Zirconium Carboxyaminophosphonate Nanosheets as Support for Ag Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zr2(PO4)H3(L)2 H2O [L = (O3PCH2)2NHCH2COO] (ZG)
2.3. Preparation of Exfoliated and Regenerated ZG
2.4. Preparation of Ag@ZG and Zn/ZG
2.5. Silver Release Test
2.6. Characterization of Antibacterial Properties
2.6.1. Sample Preparation
2.6.2. Bacterial Cultures and Agar Diffusion Assay
2.6.3. Bactericidal Activity by Direct Contact with Test Materials
2.7. Instrumental Procedures
3. Results and Discussion
3.1. Zirconium Phosphate Glycine-N,N-Bismethylphosphonate
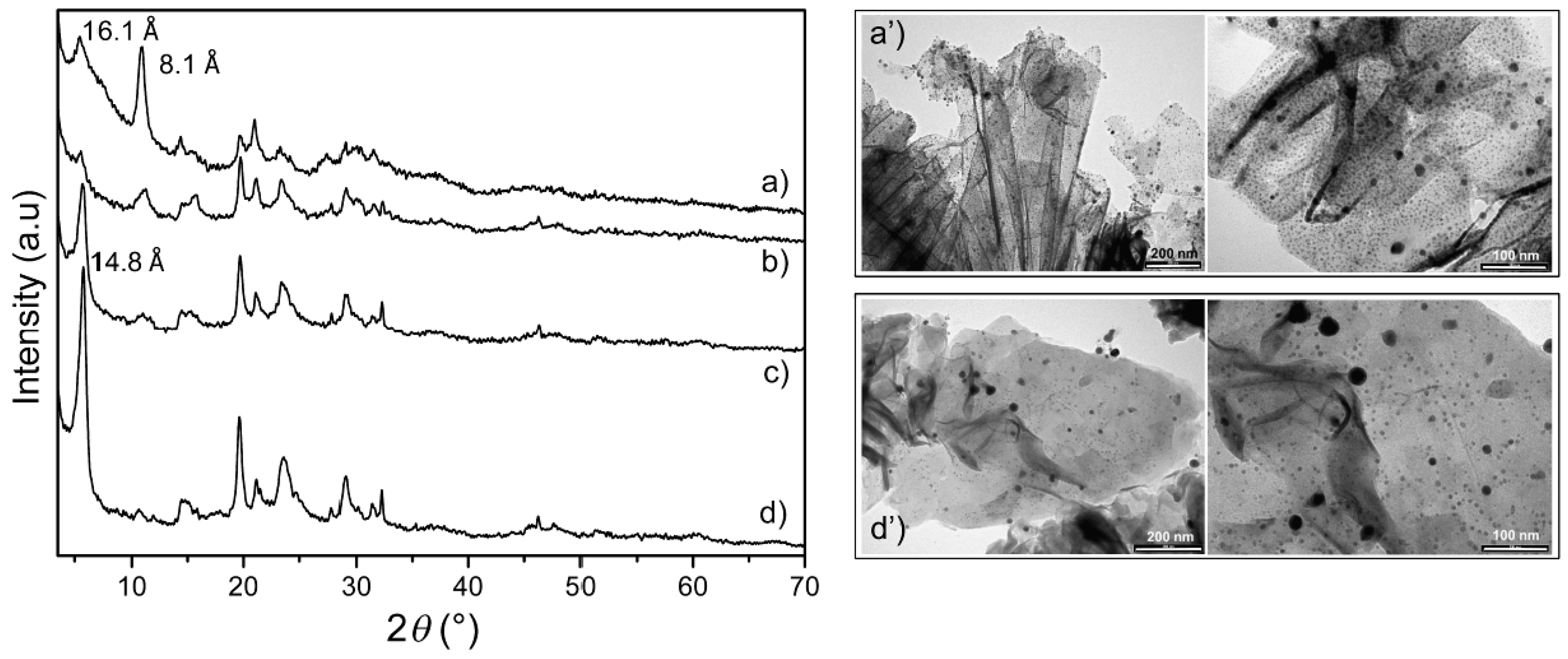
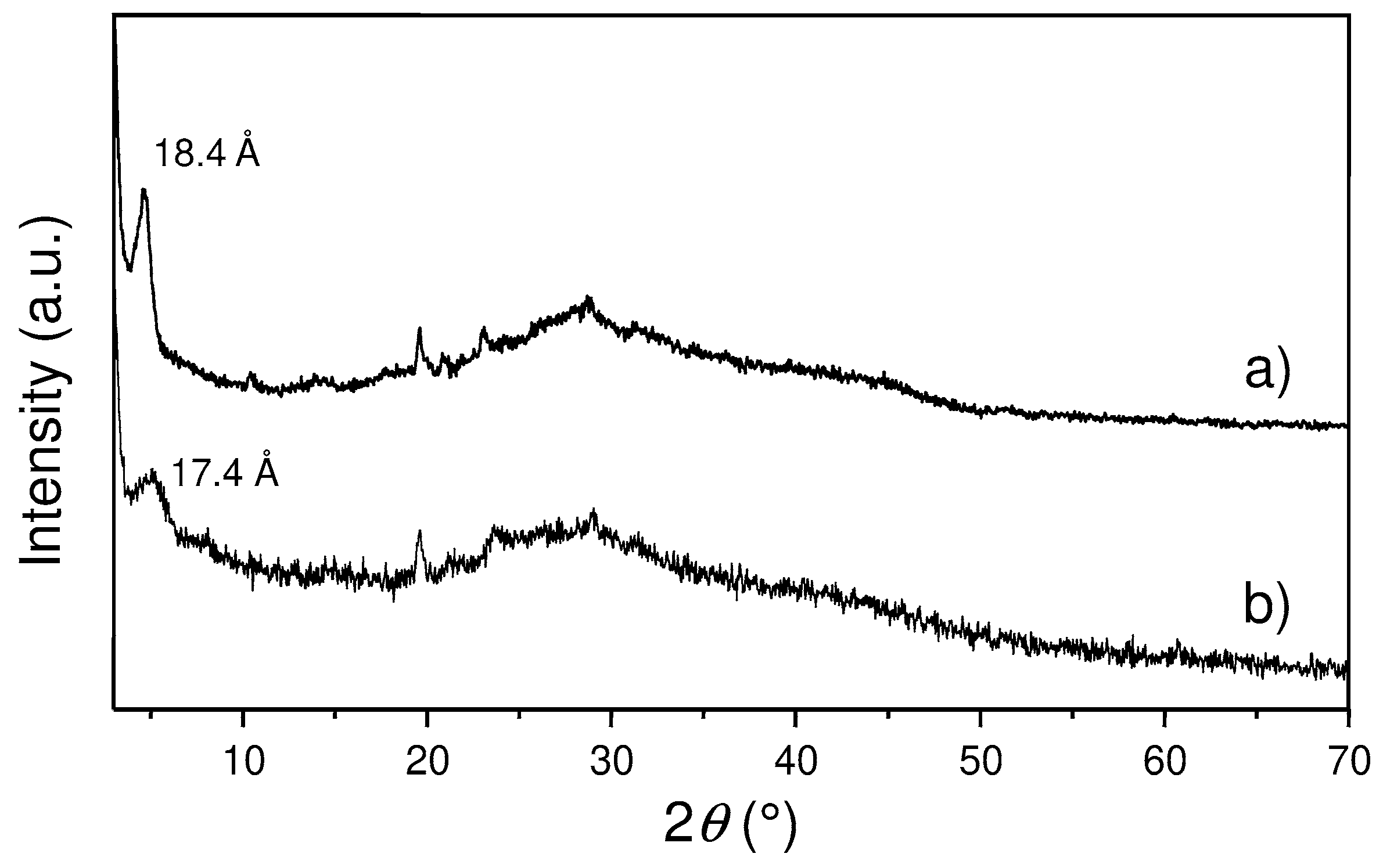
3.2. Preparation and Characterization of Ag@ZG and Zn/ZG
3.3. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature. 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet. 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag. 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef]
- Arciola, C.R.; Caramazza, R.; Pizzoferrato, A. In vitro adhesion of Staphylococcus epidermidis on heparin-surface-modified intraocular lenses. J. Cataract Refract. Surg. 1994, 20, 158–161. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA. 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Montanaro, L.; Moroni, A.; Giordano, M.; Pizzoferrato, A.; Donati, M.E. Hydroxyapatite-coated orthopaedic screws as infection resistant materials: in vitro study. Biomaterials 1999, 20, 323–327. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional coatings or films for hard-tissue applications. Materials (Basel) 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Parasuraman, P.; Antony, A.P.; B, S.L.S.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.S.; Syed, A. Antimicrobial photodynamic activity of toluidine blue encapsulated in mesoporous silica nanoparticles against Pseudomonas aeruginosa and Staphylococcus aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Raie, D.S.; Mhatre, E.; El-Desouki, D.S.; Labena, A.; El-Ghannam, G.; Farahat, L.A.; Youssef, T.; Fritzsche, W.; Kovács, Á.T. Effect of novel quercetin titanium dioxide-decorated multi-walled carbon nanotubes nanocomposite on bacillus subtilis biofilm development. Materials (Basel) 2018, 11, 157. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Taglietti, A.; Dacarro, G.; Barbieri, D.; Cucca, L.; Grisoli, P.; Patrini, M.; Arciola, C.R.; Pallavicini, P. High bactericidal self-assembled nano-monolayer of silver sulfadiazine on hydroxylated material Surfaces. Materials (Basel) 2019, 12, 2761. [Google Scholar] [CrossRef]
- Wu, S.; Li, A.; Zhao, X.; Zhang, C.; Yu, B.; Zhao, N.; Xu, F.J. Silica-coated gold−silver nanocages as photothermal antibacterial agents for combined anti-infective therapy. ACS Appl. Mater. Interfaces 2019, 11, 17177–17183. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Burdusel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef]
- Sheehy, K.; Casey, A.; Murphy, A.; Chambers, G. Antimicrobial properties of nano-silver: A cautionary approach to ionic interference. J. Colloid Interface Sci. 2015, 443, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Nano-antimicrobials based on metals. In Novel Antimicrobial Agents and Strategies; Phoenix, D.A., Harris, F., Dennison, S.R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 181–218. [Google Scholar]
- Oktar, F.N.; Yetmez, M.; Ficai, D.; Ficai, A.; Dumitru, F.; Pica, A. Molecular mechanism and targets of the antimicrobial activity of metal nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1583–1588. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Tarushi, A.; Psomas, G.; Raptopoulou, C.P.; Kessissoglou, D.P. Zinc complexes of the antibacterial drug oxolinic acid: Structure and DNA-binding properties. J. Inorg. Biochem. 2009, 103, 898–905. [Google Scholar] [CrossRef]
- Xu, J.; Ding, G.; Li, J.; Yang, S.; Fang, B.; Sun, H.; Zhou, Y. Zinc-ion implanted and deposited titanium surfaces reduce adhesion of Streptococcus mutans. Appl. Surf. Sci. 2010, 256, 7540–7544. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Recent advances in the synthesis and characterization of nano-antimicrobials. Trends Analyt. Chem. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomedicine. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Donnadio, A.; Nocchetti, M.; Costantino, F.; Taddei, M.; Casciola, M.; da Silva Lisboa, F.; Vivani, R. A layered mixed zirconium phosphate/phosphonate with exposed carboxylic and phosphonic groups: X-ray powder structure and proton conductivity properties. Inorg. Chem. 2014, 53, 13220–13226. [Google Scholar] [CrossRef]
- Costantino, F.; Vivani, R.; Bastianini, M.; Ortolani, L.; Piermatti, O.; Nocchetti, M.; Vaccaro, L. Accessing stable zirconium carboxyaminophosphonate nanosheets as support for highly active Pd nanoparticles. Chem. Commun. 2015, 51, 15990–15993. [Google Scholar] [CrossRef]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized palladium nanoparticles on zirconium carboxy-aminophosphonatesnanosheets as an efficient recoverable heterogeneous catalyst for suzuki−miyaura and heck coupling. Catalysts 2017, 7, 186. [Google Scholar] [CrossRef]
- Costantino, F.; Nocchetti, M.; Bastianini, M.; Lavacchi, A.; Caporali, M.; Liguori, F. Robust zirconium phosphate−phosphonate nanosheets containing palladium nanoparticles as efficient catalyst for alkynes and nitroarenes hydrogenation reactions. ACS Appl. Nano Mater. 2018, 1, 1750–1757. [Google Scholar] [CrossRef]
- Shearan, S.J.; Stock, N.; Emmerling, F.; Demel, J.; Wright, P.A.; Demadis, K.D.; Vassaki, M.; Costantino, F.; Vivani, R.; Sallard, S.; et al. New directions in metal phosphonate and phosphinate. Chem. Cryst. 2019, 9, 270. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U. Solid-State Supramolecular Chemistry: Two and Three-dimensional Inorganic Networks, of Comprehensive supramolecular Chemistry; Alberti, G., Bein, T., Eds.; Elsevier Science Ltd. Press, Pergamon: Oxford, UK, 1996; Chapter 1; Volume 7. [Google Scholar]
- Ambrogi, V.; Costantino, U.; Nocchetti, M.; Perioli, L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Micropor. Mesopor. Mater. 2008, 107, 149–160. [Google Scholar]
- Saxena, V.; Diaz, A.; Clearfield, A.; Batteas, J.D.; Delwar Hussain, M. Zirconium phosphate nanoplatelets: A biocompatible nanomaterial for drug delivery to cancer. Nanoscale 2013, 5, 2328–2336. [Google Scholar] [CrossRef]
- Bastianini, M.; Costenaro, D.; Bisio, C.; Marchese, L.; Costantino, U.; Vivani, R.; Nocchetti, M. On the intercalation of the iodine–iodide couple on layered double hydroxides with different particle sizes. Inorg. Chem. 2012, 51, 2560–2568. [Google Scholar] [CrossRef]
- Heli, B.; Morales-Narváez, E.; Golmohammadi, H.; Ajji, A.; Merkoçi, A. Modulation of population density and size of silver nanoparticles embedded in bacterial cellulose via ammonia exposure: Visual detection of volatile compounds in a piece of plasmonic nanopaper. Nanoscale 2016, 8, 7984–7991. [Google Scholar] [CrossRef]
- Fan, W.; Sun, Q.; Li, Y.; Tay, F.R.; Fan, B. Synergistic mechanism of Ag(+)-Zn(2+) in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J. Nanobiotechnol. 2018, 16, 10. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Shook, J.; Hammer, N.D.; Chatzistavrou, X. Resurrection of antibiotics that methicillin-resistant Staphylococcus aureus resists by silver-doped bioactive glass-ceramic microparticles. Acta Biomater. 2019, 96, 537–546. [Google Scholar] [CrossRef]





| Sample Ag@ZG -x | Ag+ Added mol/mol Zr (%IEC) | Ag+ Uptaken mol/mol Zr a | Ag (w/w %) a | Ag NPs Diameter (nm) ± SD b |
|---|---|---|---|---|
| x = 0.125 | 0.19 (12.5) | 0.18 | 4.6 | 16.7 ± 5 4.5 ± 2 |
| x = 0.25 | 0.37 (25) | 0.37 | 8.0 | 20. 3 ± 8 5.2 ± 2 |
| x = 0.5 | 0.75 (50) | 0.75 | 15.4 | 18.3 ± 6 3.8 ± 2 |
| x = 1 | 1.50 (100) | 1.50 | 26.7 | 36.3 ± 9 3.6 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocchetti, M.; Donnadio, A.; Vischini, E.; Posati, T.; Ravaioli, S.; Arciola, C.R.; Campoccia, D.; Vivani, R. Zirconium Carboxyaminophosphonate Nanosheets as Support for Ag Nanoparticles. Materials 2019, 12, 3185. https://doi.org/10.3390/ma12193185
Nocchetti M, Donnadio A, Vischini E, Posati T, Ravaioli S, Arciola CR, Campoccia D, Vivani R. Zirconium Carboxyaminophosphonate Nanosheets as Support for Ag Nanoparticles. Materials. 2019; 12(19):3185. https://doi.org/10.3390/ma12193185
Chicago/Turabian StyleNocchetti, Morena, Anna Donnadio, Eleonora Vischini, Tamara Posati, Stefano Ravaioli, Carla Renata Arciola, Davide Campoccia, and Riccardo Vivani. 2019. "Zirconium Carboxyaminophosphonate Nanosheets as Support for Ag Nanoparticles" Materials 12, no. 19: 3185. https://doi.org/10.3390/ma12193185
APA StyleNocchetti, M., Donnadio, A., Vischini, E., Posati, T., Ravaioli, S., Arciola, C. R., Campoccia, D., & Vivani, R. (2019). Zirconium Carboxyaminophosphonate Nanosheets as Support for Ag Nanoparticles. Materials, 12(19), 3185. https://doi.org/10.3390/ma12193185








