Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement
Abstract
:1. Introduction
2. Raw Materials and Experimental Methods
2.1. Raw Materials
2.2. Experimental Methods
2.2.1. Preparation of AMCSA Clinker and Cement
2.2.2. Test Method
3. Results and Analysis
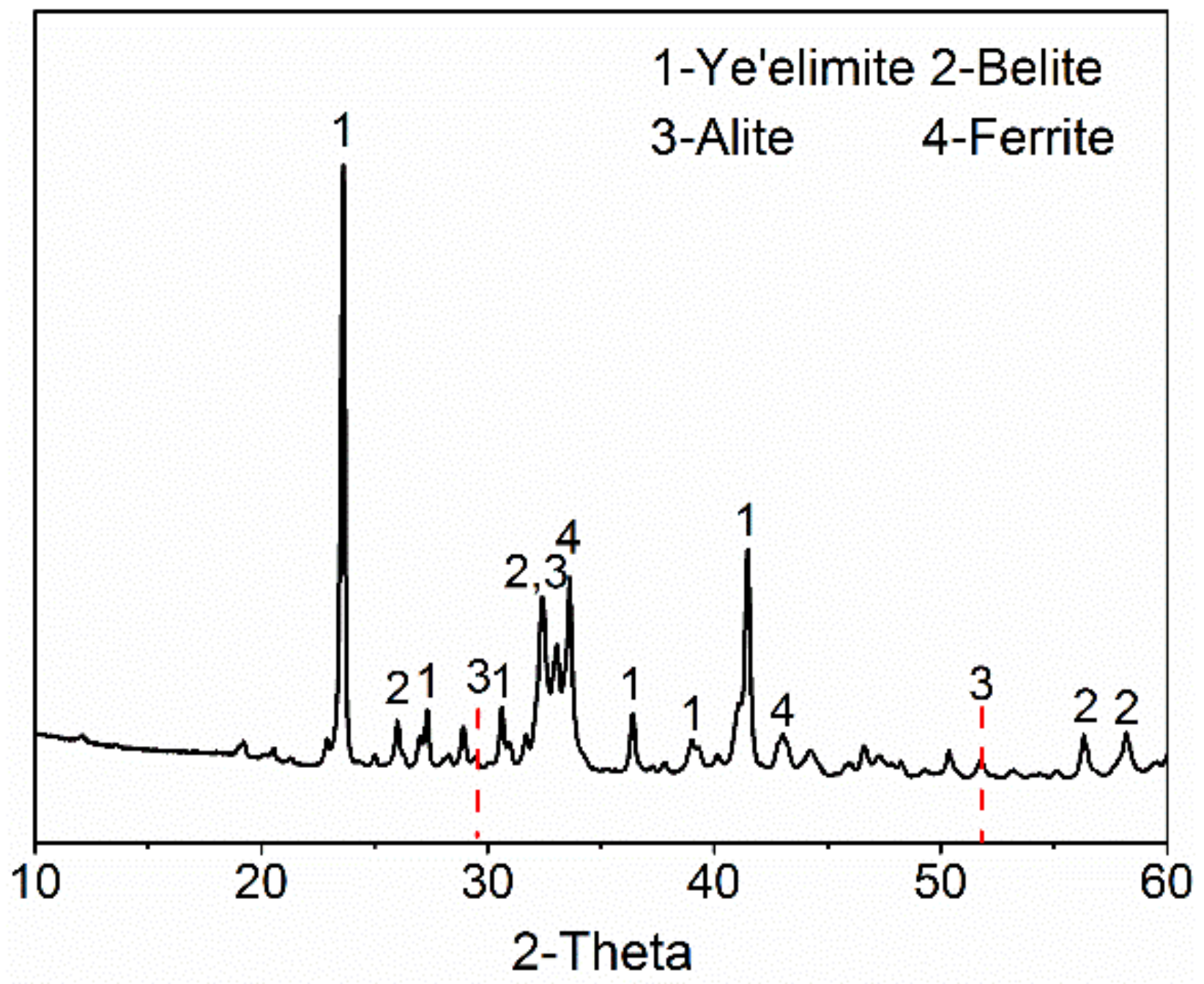
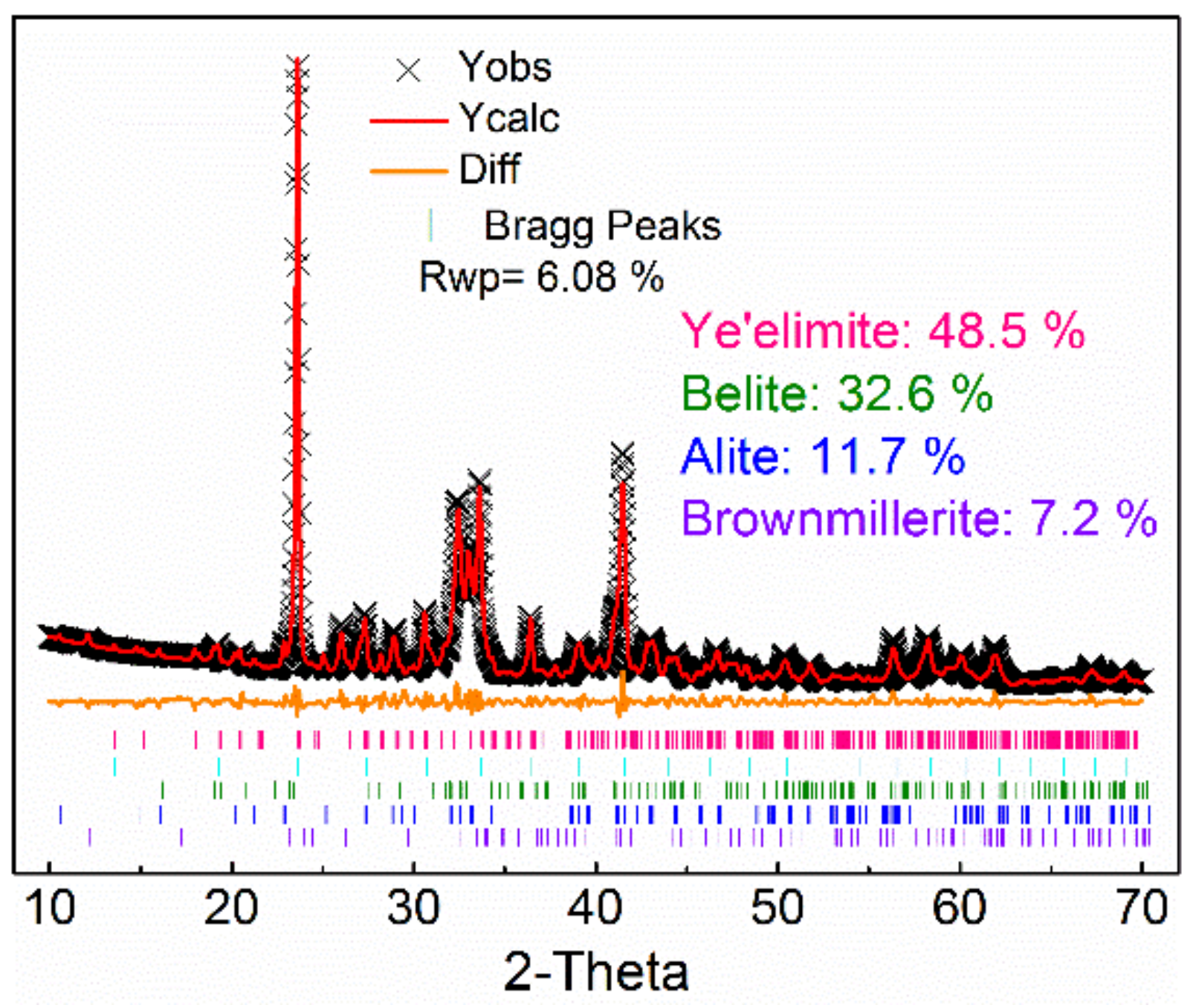
3.1. Phase Analysis of AMCSA Clinker
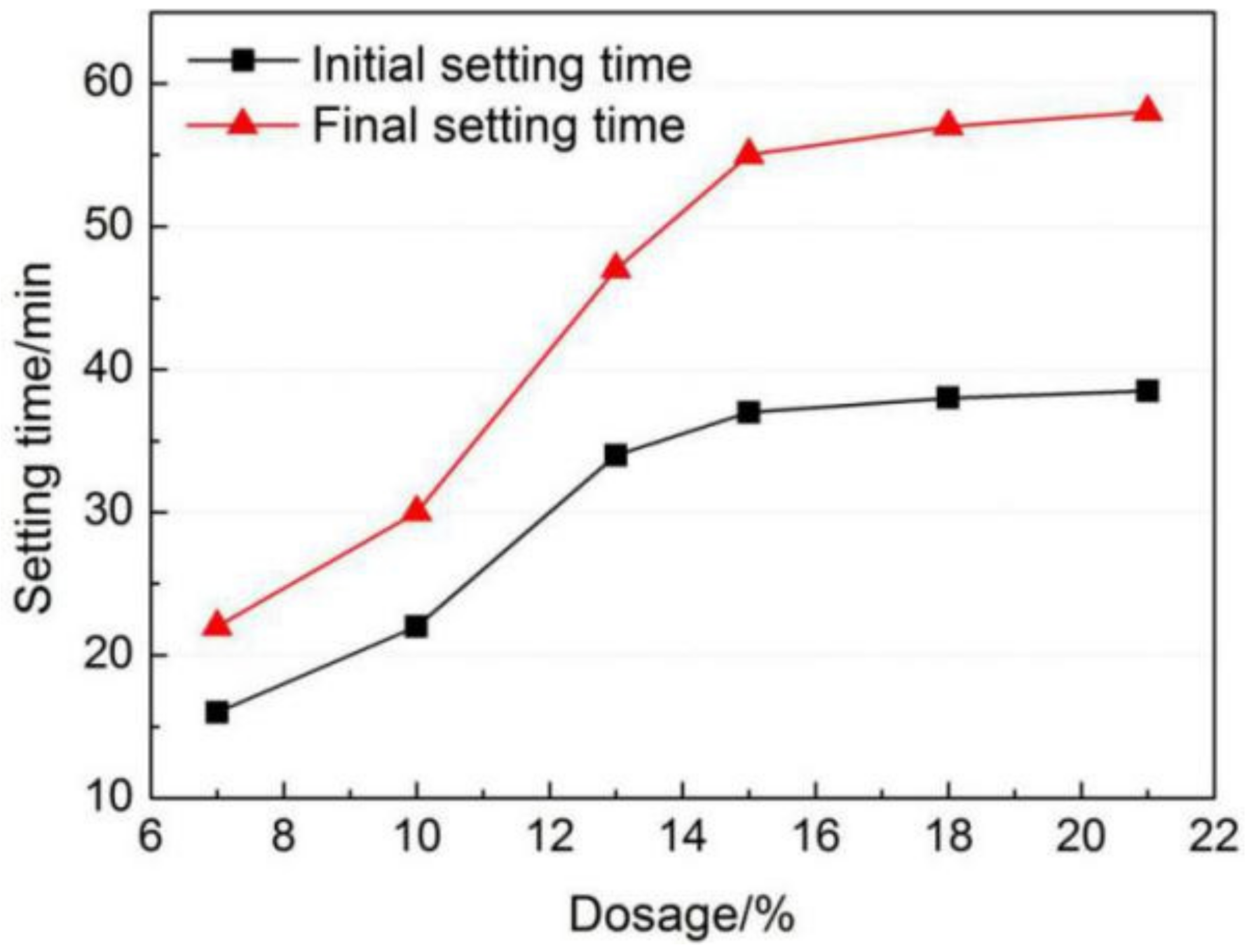
3.2. Effects of Gypsum on Setting Time of AMCSA
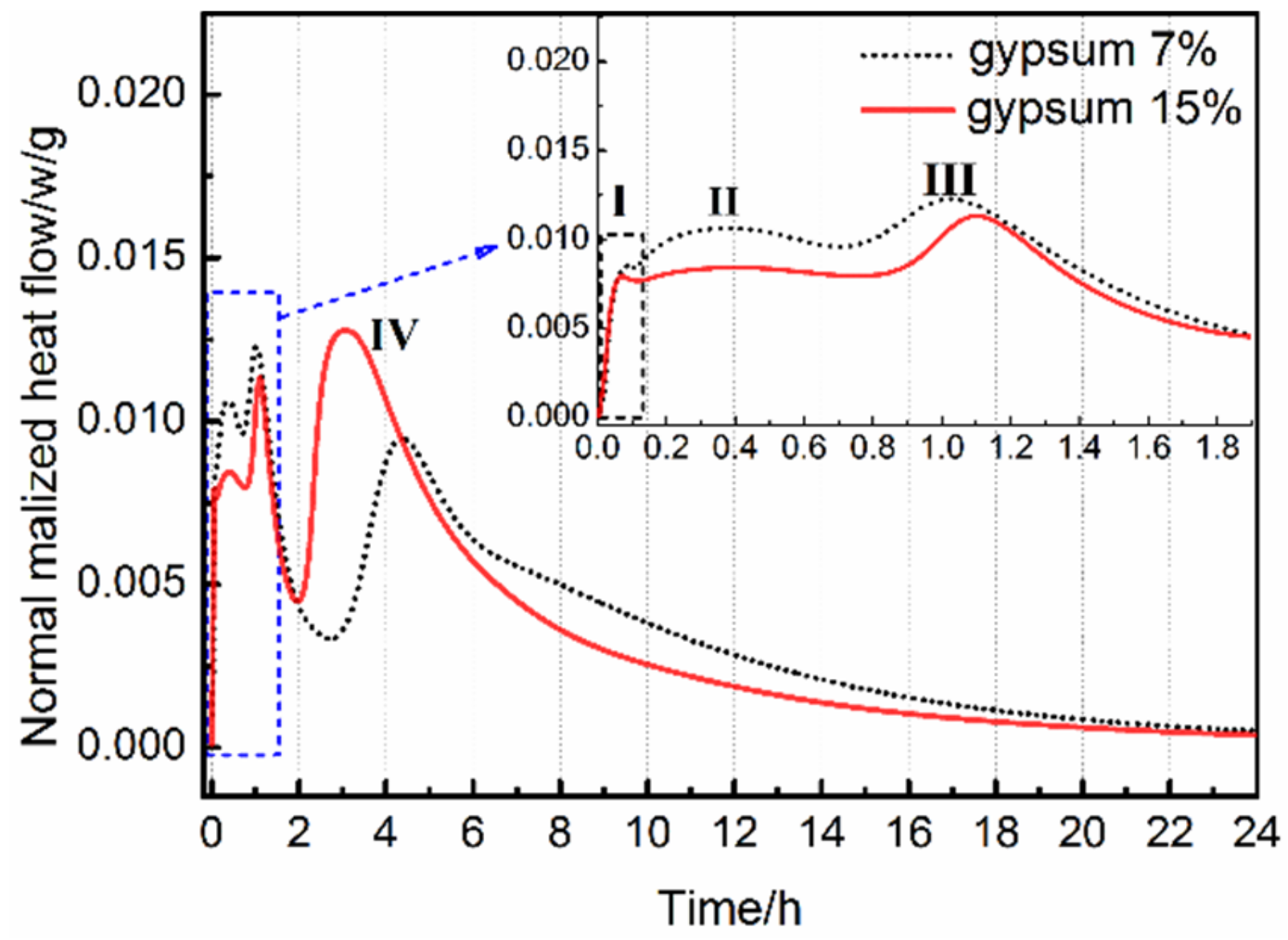
3.3. Effects of Gypsum on Hydration Rate of AMCSA
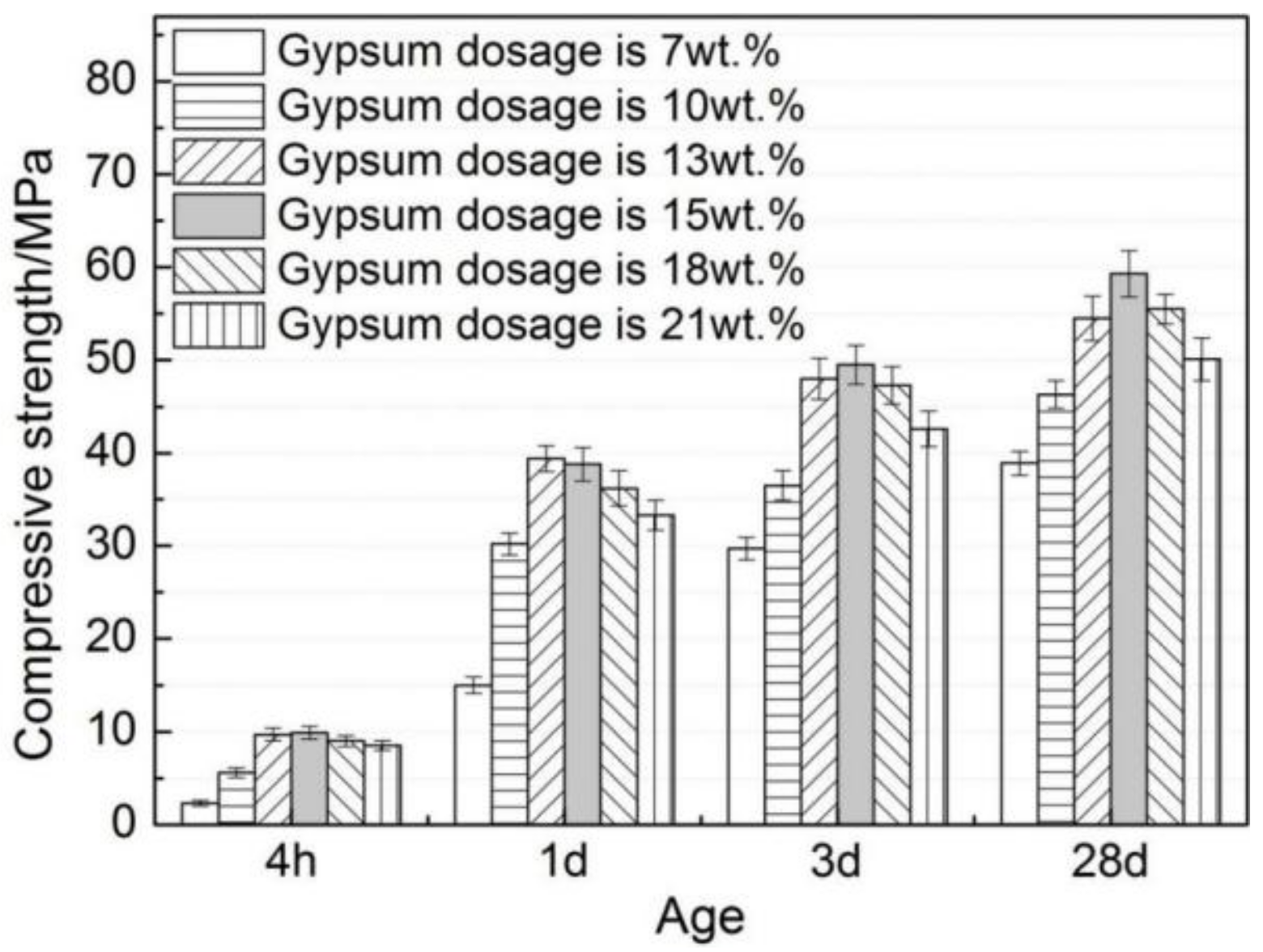
3.4. Effects of Gypsum on Compressive Strength of AMCSA Mortar
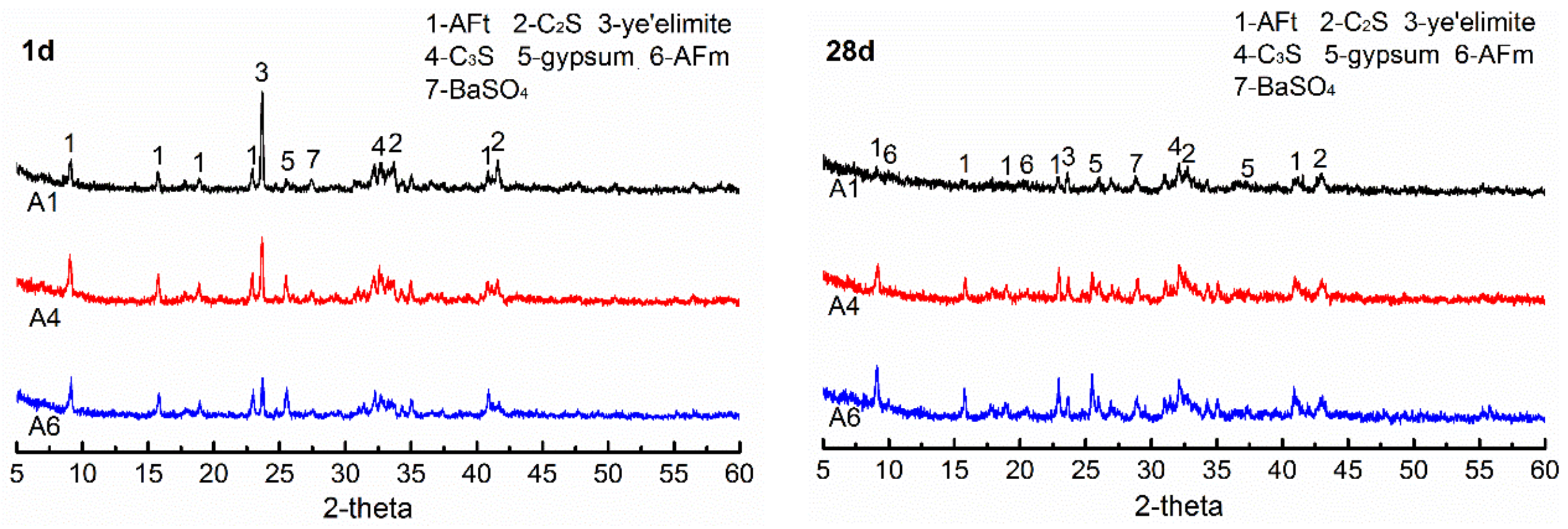
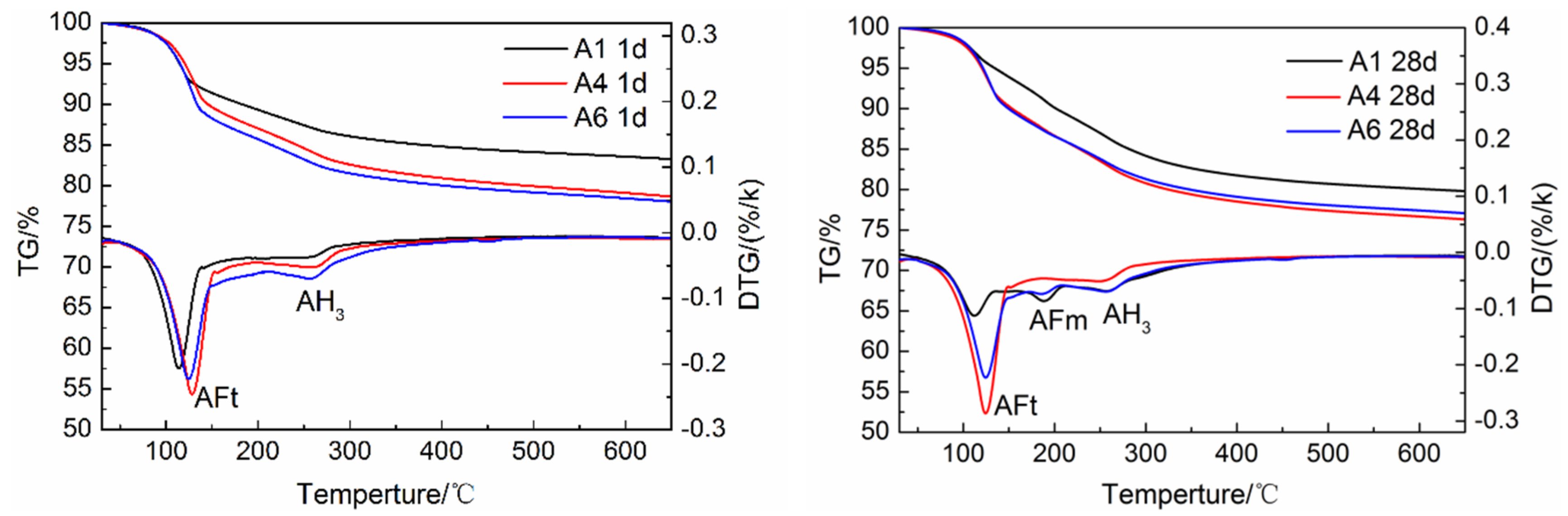
3.5. Effects of Gypsum on Hydration Products of AMCSA
3.5.1. XRD Analysis
3.5.2. TG/DTG Analysis
3.5.3. SEM Analysis
4. Conclusions
- (1)
- AMCSA clinker was calcined by liquid phase control and barium ion doping technology. The mineral content of ye’elimite, C2S, C3S and iron phase in AMCSA clinker are 48.5 wt.%, 32.6 wt.%, 11.7 wt.% and 7.2 wt.% respectively by Rietveld quantitative calculation, which are close to the designed mineral composition. The stable coexistence of ye’elimite and C3S in the same clinker system is realized.
- (2)
- In a certain range of dosage under the experimental conditions in this study, the initial and final setting time of AMCSA are prolonged with the increasing gypsum dosage. The initial and final setting time increases from 16 min and 22 min to 37 min and 55 min respectively when the gypsum dosage increases from 7 wt.% to 15 wt.%. However, the setting time of AMCSA increases slowly with the increasing gypsum dosage after the gypsum dosage exceeds 15 wt.%.
- (3)
- The trend of increasing first and then decreasing is present with the increasing gypsum dosage at all ages of the compressive strength of AMCSA mortar. The highest value of the compressive strength of AMCSA mortar is found when the gypsum dosage is 15 wt.%, and the strength of AMCSA mortar at 28 days still increases greatly compared with that at 3 days. The shortcoming of the low persistence of the strength growth in the middle and late stages of CSA is improved, which is mainly because of the role played by C3S.
Author Contributions
Funding
Conflicts of Interest
References
- Jeong, Y.; Hargis, C.; Chun, S.; Moon, J. Effect of calcium carbonate fineness on calcium sulfoaluminate-belite cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.; Jang, S.Y.; Shin, M. Combined effects of set retarders and polymer powder on the properties of calcium sulfoaluminate blended cement systems. Materials 2018, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, R.; Lu, Q. Influence of polymer latex on the setting time, mechanical properties and durability of calcium sulfoaluminate cement mortar. Constr. Build. Mater. 2018, 169, 911–922. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoaluminate cement at various temperatures by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 21, 1881–1886. [Google Scholar] [CrossRef]
- Shoude, W.; Cheng, C.; Lingchao, L.; Xin, C. Effects of slag and limestone powder on the hydration and hardening process of alite-barium calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 35, 227–231. [Google Scholar] [CrossRef]
- Cabrera, J.G.; Al-Hasan, A.S. Performance properties of concrete repair materials. Constr. Build. Mater. 1997, 11, 283–290. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; León-Reina, L.; Aranda, M.A.; De la Torre, A.G. Hydration Reactions and Mechanical Strength Developments of Iron-Rich Sulfobelite Eco-cements. Ind. Eng. Chem. Res. 2013, 52, 16606–16614. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Ludwig, H.M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Li, J.H.; Ma, H.W.; Zhao, H.W. Preparation of sulphoaluminate-alite composite mineralogical phase cement clinker from high alumina fly ash. Key Eng. Mater. 2007, 334–335, 421–424. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of calcium sulfoaluminate formation from tricalcium aluminate, calcium sulfate and calcium oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Wang, S.; Tang, M.; Shen, X. Effect of SO3 and MgO on Portland cement clinker: Formation of clinker phases and alite polymorphism. Constr. Build. Mater. 2014, 58, 182–192. [Google Scholar] [CrossRef]
- Puertas, F.; Varela, M.T.B.; Molina, S.G. Kinetics of the thermal decomposition of C4A3 in air. Cem. Concr. Res. 1995, 25, 572–580. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Altun, I.A. Effect of CaF2 and MgO on sintering of cement clinker. Cem. Concr. Res. 1999, 29, 1847–1850. [Google Scholar] [CrossRef]
- Blanco-Varela, M.T.; Puertas, F.; Vázquez, T.; Palomo, A. Modelling of the burnability of white cement raw mixes made with CaF2 and CaSO4. Cem. Concr. Res. 1996, 26, 457–464. [Google Scholar] [CrossRef]
- Ma, S.; Snellings, R.; Li, X.; Shen, X.; Scrivener, K.L. Alite-ye’elimite cement: Synthesis and mineralogical analysis. Cem. Concr. Res. 2013, 45, 15–20. [Google Scholar] [CrossRef]
- Lu, X.; Li, C.; Wang, S.; Ye, Z.; Cheng, X. Effect of ferrite phase on the formation and coexistence of 3CaO·3Al2O3·CaSO4 and 3CaO·SiO2 minerals. Ceramics-Silikáty 2018, 62, 67–73. [Google Scholar]
- Cheng, X. Barium (Strontium) Calcium Sulphoaluminate Cement; Science Press: Beijing, China, 2013. [Google Scholar]
- Wensheng, Z.; Beixing, L.; Yimin, C.; Shixi, O. Study on the stability of the mineral 3CaO·3Al2O3·BaSO4. J. Chin. Ceram. Soc. 2000, 28, 497–500. [Google Scholar]
- Winnefeld, F.; Martin, L.H.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Majling, J.; Znášik, P.; Gabrišová, A.; Svetík, Š. The influence of anhydrite activity upon the hydration of calcium sulfoaluminate cement clinker. Thermochim. Acta 1985, 92, 349–352. [Google Scholar] [CrossRef]
- Hanic, F.; Kapralik, I.; Gabrisova, A. Mechanism of hydration reactions in the system C4A3-CS-CaO-H2O referred to hydration of sulfoaluminate cements. Cem. Concr. Res. 1989, 19, 671–682. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements-experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- García-Maté, M.; Angeles, G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.; De la Torre, A.G. Hydration of belite-ye’elimite-ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Allevi, S.; Marchi, M.; Scotti, F.; Bertini, S.; Cosentino, C. Hydration of calcium sulphoaluminate clinker with additions of different calcium sulphate sources. Mater. Struct. 2016, 49, 453–466. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Stanek, T.; Sulovaky, P. The influence of the alite polymorphism on the strength of the Portland cement. Cem. Concr. Res. 2002, 32, 1169–1175. [Google Scholar] [CrossRef]
- Li, H.X.; Wang, P.M.; Wu, J.G. Formation of tricalcium silicate with different content of SO3. Bull. Chin. Ceram. Soc. 2007, 26, 685–690. [Google Scholar]
- Dreele, R.B.V. Quantitative texture analysis by Rietveld refinement. J. Appl. Crystallogr. 1997, 30, 517–525. [Google Scholar] [CrossRef]
- Tong, M.J. Study on the Relationship of Petrographic Structure and Strength of Portland Cement Clinker; Southwest University of Science and Technology: Mianyang, China, 2014. [Google Scholar]
- Wang, Y.J. The Influence of Fe2O3 on the Formation of Clinker Mineral and Performance of Sulphoaluminate Cement; Wuhan University of Technology: Wuhan, China, 2015. [Google Scholar]
- Pelletier, L.; Winnefeld, F.; Lothenbach, B. The ternary system Portland cement–calcium sulphoaluminate clinker–anhydrite: Hydration mechanism and mortar properties. Cem. Concr. Compos. 2010, 32, 497–507. [Google Scholar] [CrossRef]
- Wei, D.B.; Ding, M.; Ren, G.B.; Wu, L.D.; Li, X.M.; Wang, Q.C. Study on hydration properties of composite cementitious material based on sulphoaluminate. Mater. Rev. 2018, 32 (Suppl. 2), 492–497. [Google Scholar]
- Xu, L.L.; Zhou, X.Y.; Li, N.; Wang, P. Impact of calcium sulfate on hydration features of calcium sulfoaluminate cement. J. Tongji Univ. (Nat. Sci.) 2017, 45, 885–890. [Google Scholar]


| Raw Materials | L.O.I | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | BaO |
|---|---|---|---|---|---|---|---|---|
| limestone | 42.63 | 51.99 | 1.80 | 0.51 | 0.35 | / | 2.41 | / |
| bauxite | 16.01 | 2.05 | 16.92 | 58.58 | 2.70 | / | 0.24 | / |
| gypsum | 9.67 | 37.66 | 0.23 | 1.42 | 0.47 | 45.46 | 3.53 | / |
| barite | 6.45 | 1.09 | 2.98 | 0.88 | 0.24 | 27.27 | 0.39 | 59.56 |
| tailing sand | 2.53 | 4.00 | 60.69 | 3.95 | 23.06 | / | 4.20 | / |
| C3S | C2S | C2F | ||
|---|---|---|---|---|
| 21.7 | 24.3 | 10.0 | 35.0 | 7.0 |
| No. | Clinker Dosage | Gypsum Dosage |
|---|---|---|
| A1 | 93 | 7 |
| A2 | 90 | 10 |
| A3 | 87 | 13 |
| A4 | 85 | 15 |
| A5 | 82 | 18 |
| A6 | 79 | 21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ma, Z.; Zhang, Z.; Li, X.; Lu, X.; Hou, P.; Du, P. Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials 2019, 12, 3131. https://doi.org/10.3390/ma12193131
Li P, Ma Z, Zhang Z, Li X, Lu X, Hou P, Du P. Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials. 2019; 12(19):3131. https://doi.org/10.3390/ma12193131
Chicago/Turabian StyleLi, Pei, Zhiqiang Ma, Zhong Zhang, Xumin Li, Xiaolei Lu, Pengkun Hou, and Peng Du. 2019. "Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement" Materials 12, no. 19: 3131. https://doi.org/10.3390/ma12193131
APA StyleLi, P., Ma, Z., Zhang, Z., Li, X., Lu, X., Hou, P., & Du, P. (2019). Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials, 12(19), 3131. https://doi.org/10.3390/ma12193131





