Magnetic Properties of Manganese-Zinc Soft Ferrite Ceramic for High Frequency Applications
Abstract
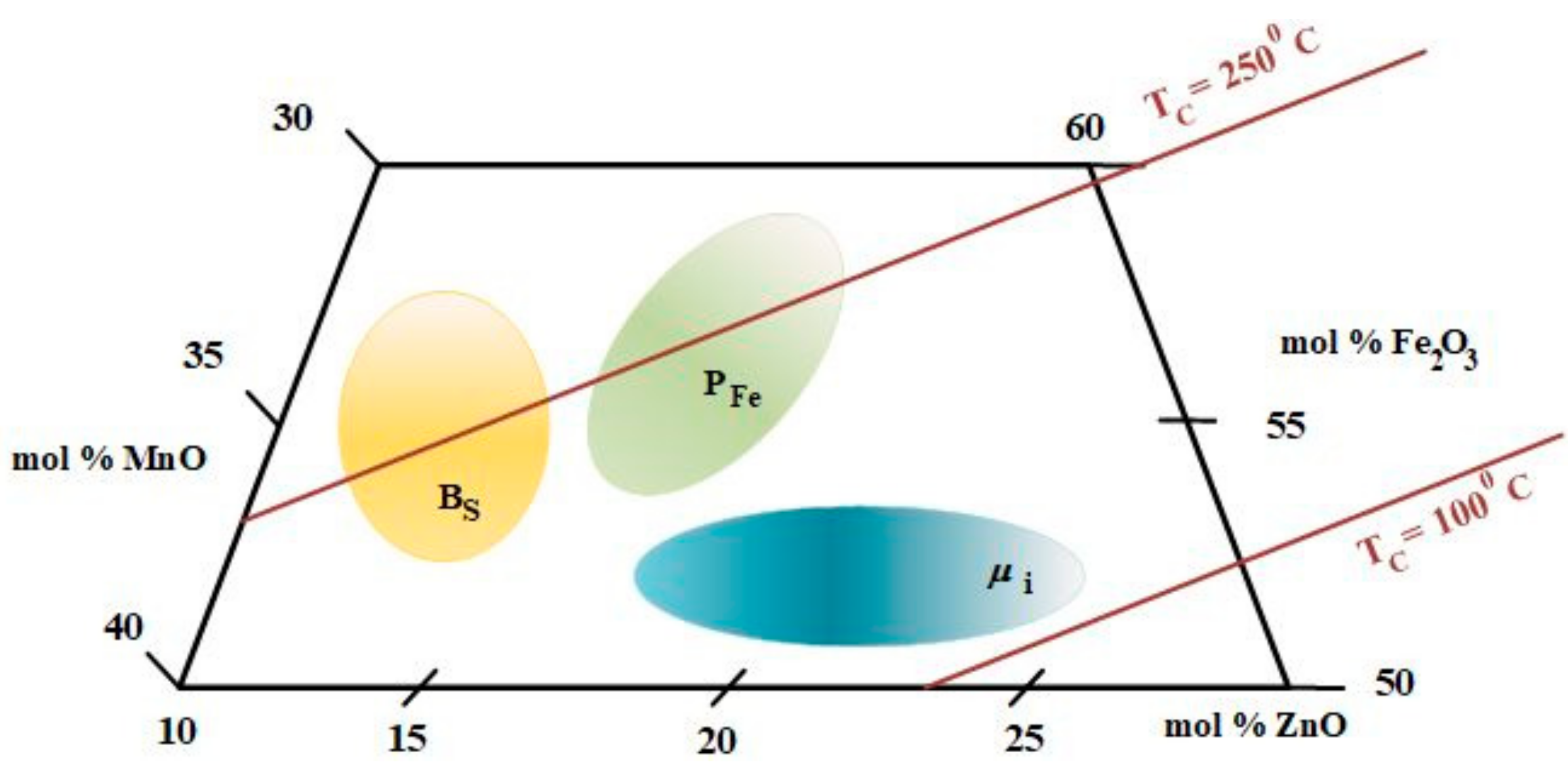
1. Introduction
2. Experimental
2.1. Materials and Synthesis
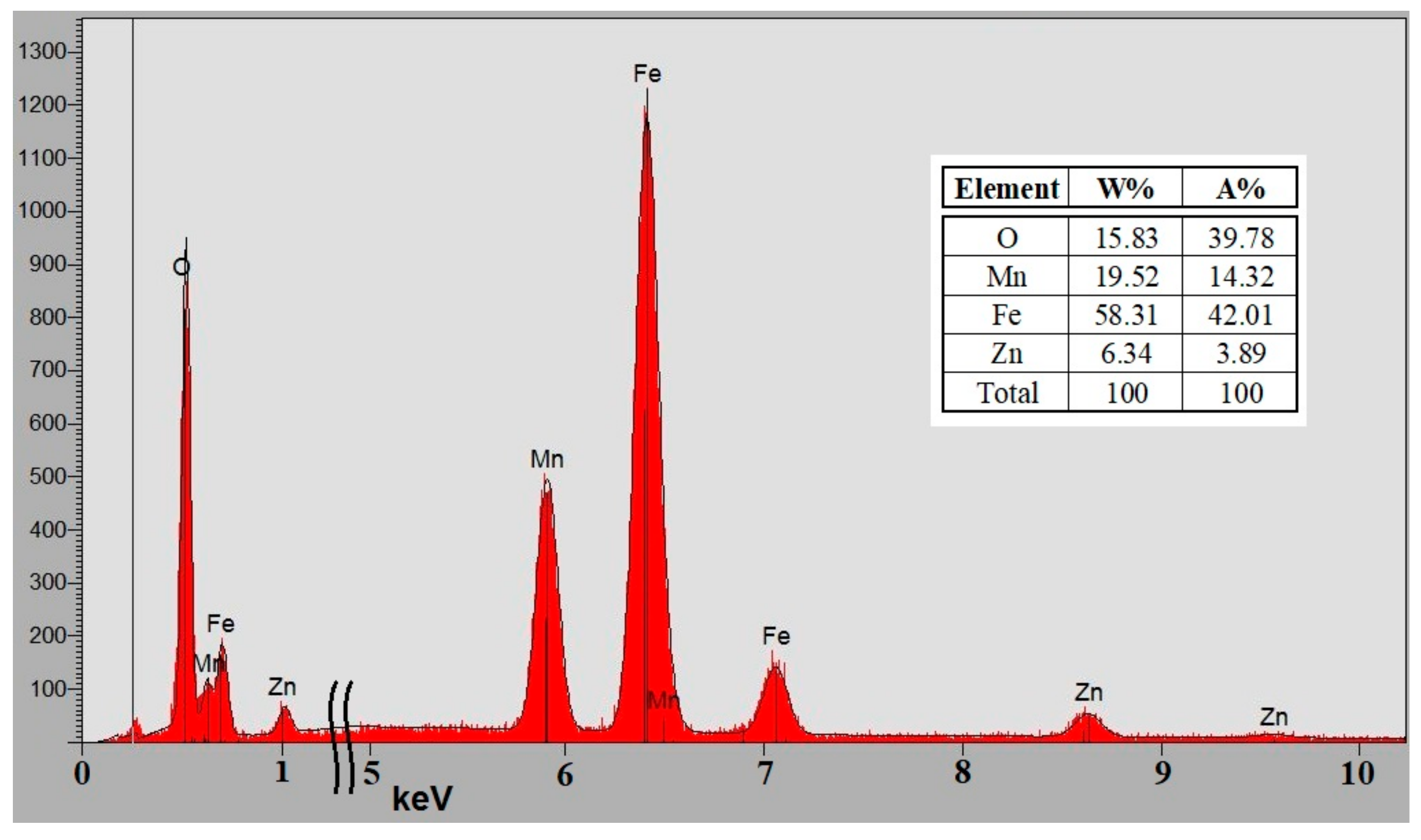
2.2. Chemical, Morphological & Structural Analysis, and Magnetic Properties
3. Results and Discussion
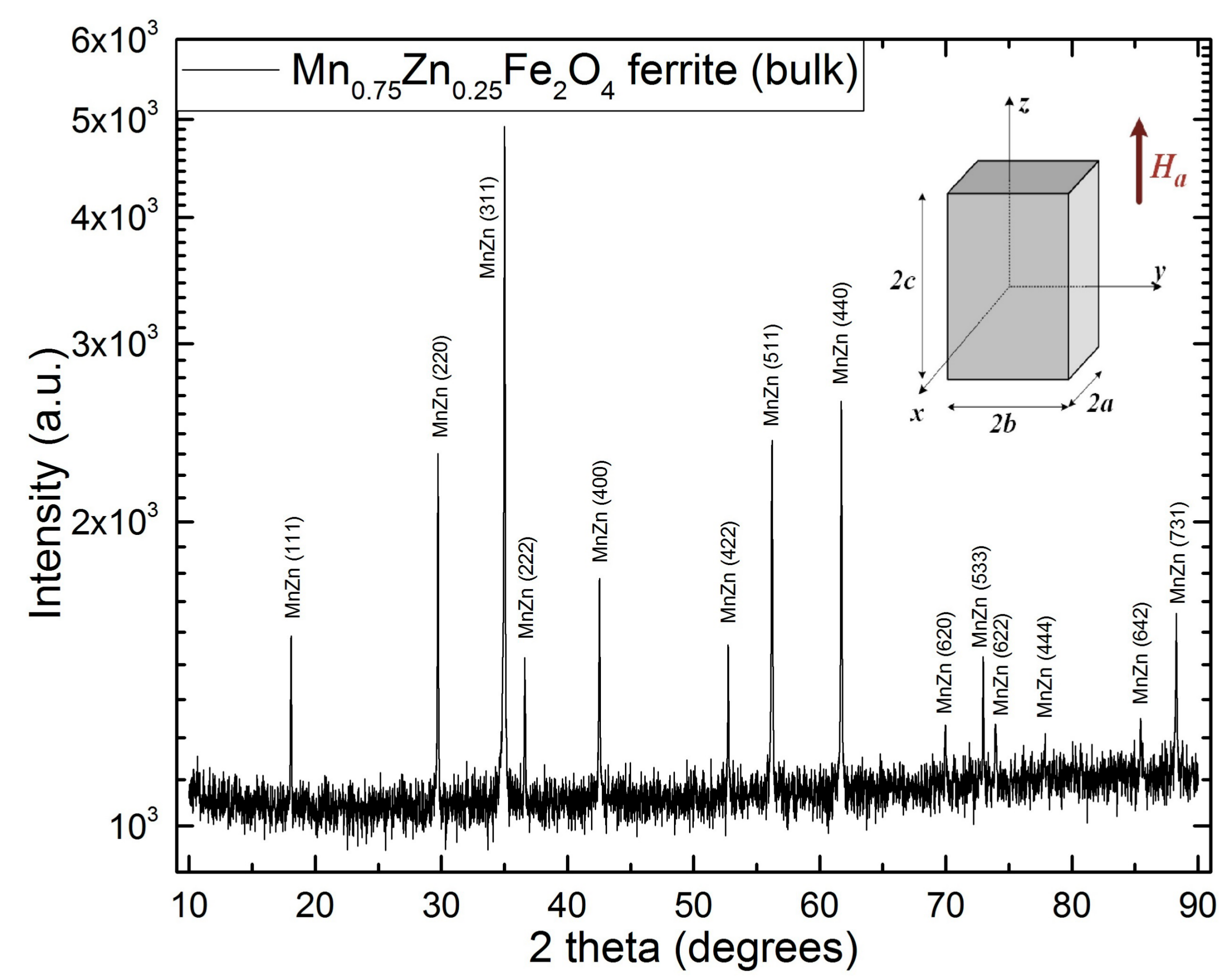
3.1. Structure and Magnetic Domains Size
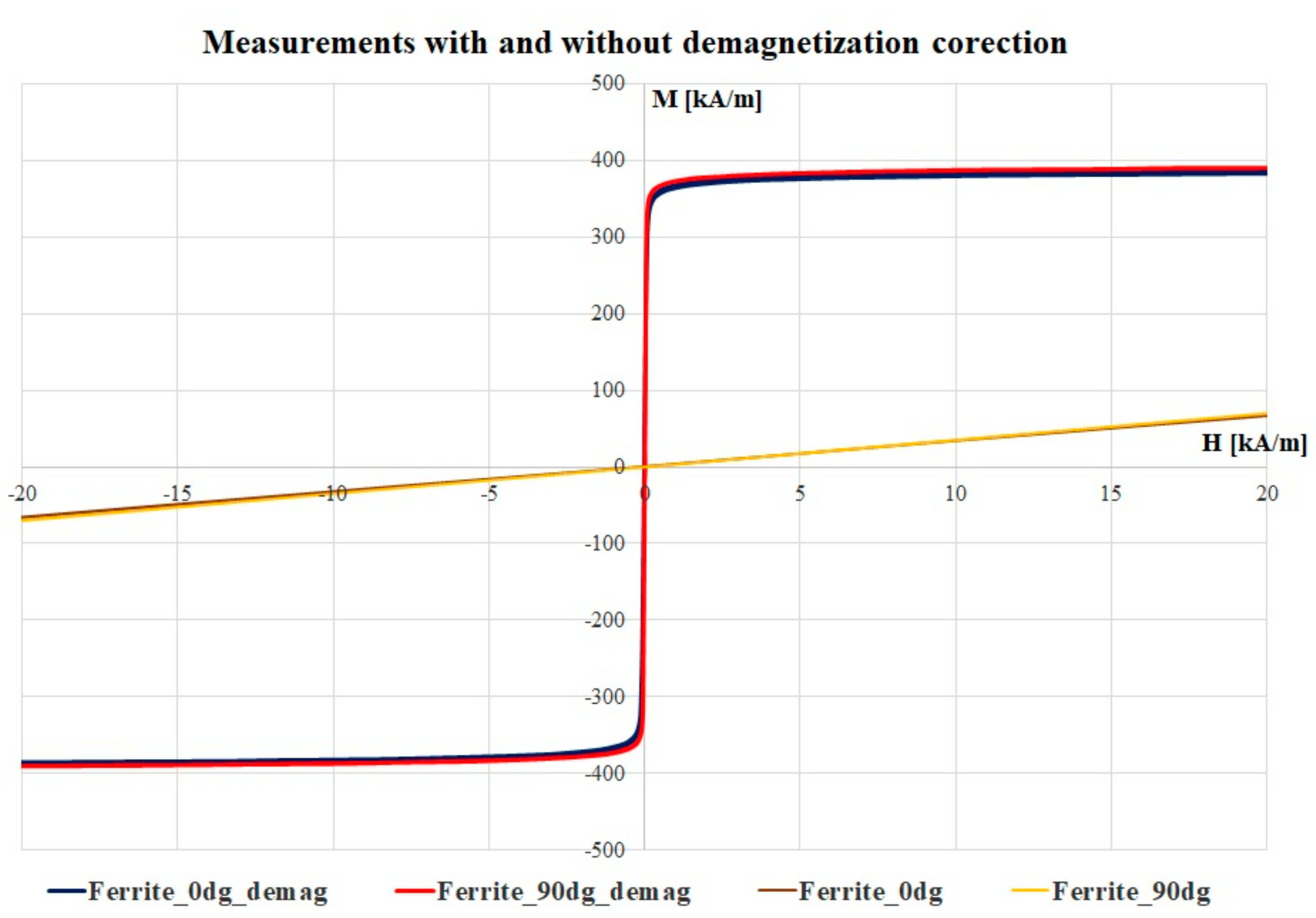
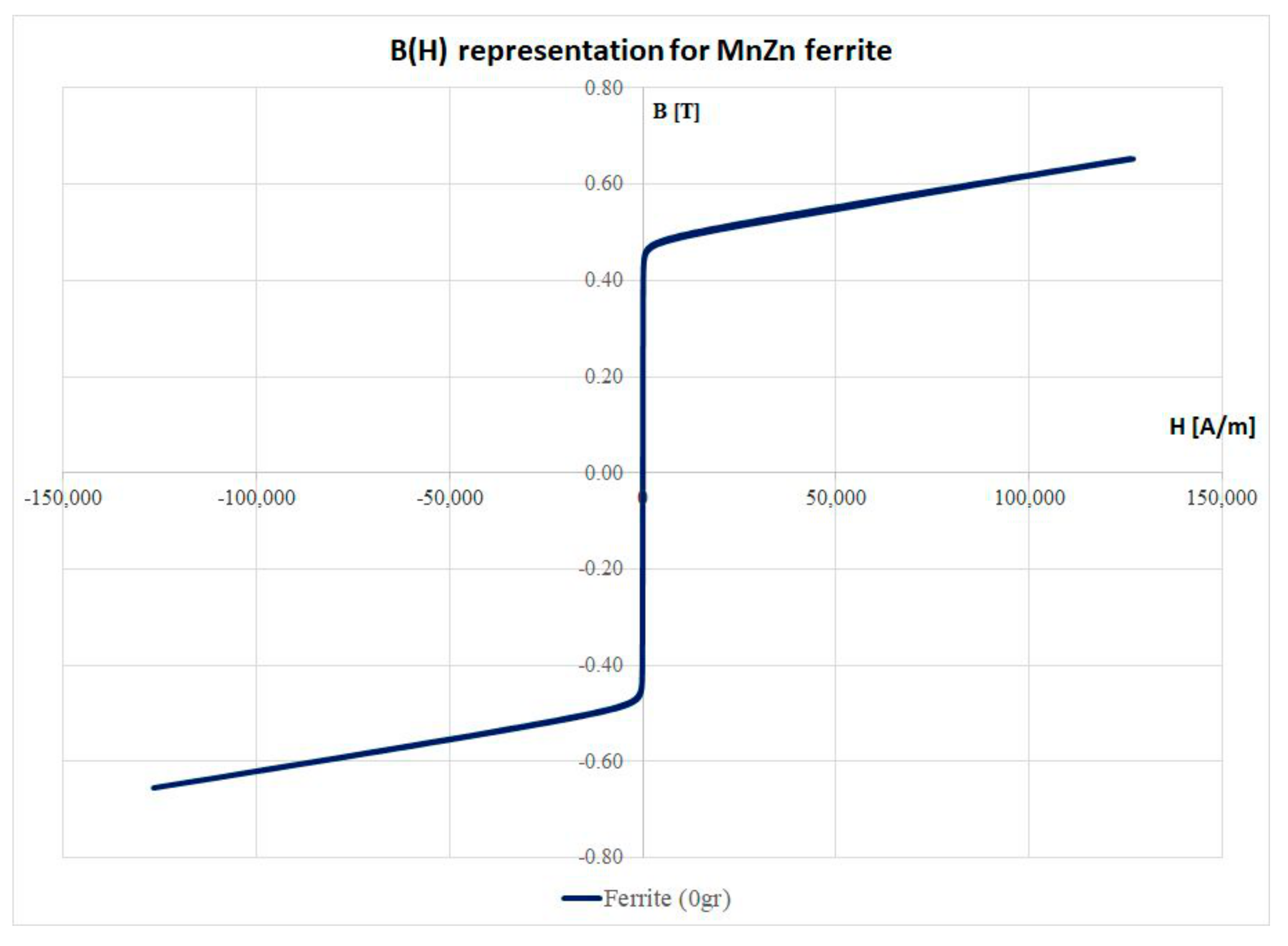
3.2. Magnetic Characteristics and Correction Fact
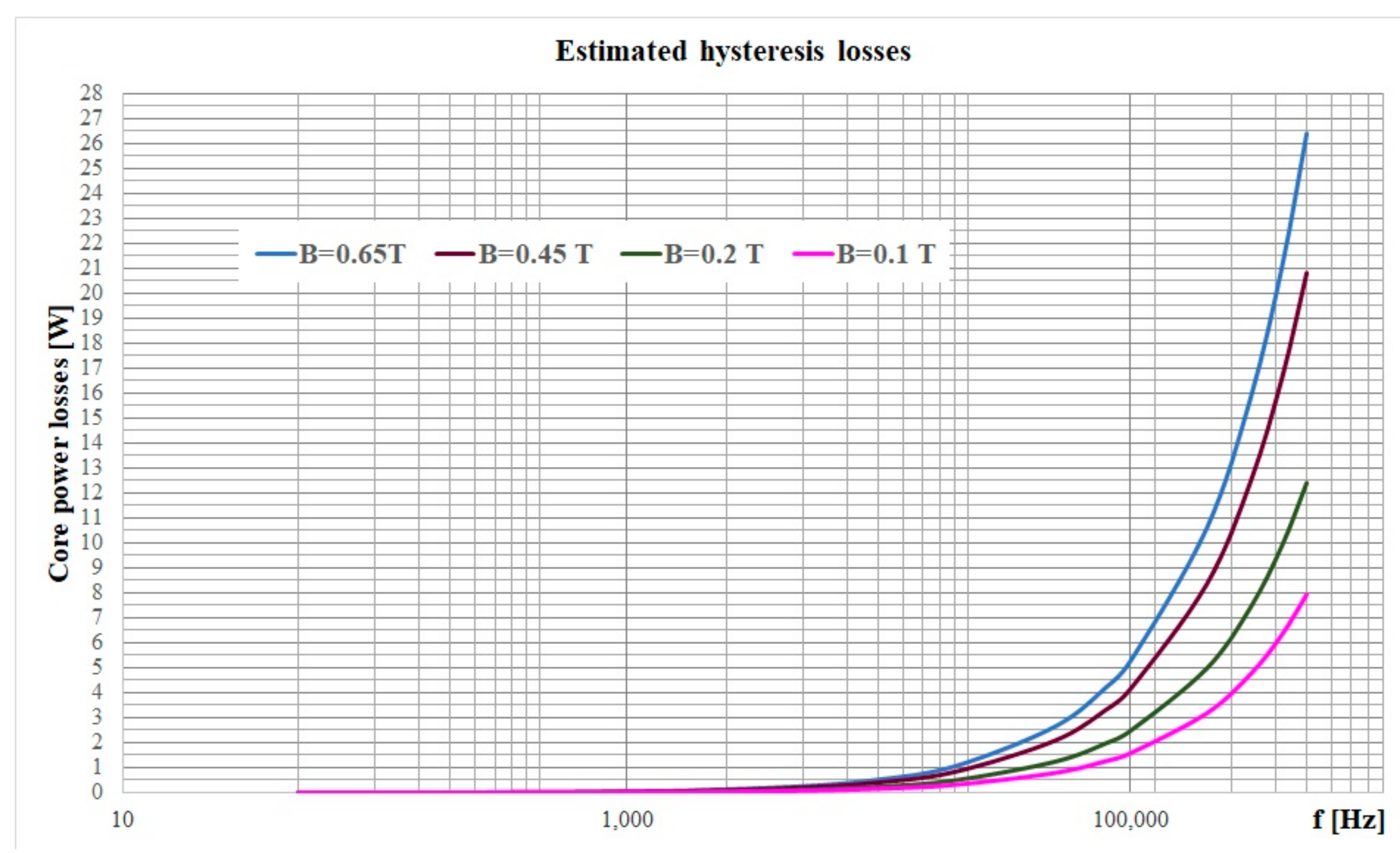
3.3. Losses Estimation in High Frequency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McLyman, W.T. Transformer and Inductor Design Handbook, 4th ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2016; ISBN 9781439836880. [Google Scholar]
- Harper, C.A. Electronic Materials and Processes Handbook, 3rd ed.; McGraw-Hill Companies, Inc.: New York, NY, USA, 2004; ISBN 9780071402149. [Google Scholar]
- Khandpur, R.S. Printed Circuit Boards: Design, Fabrication, Assembly and Testing; Tata McGraw-Hill Companies, Inc.: New York, NY, USA, 2005; ISBN 0-07-058814-7. [Google Scholar]
- Ouyang, Z.; Sen, G.; Thomsen, O.C.; Andersen, M.A.E. Analysis and design of fully integrated planar magnetics for primary–parallel isolated boost converter. IEEE T. Ind. Electron. 2013, 60, 494–508. [Google Scholar] [CrossRef]
- Wong, F.; Lu, J. Design Consideration of High Frequency Planar Transformer. In Proceedings of the 2002 IEEE International Magnetics Conference (INTERMAG), Amsterdam, The Netherlands, 28 April–2 May 2002. [Google Scholar]
- Rao, B.; Zhao, Y.; Yang, Y.; Gao, J.; Zhang, Z.; Zhou, S.; Zhang, M.; Chen, Z. Reduction of leakage inductance and AC resistance of planar transformers by optimising the current distribution. IET Power Electron. 2018, 11, 501–506. [Google Scholar] [CrossRef]
- Magambo, J.S.N.T.; Bakri, R.; Margueron, X.; Le Moigne, P.; Mahe, A.; Guguen, S.; Bensalah, T. Planar magnetic components in more electric aircraft: Review of technology and key parameters for DC–DC power electronic converter. IEEE T. Transp. Electr. 2017, 3, 831–842. [Google Scholar] [CrossRef]
- Angadi, V.J.; Anupama, A.V.; Kumar, R.; Choudhary, H.K.; Matteppanavar, S.; Somashekarappa, H.M.; Rudraswamy, B.; Sahoo, B. Composition dependent structural and morphological modifications. Mater. Chem. Phys. 2017, 199, 313–321. [Google Scholar] [CrossRef]
- Petrescu, L.; Cheșca, B.; Cazacu, E.; Petrescu, C. Planar Transformer Windings losses at Different Waveforms. In Proceedings of the 2017 10th International Symposium on Advanced Topics in Electrical Engineering (ATEE), Bucharest, Romania, 23–25 March 2017; pp. 350–353. [Google Scholar]
- Quirke, M.T.; Barrett, J.J.; Hayes, M. Planar magnetic component technology—A review. IEEE T. Comp. Hybrids Manuf. Technol. 1992, 15, 884–892. [Google Scholar] [CrossRef]
- Fiorillo, F. Measurement and Characterization of Magnetic Materials; Elsevier/Academic Press: Amsterdam, The Netherlands, 2004; ISBN 9780080528922. [Google Scholar]
- Ionita, V.; Cazacu, E. Correction of measured magnetization curves using finite element method. IEEE Trans. Magn. 2013, 45, 1174–1177. [Google Scholar] [CrossRef]
- Dong, L.; Han, Z.; Zhang, Y.; Wu, Z.; Zhang, X. Preparation and sinterability of Mn-Zn ferrite powders by sol-gel method. J. Rare Earth. 2006, 24, 54–56. [Google Scholar]
- Szczygieł, I.; Winiarska, K. Synthesis and characterization of manganese–zinc ferrite obtained by thermal decomposition from organic precursors. J. Therm. Anal. Calorim. 2014, 115, 471–477. [Google Scholar] [CrossRef][Green Version]
- Mitrović, N.S.; Djukić, S.R.; Randjić, S.; Ristanović, Z.; Danninger, H. Soft magnetic properties of MnZn ferrites prepared by powder injection moulding. Sci. Sinter. 2012, 44, 355–364. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Kamari, H.M.; Shaari, A.H.; Saion, E. Fabrication and characterization of Manganese–Zinc Ferrite nanoparticles produced utilizing heat treatment technique. Results Phys. 2019, 12, 1821–1825. [Google Scholar] [CrossRef]
- Shriver, D.; Weller, M.; Overton, T.; Armstrong, F.; Rourke, J. Inorganic Chemistry, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2014; ISBN 9781429299060. [Google Scholar]
- Kamali, S.; Shih, K.; Barbiellini, B.; Wang, Y.J.; Kaprzyk, S.; Itou, M.; Bansil, A.; Sakurai, Y. Extracting the cation distributions in NiFe2−xAlxO4 solid solutions using magnetic Compton scattering. J. Phys. Condens. Mat. 2015, 27, 45600. [Google Scholar] [CrossRef] [PubMed]
- Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-step synthesis of long term stable superparamagnetic colloid of zinc ferrite nanorods in water. Materials 2019, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mitran, G.; Chen, S.; Seo, D.K. Molybdenum dopped copper ferrites as active catalysts for alcohols oxidative coupling. Materials 2019, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.A. Demagnetizing factors of a general ellipsoid. Phys. Rev. 1945, 67, 351–357. [Google Scholar] [CrossRef]
- Chen, D.X.; Brug, J.A.; Golfarb, R.B. Demagnetization factors for cylinders. IEEE Trans. Magn. 1991, 27, 3601–3619. [Google Scholar] [CrossRef]
- Aharoni, A. Demagnetization factor for rectangular prisms. J. Appl. Phys. 1998, 83, 3432–3434. [Google Scholar] [CrossRef]
- Graham, C.D.; Lorenz, B.E. Experimental demagnetizing factors for disk samples magnetized along a diameter. IEEE Trans. Magn. 2007, 43, 2743–2745. [Google Scholar] [CrossRef]
- Chen, D.X.; Pardo, E.; Sanchez, A. Demagnetization factors for rectangular prims. IEEE Trans. Magn. 2005, 41, 2077–2088. [Google Scholar] [CrossRef]
- TDK Catalogue, Mn-Zn Ferrite—Material Characteristics, June 2019. Available online: https://product.tdk.com/info/en/catalog/datasheets/ferrite_mn-zn_material_characteristics_en.pdf (accessed on 20 July 2019).
- Steinmetz, C.P. On the law of hysteresis. Trans. Am. Inst. Electr. Eng. 1892, 9, 3–64. [Google Scholar] [CrossRef]
- Petrescu, L.; Ionita, V.; Cazacu, E.; Petrescu, C. Steinmetz’ Parameters Fitting Procedure for The Power Losses Estimation in Soft Magnetic Materials. In Proceedings of the 2017 International Conference on Optimization of Electrical and Electronic Equipment (OPTIM) & 2017 Intl Aegean Conference on Electrical Machines and Power Electronics (ACEMP), Brasov, Romania, 25–27 May 2017; pp. 208–213. [Google Scholar]
- Ghiță, C.; Deaconu, I.D.; Chirilă, A.I.; Năvrăpescu, V.; Ilina, I.D. D analysis of electrical transformer’s field. Rev. Roum. Sci. Tech.-El 2009, 54, 233–242. [Google Scholar]
- Sakaki, Y.; Matsuoka, T. Hysteresis Losses in Mn-Zn Ferrite Cores. IEEE Trans. Magn. 1986, 22, 623–625. [Google Scholar] [CrossRef]
- Petrescu, L. Comparison between frequently used Hysteresis Models. Rev. Roum. Sci. Tech.–El. 2007, 52, 311–320. [Google Scholar]
- Constantinescu, C.; Scarisoreanu, N.; Moldovan, A.; Dinescu, M.; Petrescu, L.; Epureanu, G. Thin films of NdFeB deposited by PLD technique. Appl. Surf. Sci. 2007, 253, 8192–8196. [Google Scholar] [CrossRef]
- Fiorillo, F.; Beatrice, C.; Bottauscio, O.; Patroi, E. Measuring the hysteresis loop of permanent magnets with the pulsed field magnetometer. IEEE Trans. Magn. 2007, 43, 3159–3164. [Google Scholar] [CrossRef]
- Constantinescu, C.; Patroi, E.; Codescu, M.; Dinescu, M. Effect of nitrogen environment on NdFeB thin films grown by radio frequency plasma beam assisted pulsed laser deposition. Mater. Sci. Eng. B 2013, 178, 267–271. [Google Scholar]
- Constantinescu, C.; Ion, V.; Codescu, M.; Rotaru, P.; Dinescu, M. Optical, morphological and thermal behaviour of NdFeB magnetic thin films grown by radiofrequency plasma-assisted pulsed laser deposition. Curr. Appl. Phys. 2013, 13, 2019–2025. [Google Scholar] [CrossRef]


| File (pdf #) | Chemical Stoichiometry | CrystallineStructure | Observation |
|---|---|---|---|
| 04-016-2782 | Mn0.80Zn0.18Fe2.02O4 | Cubic, a = 8.5 | Manganese zinc iron oxide |
| 04-002-0493 | Mn0.7Zn0.3Fe2O4 | Cubic, a = 8.5 | Zinc manganese iron oxide |
| 04-002-0227 | Mn0.69Zn0.31Fe2O4 | Cubic, a = 8.474 | Zinc manganese iron oxide |
| 01-085-1202 | (Mn0.612Zn0.288Fe0.1)1(Fe9.835Mn0.0165)2O4 | Cubic, a = 8.4915 | Manganese zinc iron oxide |
| 01-074-2401 | Mn0.6Zn0.4Fe2O4 | Cubic, a = 8.4975 | Jacobsit, Zincian |
| 00-068-0293 | Mn0.5Zn0.5Fe2O4 | Cubic, a = 8.4344 | Manganese zinc ferrite, Manganese zinc iron oxide |
| 01-080-6581 | (Mn0.5Zn0.5)Fe2O4 | Cubic, a = 8.462 | Franklinite, Manganoan |
| 01-074-2400 | Mn0.4Zn0.6Fe2O4 | Cubic, a = 8.4794 | Franklinite, Manganoan |
| 01-074-2399 | Mn0.2Zn0.8Fe2O4 | Cubic, a = 8.4616 | Franklinite, Manganoan |
| 01-074-2398 | Mn0.1Zn0.9Fe2O4 | Cubic, a = 8.453 | Franklinite, Manganoan |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrescu, L.-G.; Petrescu, M.-C.; Ioniță, V.; Cazacu, E.; Constantinescu, C.-D. Magnetic Properties of Manganese-Zinc Soft Ferrite Ceramic for High Frequency Applications. Materials 2019, 12, 3173. https://doi.org/10.3390/ma12193173
Petrescu L-G, Petrescu M-C, Ioniță V, Cazacu E, Constantinescu C-D. Magnetic Properties of Manganese-Zinc Soft Ferrite Ceramic for High Frequency Applications. Materials. 2019; 12(19):3173. https://doi.org/10.3390/ma12193173
Chicago/Turabian StylePetrescu, Lucian-Gabriel, Maria-Cătălina Petrescu, Valentin Ioniță, Emil Cazacu, and Cătălin-Daniel Constantinescu. 2019. "Magnetic Properties of Manganese-Zinc Soft Ferrite Ceramic for High Frequency Applications" Materials 12, no. 19: 3173. https://doi.org/10.3390/ma12193173
APA StylePetrescu, L.-G., Petrescu, M.-C., Ioniță, V., Cazacu, E., & Constantinescu, C.-D. (2019). Magnetic Properties of Manganese-Zinc Soft Ferrite Ceramic for High Frequency Applications. Materials, 12(19), 3173. https://doi.org/10.3390/ma12193173







