
- Kumar, K.A.; Kalaignan, G.P.; Muralidharan, V.S. Pulse and Pulse Reverse Current Electrodeposition and Characterization of Ni–W–TiN Composites. Sci. Adv. Mater. 2012, 4, 1039–1046. [Google Scholar] [CrossRef]
- Chen, F.C.; Liu, X.J. Study on Electrodeposition of Ni-Mo Alloy. J. Hunan Univ. Nat. Sci. 2014, 41, 44–67. [Google Scholar]
- Antenucci, A.; Guarino, S.; Tagliaferri, V.; Ucciardello, N. Improvement of the mechanical and thermal characteristics of open cell aluminum foams by the electrodeposition of Cu. Mater. Des. 2014, 59, 124–129. [Google Scholar] [CrossRef]
- Li, B.S.; Li, X.; Huan, Y.X.; Xia, W.Z.; Zhang, W.W. Influence of alumina nanoparticles on microstructure and properties of Ni-B composite coating. J. Alloy Compd. 2018, 762, 132–142. [Google Scholar] [CrossRef]
- Smith, B.H.; Szyniszewski, S.; Hajjar, J.F.; Schafer, B.W.; Arwade, S.R. Steel Foam for Structures: A Review of Applications, Manufacturing and Material Properties. J. Constr. Steel Res. 2012, 71, 1–10. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kyriakides, S.; Kraynik, A.M. On the compressive strength of open-cell metal foams with Kelvin and random cell structures. Int. J. Solids Struct. 2010, 47, 2872–2883. [Google Scholar] [CrossRef]
- Lan, F.C.; Zeng, F.B.; Zhou, Y.J.; Chen, J.Q. Progress on Research of Mechanical Properties of Closed-cell Aluminum Foams and Its Applications in Automobile Crashworthiness. Chin. J. Mech. Eng. 2014, 50, 97–112. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids Structures and Properties-Second Edition; Cambridge University Press: Cambridge, UK, 1999; p. 528. [Google Scholar]
- Li, N.; Gao, C.H. Microstructure and electrochemical properties of the electrodeposited Ni-Mo/ZrO2 Alloy coating. Mater. Sci. Technol. Lond. 2011, 19, 104–109. [Google Scholar]
- Xu, Y.; Ma, S.; Fan, M.Y.; Chen, Y.; Song, X.; Hao, J. Design and properties investigation of Ni-Mo composite coating reinforced with duplex nanoparticles. J. Surf. Coat. Technol. 2019, 363, 51–60. [Google Scholar] [CrossRef]

| Bath Composition | Concentration | Purpose |
|---|---|---|
| NiSO4·6H2O | 0.27 mol· | Ni source |
| Na2MoO4·2H2O | 0.032 mol· | Mo source |
| Na3C6H5O7·2H2O | 0.52 mol· | Complexing agent |
| NH4Cl | 0.65 mol· | Buffer |
| SDS | 0.1 g· | Surfactant |
| SiC | 5 g· | Composite phase |
| TiN | 5 g· | Composite phase |
| Coatings (Electrodeposition Time) | Crystallite Size (nm) |
|---|---|
| Ni–Mo (10 min) | 13.31 |
| Ni–Mo–SiC–TiN (10 min) | 12.14 |
| Ni–Mo–SiC–TiN (20 min) | 17.01 |
| Ni–Mo–SiC–TiN (30 min) | 20.36 |
| Ni–Mo–SiC–TiN (40 min) | 24.96 |
| Samples | Deposition Time t (min) | Coefficient of Variation P% (%) | Yield Strength σs (MPa) | Elastic Modulus (MPa) | Wv (J) | ||
|---|---|---|---|---|---|---|---|
| Substrate | 0 | / | / | / | 1.06 | 43.67 | 0.852 |
| Ni–Mo–SiC–TiN | 10 | 0.4562 | 0.5051 | 10.7 | 1.93 | 54.39 | 1.237 |
| Ni–Mo–SiC–TiN | 20 | 0.4553 | 0.5623 | 23.5 | 2.38 | 106.27 | 1.795 |
| Ni–Mo–SiC–TiN | 30 | 0.4320 | 0.5996 | 38.8 | 2.59 | 248.63 | 2.146 |
| Ni–Mo | 30 | 0.4326 | 0.5976 | 38.2 | 2.52 | 240.46 | 2.069 |
| Ni–Mo–SiC–TiN | 40 | 0.4612 | 0.7075 | 53.4 | 2.99 | 344.75 | 2.520 |
| Passivation Parameters | Substrate | Ni–Mo | Ni–Mo–SiC–TiN |
|---|---|---|---|
| Potential for primary passivation (Epp, mV) | −1130 | −905 | −829 |
| Breakdown potential (Eb, mV) | −653 | −605 | −554 |
| Corrosion potential (Ecorr, mV) | −1160 | −937 | −866 |
| Corrosion current density (Icorr, A/cm2) | 4.48 × 10−5 | 3.90 × 10−5 | 2.72 × 10−5 |
| (mV/decade) | 69.08 | 47.14 | 33.32 |
| (mV/decade) | 25.47 | 29.88 | 40.45 |
| Corrosion rate (g/cm2·h) | 3.8643 × 10−4 | 1.8583 × 10−4 | 1.0643 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ma, S.; Fan, M.; Zheng, H.; Chen, Y.; Song, X.; Hao, J. Mechanical and Corrosion Resistance Enhancement of Closed-Cell Aluminum Foams through Nano-Electrodeposited Composite Coatings. Materials 2019, 12, 3197. https://doi.org/10.3390/ma12193197
Xu Y, Ma S, Fan M, Zheng H, Chen Y, Song X, Hao J. Mechanical and Corrosion Resistance Enhancement of Closed-Cell Aluminum Foams through Nano-Electrodeposited Composite Coatings. Materials. 2019; 12(19):3197. https://doi.org/10.3390/ma12193197
Chicago/Turabian StyleXu, Yiku, Shuang Ma, Mingyuan Fan, Hongbang Zheng, Yongnan Chen, Xuding Song, and Jianmin Hao. 2019. "Mechanical and Corrosion Resistance Enhancement of Closed-Cell Aluminum Foams through Nano-Electrodeposited Composite Coatings" Materials 12, no. 19: 3197. https://doi.org/10.3390/ma12193197
APA StyleXu, Y., Ma, S., Fan, M., Zheng, H., Chen, Y., Song, X., & Hao, J. (2019). Mechanical and Corrosion Resistance Enhancement of Closed-Cell Aluminum Foams through Nano-Electrodeposited Composite Coatings. Materials, 12(19), 3197. https://doi.org/10.3390/ma12193197




