Laser Synthesis of Iridium Nanospheres for Overall Water Splitting
Abstract
1. Introduction
2. Materials and Methods
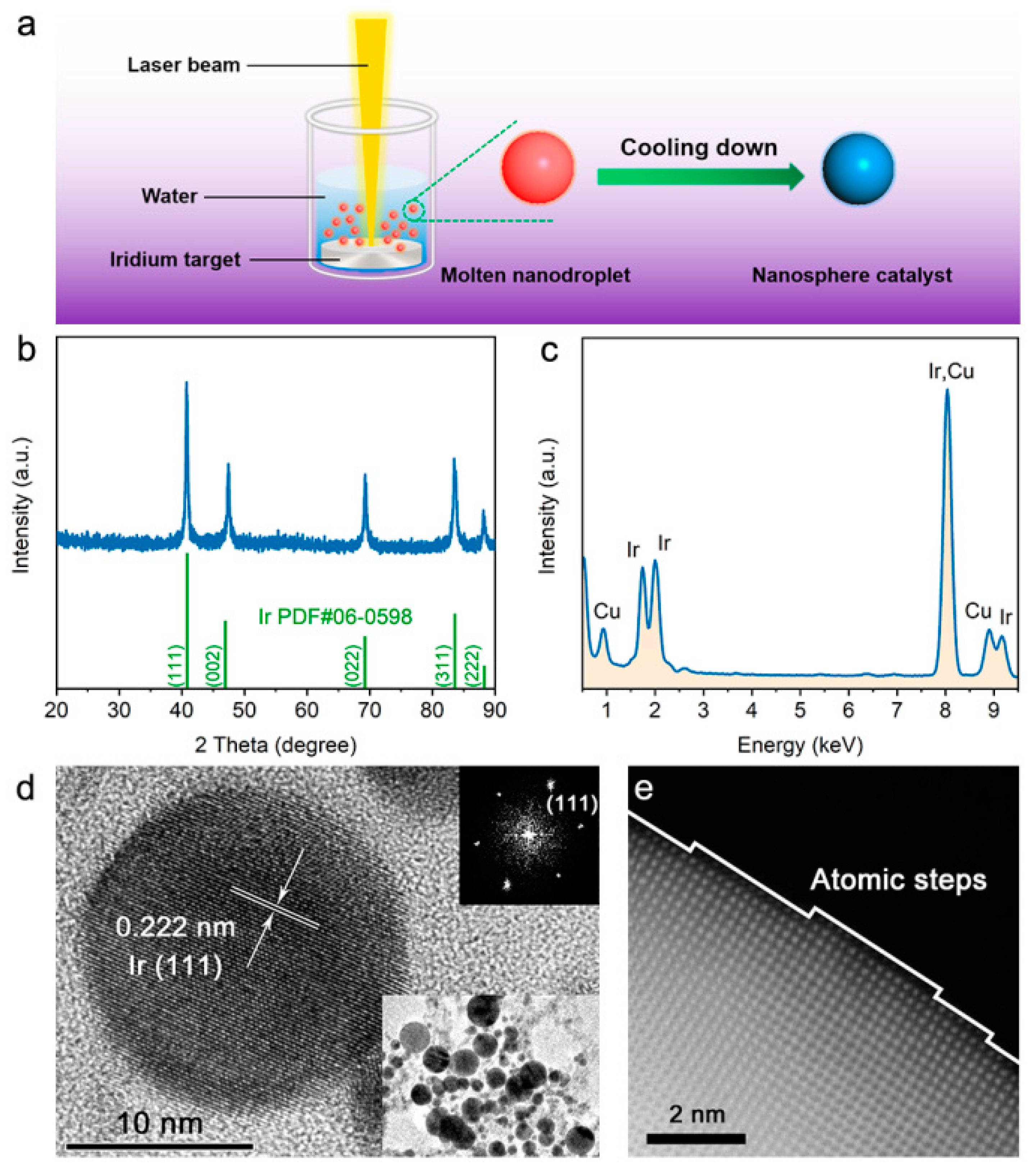
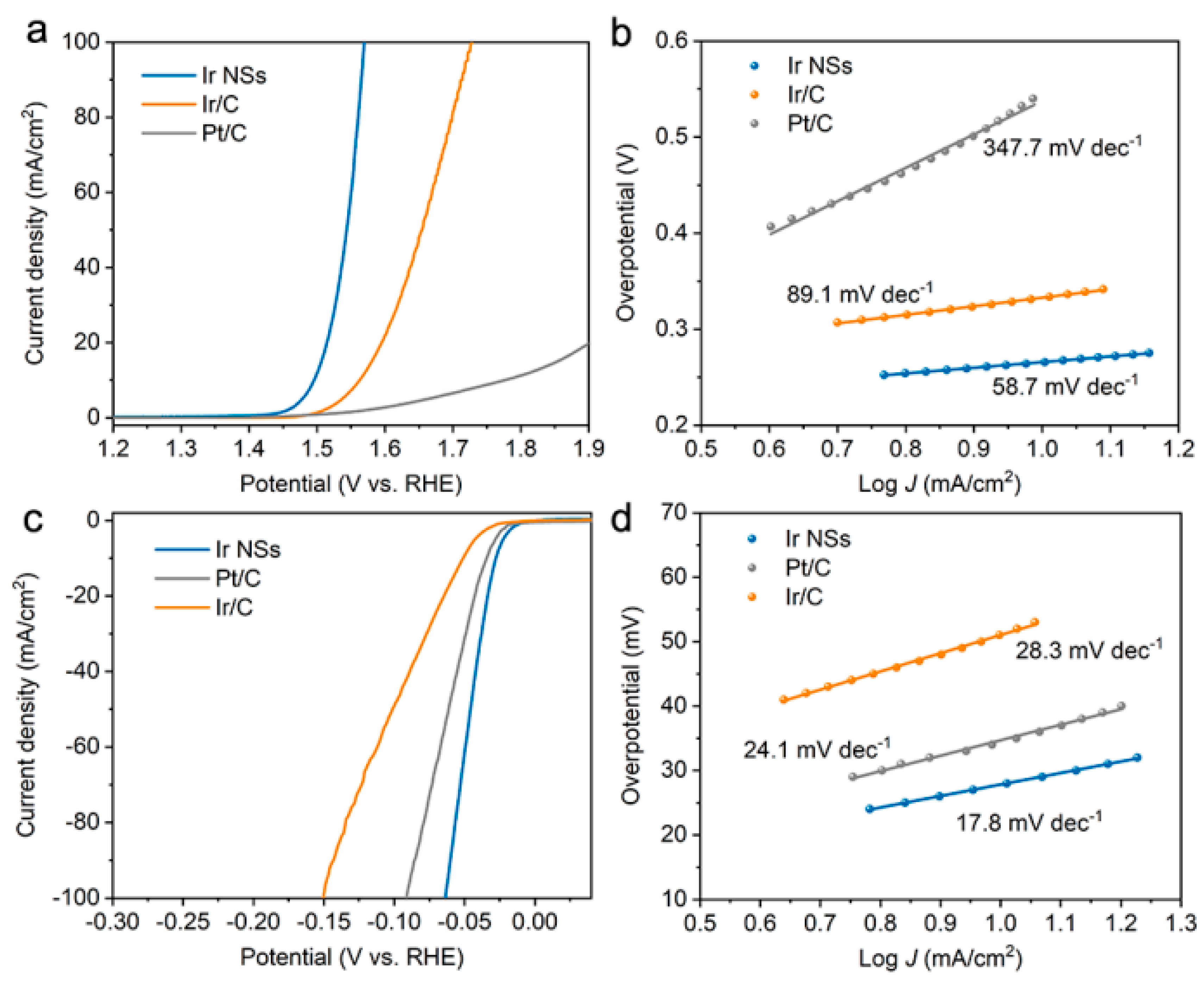
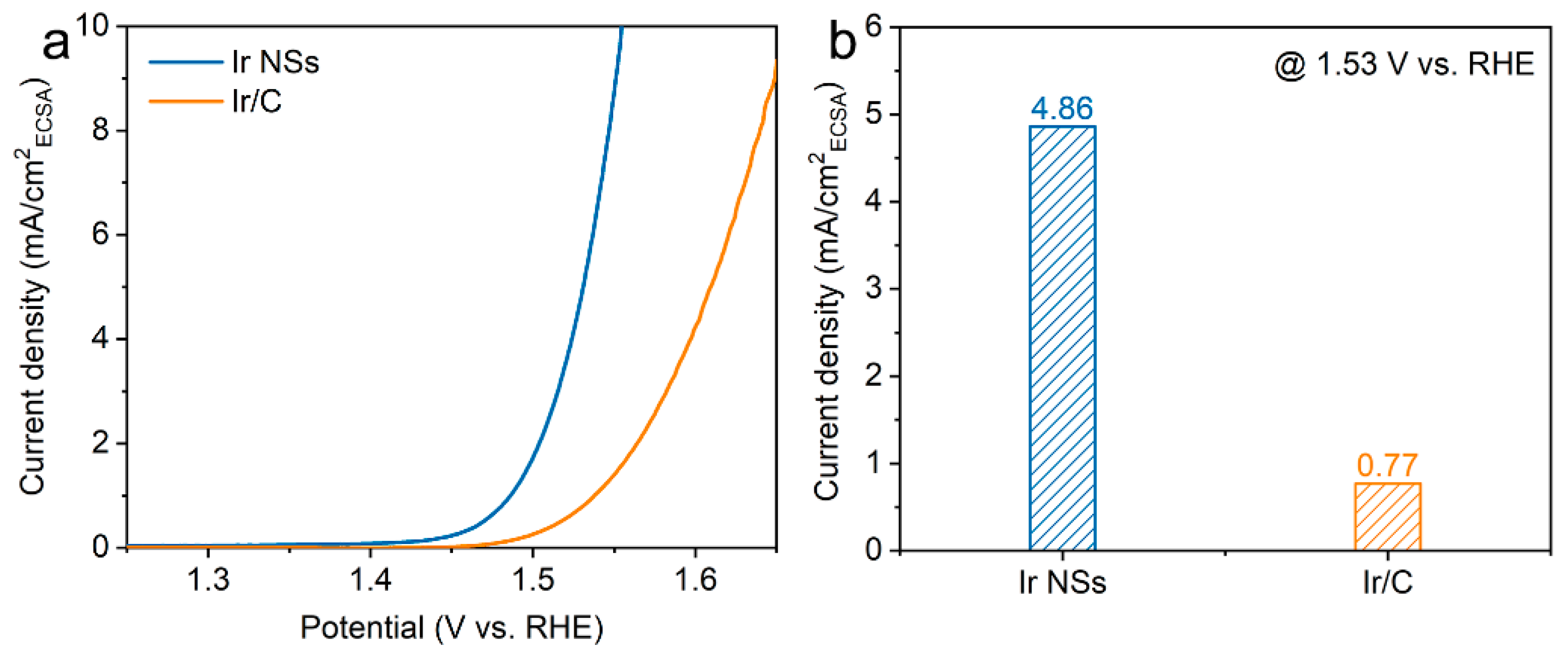
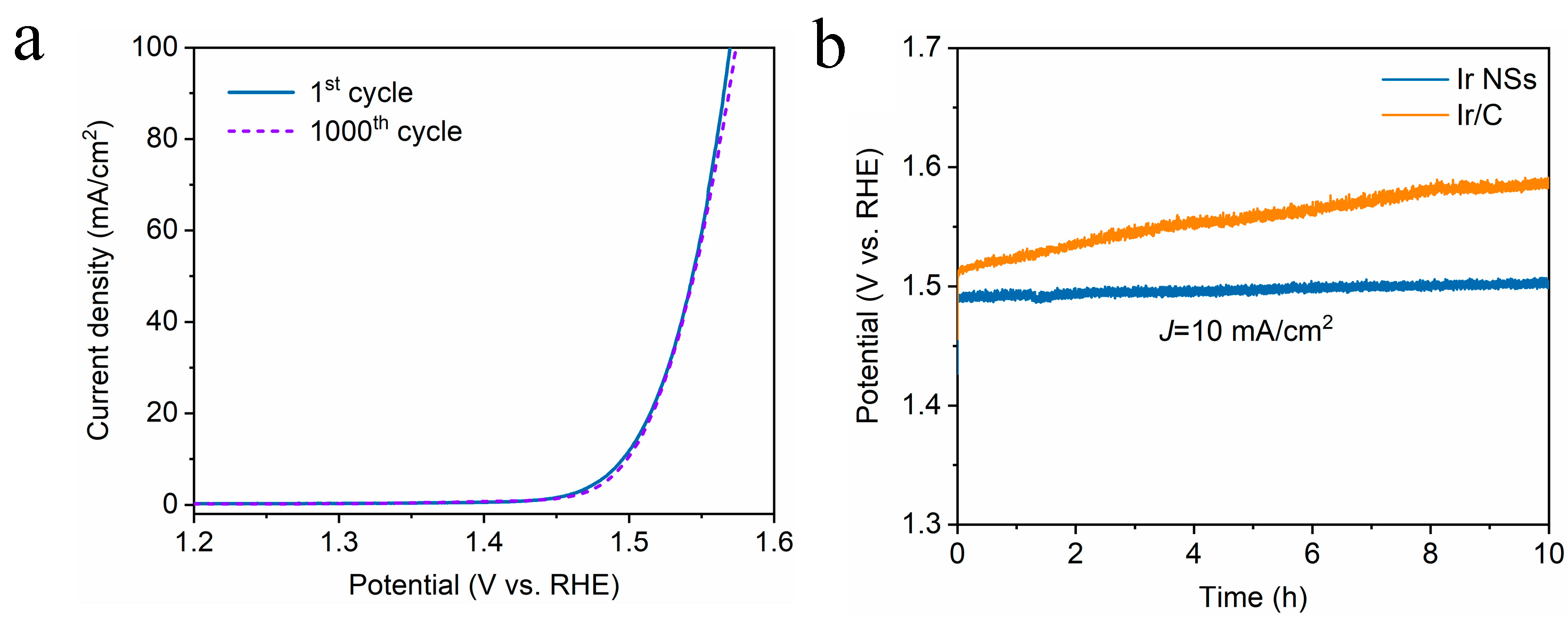
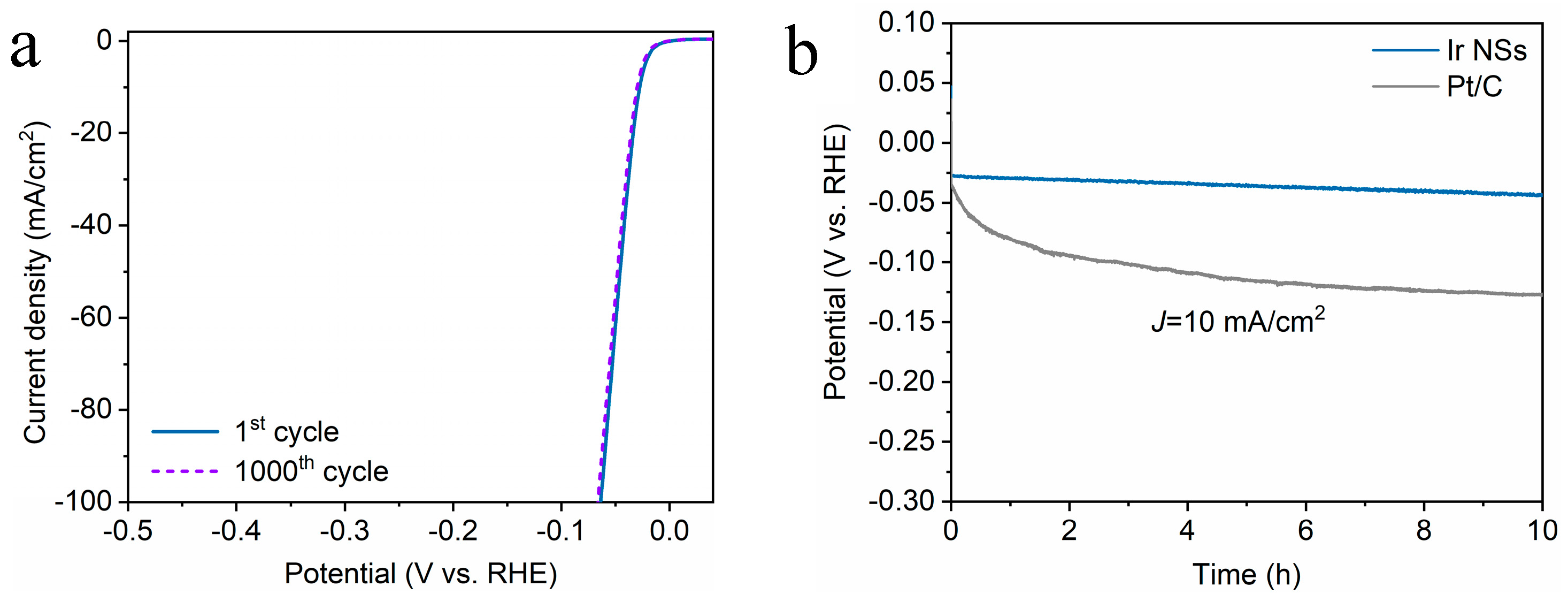
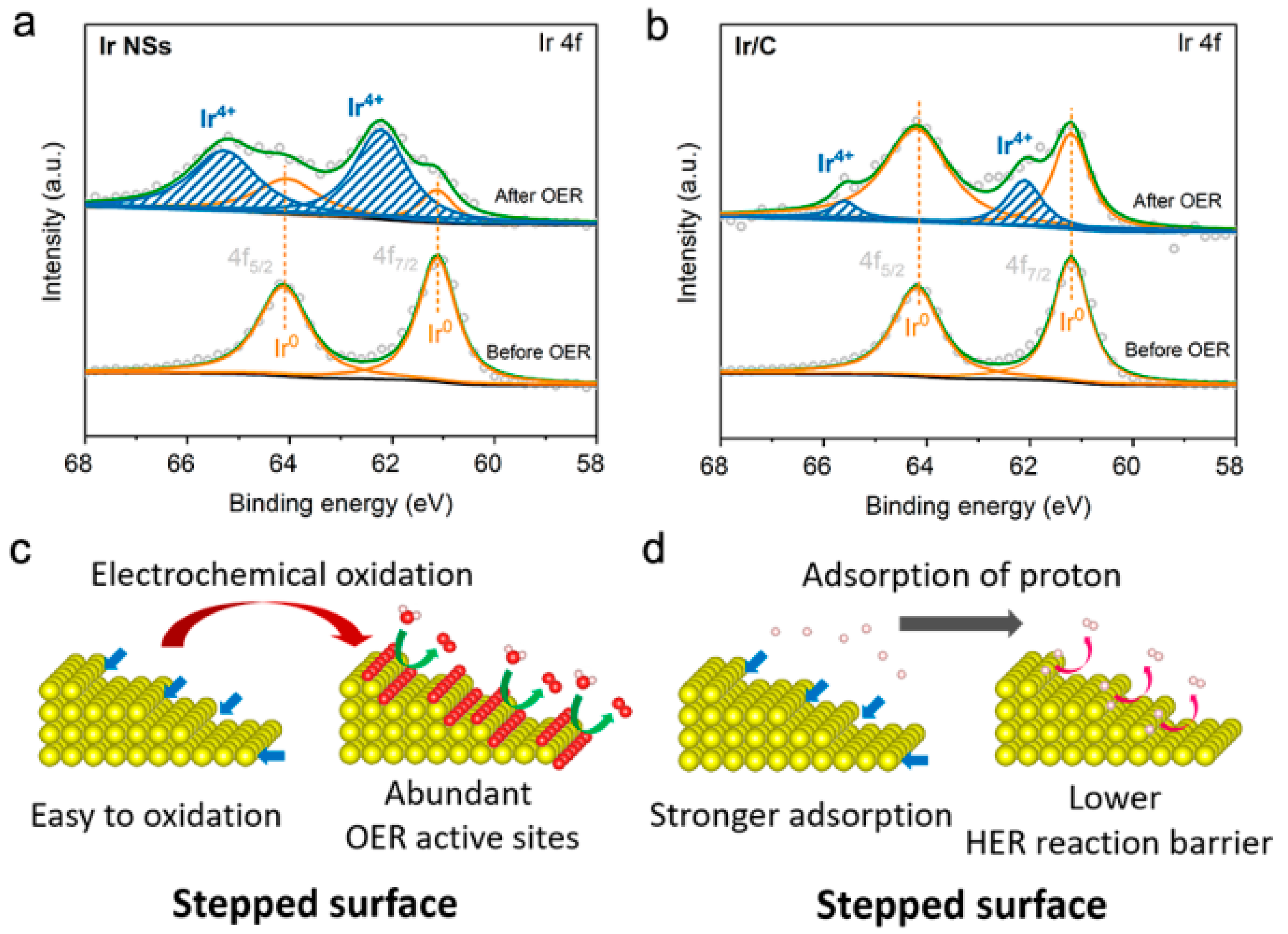
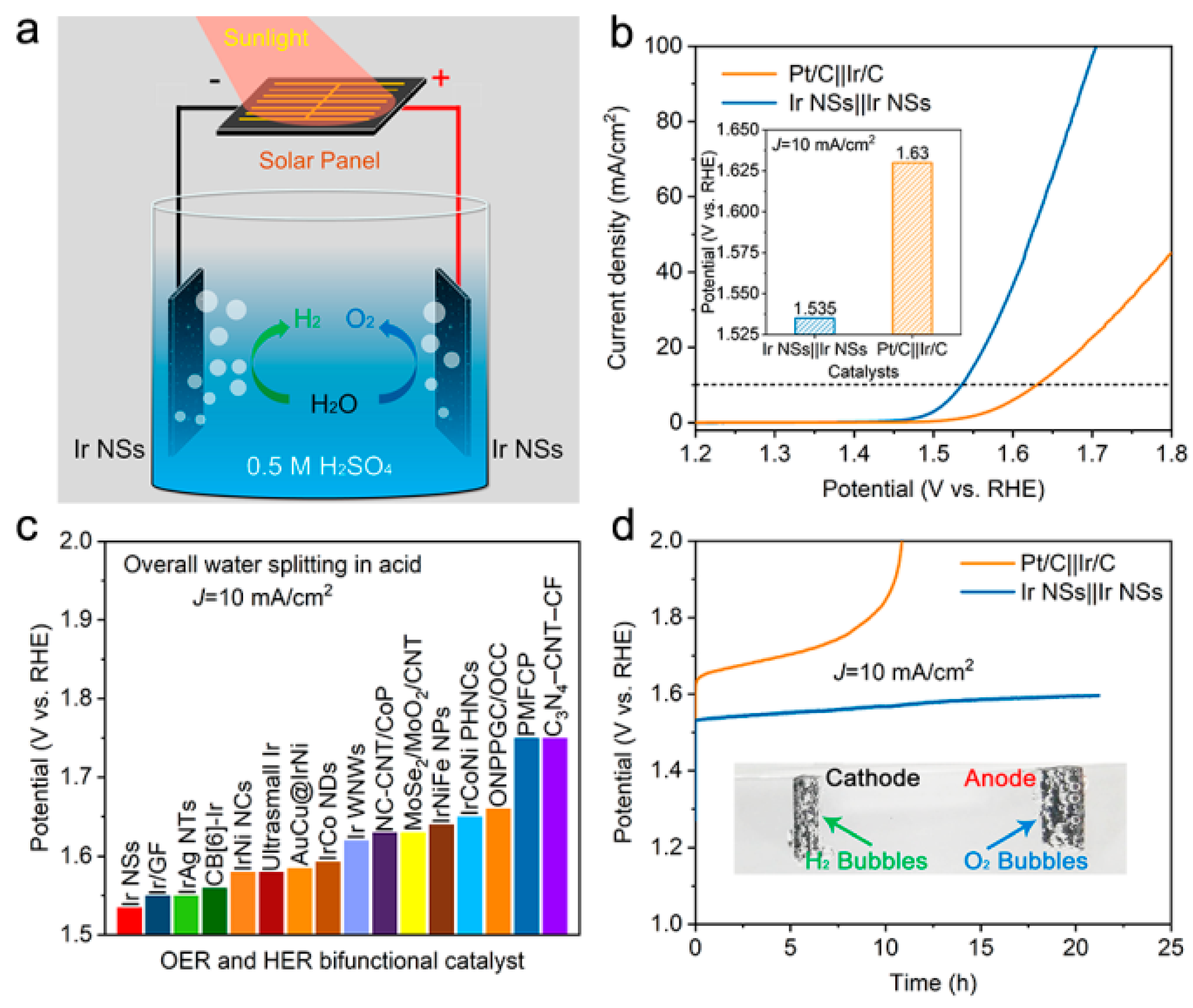
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hunter, B.M.; Gray, H.B.; Müller, A.M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.T.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S.J.; Lu, G.; Yao, J.L.; Huang, X.Q. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun. 2017, 8, 14580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Cui, W.; Liu, Q.; Xing, Z.C.; Asiri, A.M.; Sun, X.P. Recent Progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Hwang, X.; Sa, Y.J.; Park, J.; Baik, H.; Joo, S.H.; Lee, K. Cobalt assisted synthesis of IrCu hollow octahedral nanocages as highly active electrocatalysts toward oxygen evolution reaction. Adv. Funct. Mater. 2017, 27, 1604688. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Nong, H.N.; Reier, T.; Bergmann, A.; Gliech, M.; Araujo, J.F.; Willinger, E.; Schlögl, R.; Teschner, D.; Strasser, P. Electrochemical catalyst–support effects and their stabilizing role for IrOx nanoparticle catalysts during the oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 12552–12563. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Yang, F.L.; Cheng, G.Z.; Luo, W. Ultrathin Ir nanowires as high-performance electrocatalysts for efficient water splitting in acidic media. Nanoscale 2018, 10, 1892–1897. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Oh, A.; Jin, H.; Joo, J.; Baik, H.; Lee, K. Hemi-core@frame AuCu@IrNi nanocrystals as active and durable bifunctional catalysts for the water splitting reaction in acidic media. Nanoscale Horiz. 2019, 4, 727–734. [Google Scholar] [CrossRef]
- Li, S.; Xi, C.; Jin, Y.; Wu, D.Y.; Wang, J.Q.; Liu, T.; Wang, H.B.; Dong, C.; Liu, H.; Kulinich, S.A.; et al. Ir-O-V catalytic group in Ir-doped NiV(OH)2 for overall water splitting. ACS Energy Lett. 2019, 4, 1823–1829. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 146. [Google Scholar] [CrossRef]
- Lv, F.; Feng, J.R.; Wang, K.; Dou, Z.P.; Zhang, W.Y.; Zhou, J.H.; Yang, C.; Luo, M.C.; Yang, Y.; Li, Y.J.; et al. Iridium–tungsten alloy nanodendrites as pH-universal water-splitting electrocatalysts. ACS Cent. Sci. 2018, 4, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Chen, Z.T.; Xie, M.H.; Lyu, Z.H.; Chi, M.F.; Mavrikakis, M.; Jin, W.Q.; Xia, Y.N. Iridium-based cubic nanocages with 1.1-nm-thick walls: A highly efficient and durable electrocatalyst for water oxidation in an acidic medium. Angew. Chem. Int. Ed. 2019, 58, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Zeng, X.; Huang, C.Z.; Cai, P.; Cheng, G.Z.; Luo, W. Ultrasmall Ir nanoparticles for efficient acidic electrochemical water splitting. Inorg. Chem. Front. 2018, 5, 1121–1125. [Google Scholar] [CrossRef]
- Shi, Q.R.; Zhu, C.Z.; Zhong, H.; Su, D.; Li, N.; Engelhard, M.H.; Xia, H.B.; Zhang, Q.; Feng, S.; Beckman, S.P.; et al. Nanovoid incorporated IrxCu metallic aerogels for oxygen evolution reaction catalysis. ACS Energy Lett. 2018, 3, 2038–2044. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Liao, Z.Q.; Zhang, P.P.; Wang, F.X.; Zhuang, X.D.; Zschech, E.; Feng, X.L. Iridium nanoparticles anchored on 3D graphite foam as a bifunctionalelectrocatalyst for excellent overall water splitting in acidic solution. Nano Energy 2017, 40, 27–33. [Google Scholar] [CrossRef]
- You, H.H.; Wu, D.S.; Chen, Z.N.; Sun, F.F.; Zhang, H.; Chen, Z.H.; Cao, M.N.; Zhuang, W.; Cao, R. Highly active and stable water splitting in acidic media using a bifunctional iridium/cucurbit[6]uril catalyst. ACS Energy Lett. 2019, 4, 1301–1307. [Google Scholar] [CrossRef]
- Feng, J.R.; Lv, F.; Zhang, W.Y.; Li, P.H.; Wang, K.; Yang, C.; Wang, B.; Yang, Y.; Zhou, J.H.; Lin, F.; et al. Iridium-based multimetallic porous hollow nanocrystals for efficient overall-water-splitting catalysis. Adv. Mater. 2017, 29, 1703798. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.Q.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S.Z. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments. Adv. Mater. 2019, 31, 1900510. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Cheng, G.Z.; Luo, W. Colloidal synthesis of monodisperse trimetallic IrNiFe nanoparticles as highly active bifunctional electrocatalysts for acidic overall water splitting. J. Mater. Chem. A 2017, 5, 24836–24841. [Google Scholar] [CrossRef]
- Pi, Y.C.; Shao, Q.; Zhu, X.; Huang, X.Q. Dynamic structure evolution of composition segregated iridium-nickel rhombic dodecahedra toward efficient oxygen evolution electrocatalysis. ACS Nano 2018, 12, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Wu, D.X.; Zou, L.F.; Yin, Q.Y.; Zhang, H.L.; Zakharov, D.N.; Stach, E.A.; Zhou, G.W. In situ atomic-scale observation of inhomogeneous oxide reduction. Chem. Commun. 2018, 54, 7342–7345. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, B.; Ni, Z.Y.; Deng, Y.D.; Han, X.P.; Hu, W.B.; Zhong, C. Improving the electrocatalytic activity of Pt monolayer catalysts for electrooxidation of methanol, ethanol and ammmonia by tailoring the surface morphology of the supporting core. ChemElectroChem 2016, 3, 537–551. [Google Scholar] [CrossRef]
- Lee, S.W.; Chen, S.; Sheng, W.C.; Yabuuchi, N.; Kim, Y.T.; Mitani, T.; Vescovo, E.; Shao-Horn, Y. Roles of surface steps on Pt nanoparticles in electro-oxidation of carbon monoxide and methanol. J. Am. Chem. Soc. 2009, 131, 15669–15677. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Fan, Y.J.; Wang, H.H.; Tian, N.; Zhou, Z.Y.; Sun, S.G. Electrochemically shape-controlled synthesis in deep eutectic solvents of Pt nanoflowers with enhanced activity for ethanol oxidation. Electrochim. Acta 2012, 76, 468–474. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhang, X.; Luo, M.C.; Chen, X.; Wang, L.; Li, Y.J.; Li, M.Q.; Qin, Y.N.; Li, C.J.; Xu, N.Y.; et al. Ultrathin PtPd-based nanorings with abundant step atoms enhance oxygen catalysis. Adv. Mater. 2018, 30, 1802136. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Yang, K.D.; Yoon, S.M.; Ahn, H.Y.; Lee, Y.Y.; Chang, H.; Jeong, D.H.; Lee, Y.S.; Kim, M.Y.; Nam, K.T. Concave rhombic dodecahedral Au nanocatalyst with multiple high-index facets for CO2 reduction. ACS Nano 2015, 9, 8384–8393. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.C.; Guo, J.; Shao, Q.; Huang, X.Q. Highly efficient acidic oxygen evolution electrocatalysis enabled by porous Ir–Cu nanocrystals with three-dimensional electrocatalytic surfaces. Chem. Mater. 2018, 30, 8571–8578. [Google Scholar] [CrossRef]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.Y.; Hu, J.S. Hydrogen evolution: Scalable solid-state synthesis of highly dispersed uncapped Metal (Rh, Ru, Ir) nanoparticles for efficient hydrogen evolution. Adv. Energy Mater. 2018, 8, 1801698. [Google Scholar] [CrossRef]
- Wang, C.; Sui, Y.M.; Xiao, G.J.; Yang, X.Y.; Wei, Y.J.; Zou, G.T.; Zou, B. Synthesis of Cu–Ir nanocages with enhanced electrocatalytic activity for the oxygen evolution reaction. J. Mater. Chem. A 2015, 3, 19669–19673. [Google Scholar] [CrossRef]
- Zeng, H.B.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.K.; He, J.P.; Cai, W.P. Nanomaterials via laser ablation/ irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454. [Google Scholar] [CrossRef]
- Gavrilenko, E.A.; Goncharova, D.A.; Lapin, I.N.; Nemoykina, A.L.; Svetlichnyi, V.A.; Aljulaih, A.A.; Mintcheva, N.; Kulinich, S.A. Comparative study of physicochemical and antibacterial properties of ZnO nanoparticles prepared by laser ablation of Zn target in water and air. Materials 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, Y.L.; Cui, L.; Kulinich, S.A.; Du, X.W. Spheroidization of nickel powder and coating with carbon layer through laser heating. Materials 2018, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Mintcheva, N.; Aljulaih, A.A.; Bito, S.; Honda, M.; Kondo, T.; Iwamori, S.; Kulinich, S.A. Nanomaterials produced by laser beam ablating Sn-Zn alloy in water. J. Alloys Compd. 2018, 747, 166–175. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-ablated ZnO nanoparticles and their photocatalytic activity towards organic pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sato, Y.; Kinoshita, M.; Shankar, P.; Mintcheva, N.N.; Honda, M.; Iwamori, S.; Kulinich, S.A. Room temperature ethanol sensor based on ZnO prepared via laser ablation in water. Jpn. J. Appl. Phys. 2017, 56, 080304. [Google Scholar] [CrossRef]
- Honda, M.; Goto, T.; Owashi, T.; Rozhin, A.G.; Yamaguchi, S.; Ito, T.; Kulinich, S.A. ZnO nanorods prepared via ablation of Zn with millisecond laser in liquid media. Phys. Chem. Chem. Phys. 2016, 18, 23628–23637. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Kondo, T.; Shimizu, Y.; Ito, T. Pressure effect on ZnO nanoparticles prepared via laser ablation in water. J. Appl. Phys. 2013, 113, 033509. [Google Scholar] [CrossRef]
- Svetlichnyi, V.A.; Shabalina, A.V.; Lapin, I.N.; Goncharova, D.A.; Kharlamova, T.S.; Stadnichenko, A.I. Comparative study of magnetite nanoparticles obtained by pulsed laser ablation in water and air. Appl. Surf. Sci. 2019, 467–468, 402–410. [Google Scholar] [CrossRef]
- Brandiele, R.; Amendola, V.; Guadagnini, A.; Rizzi, G.A.; Badocco, D.; Pastore, P.; Isse, A.A.; Durante, C.; Gennaro, A. Facile synthesis of Pd3Y alloy nanoparticles for electrocatalysis of the oxygen reduction reaction. Electrochim. Acta 2014, 320, 134563. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.K.; Wang, J.Q.; Kulinich, S.A.; Du, X.W. Laser-prepared CuZn alloy catalyst for selective electrochemical reduction of CO2 to ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of surfactant-free colloidal nanoparticles in heterogeneous catalysis. ChemCatChem 2019, 11, 1–31. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-B.; Wang, J.-Q.; Mintcheva, N.; Wang, M.; Li, S.; Mao, J.; Liu, H.; Dong, C.-K.; Kulinich, S.A.; Du, X.-W. Laser Synthesis of Iridium Nanospheres for Overall Water Splitting. Materials 2019, 12, 3028. https://doi.org/10.3390/ma12183028
Wang H-B, Wang J-Q, Mintcheva N, Wang M, Li S, Mao J, Liu H, Dong C-K, Kulinich SA, Du X-W. Laser Synthesis of Iridium Nanospheres for Overall Water Splitting. Materials. 2019; 12(18):3028. https://doi.org/10.3390/ma12183028
Chicago/Turabian StyleWang, Hai-Bin, Jia-Qi Wang, Neli Mintcheva, Min Wang, Shuang Li, Jing Mao, Hui Liu, Cun-Ku Dong, Sergei A. Kulinich, and Xi-Wen Du. 2019. "Laser Synthesis of Iridium Nanospheres for Overall Water Splitting" Materials 12, no. 18: 3028. https://doi.org/10.3390/ma12183028
APA StyleWang, H.-B., Wang, J.-Q., Mintcheva, N., Wang, M., Li, S., Mao, J., Liu, H., Dong, C.-K., Kulinich, S. A., & Du, X.-W. (2019). Laser Synthesis of Iridium Nanospheres for Overall Water Splitting. Materials, 12(18), 3028. https://doi.org/10.3390/ma12183028






