Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review
Abstract
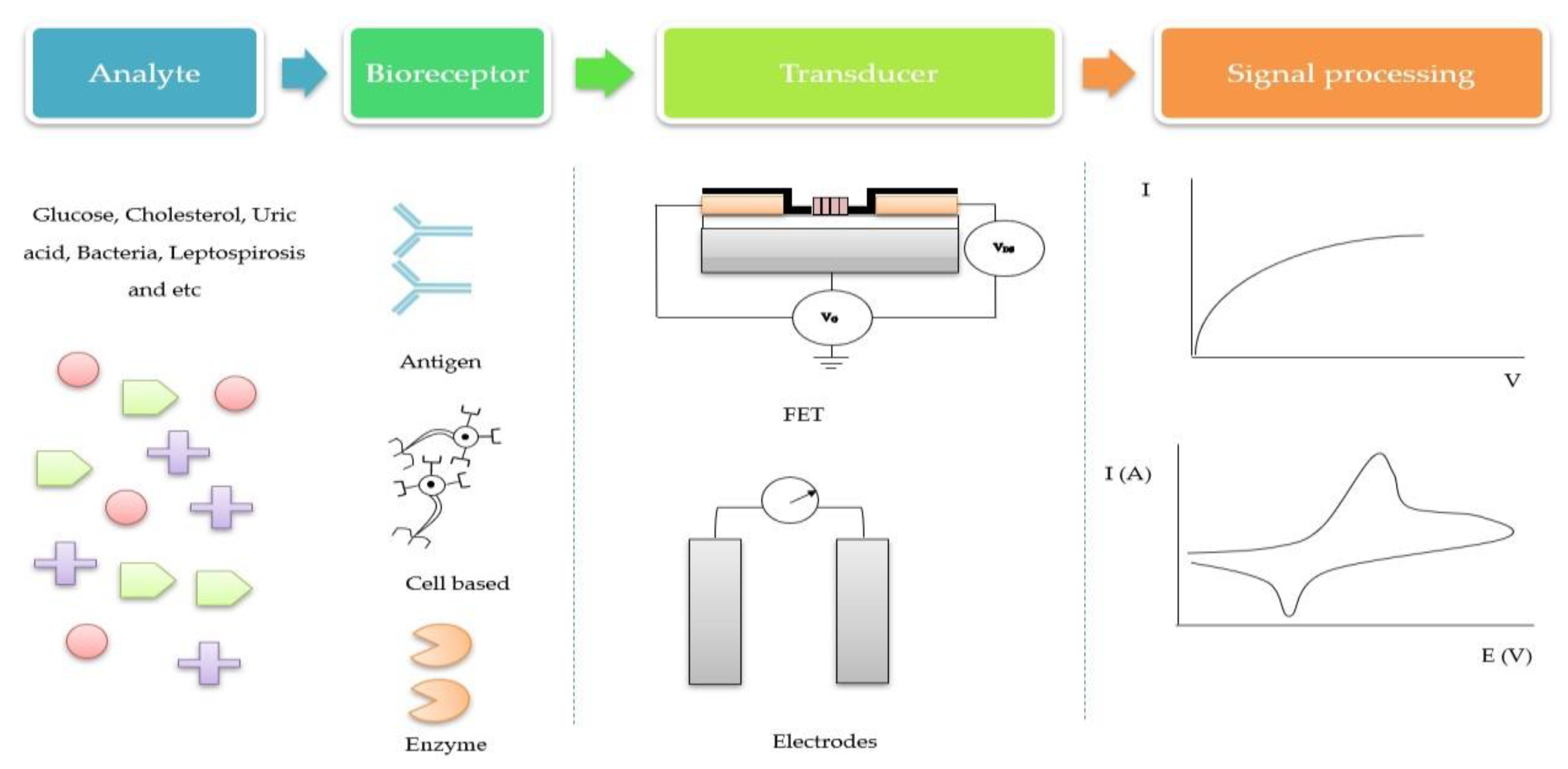
1. Introduction
ZnO-Based Electrochemical Biosensors
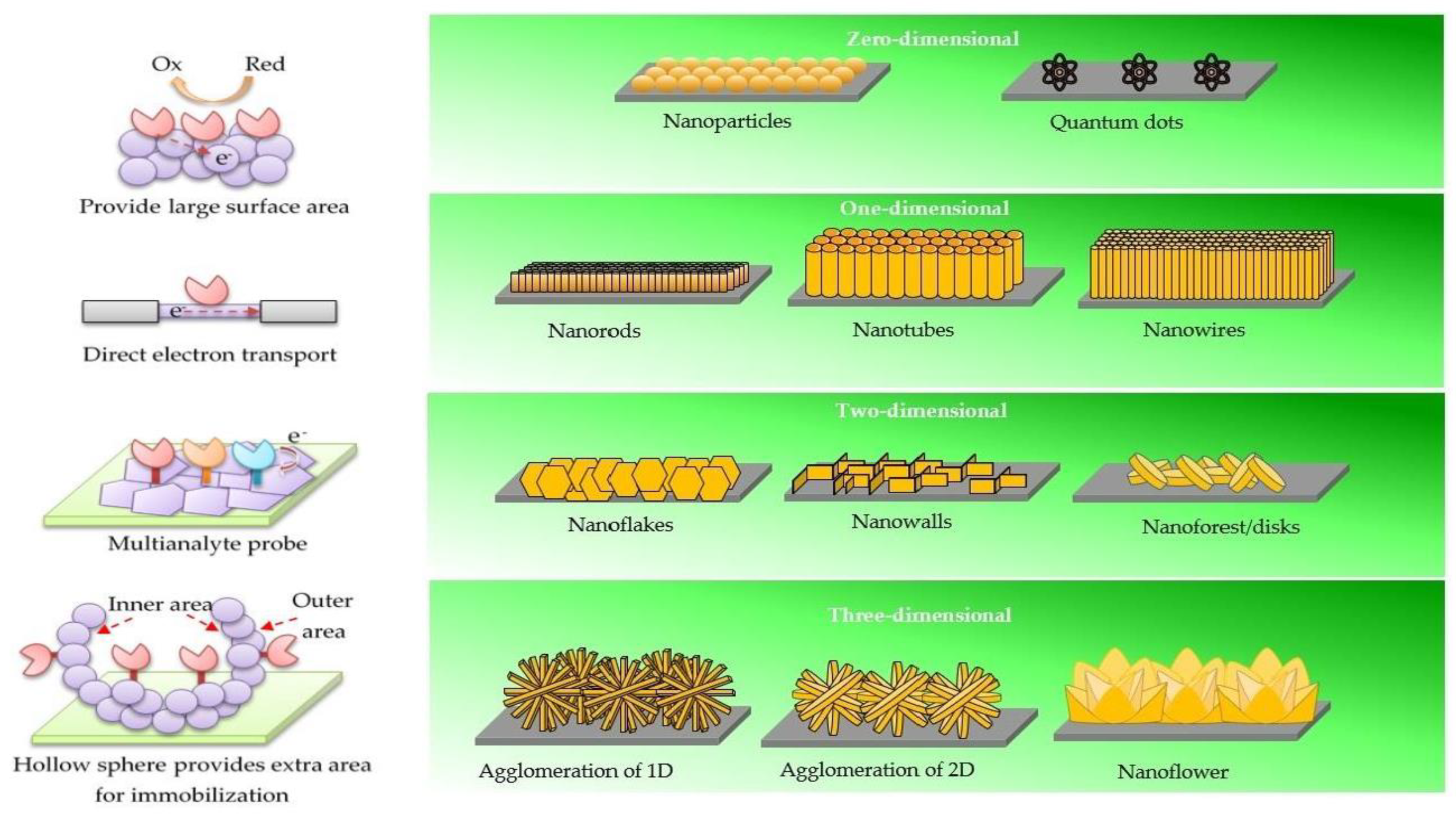
2. Biosensors with Different ZnO Morphologies
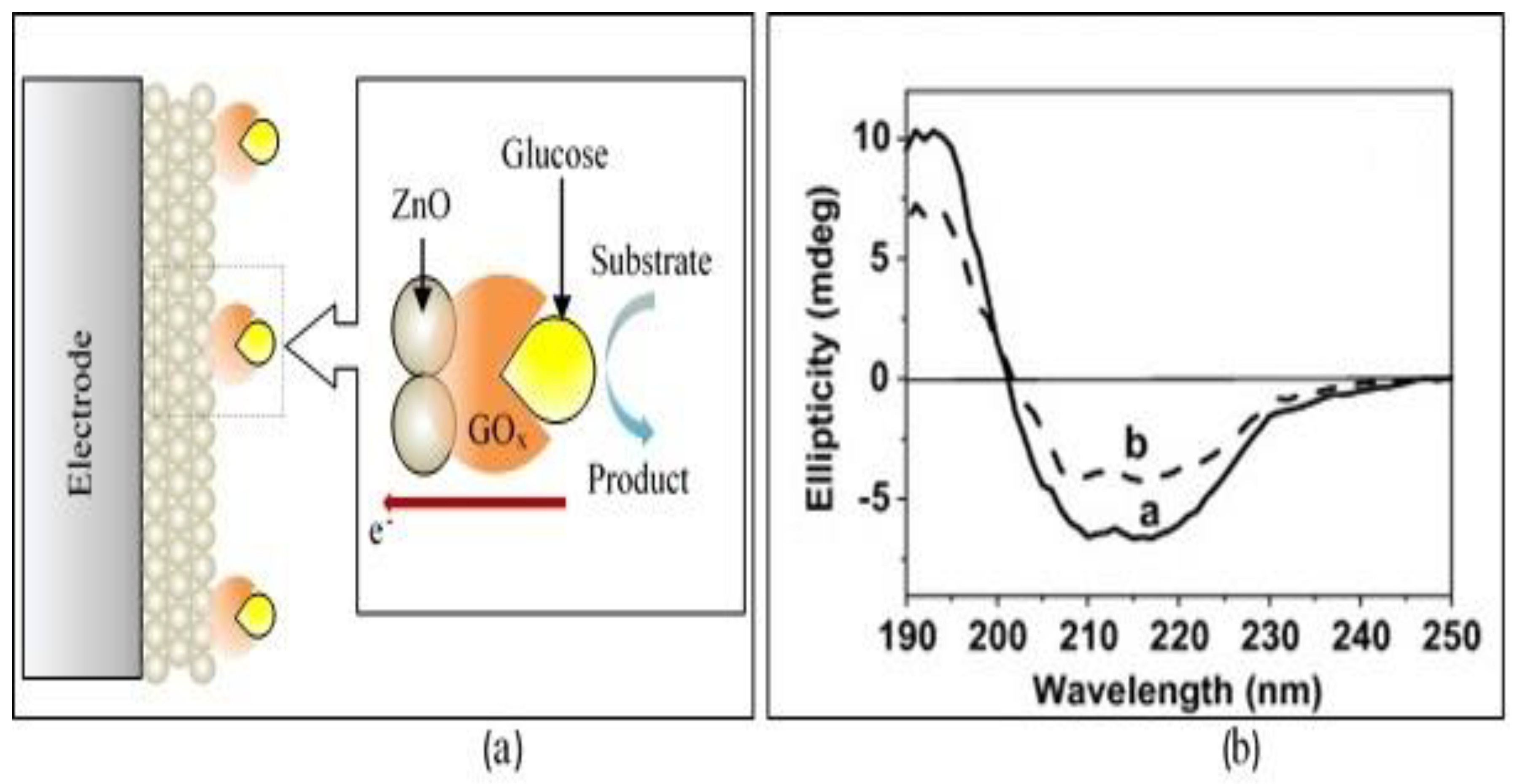
2.1. Zero-Dimensional ZnO Nanostructures (0-D ZnO)
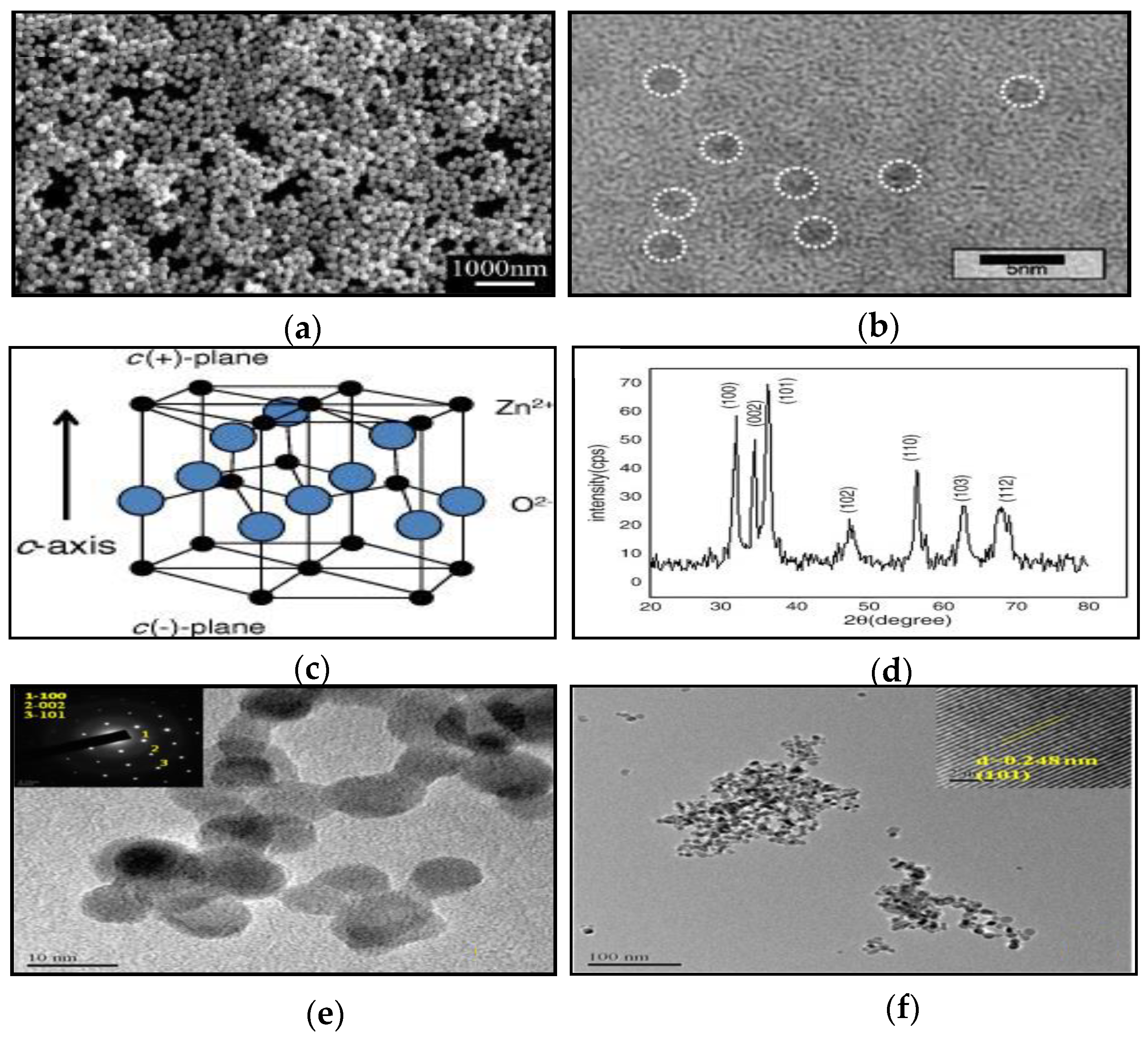
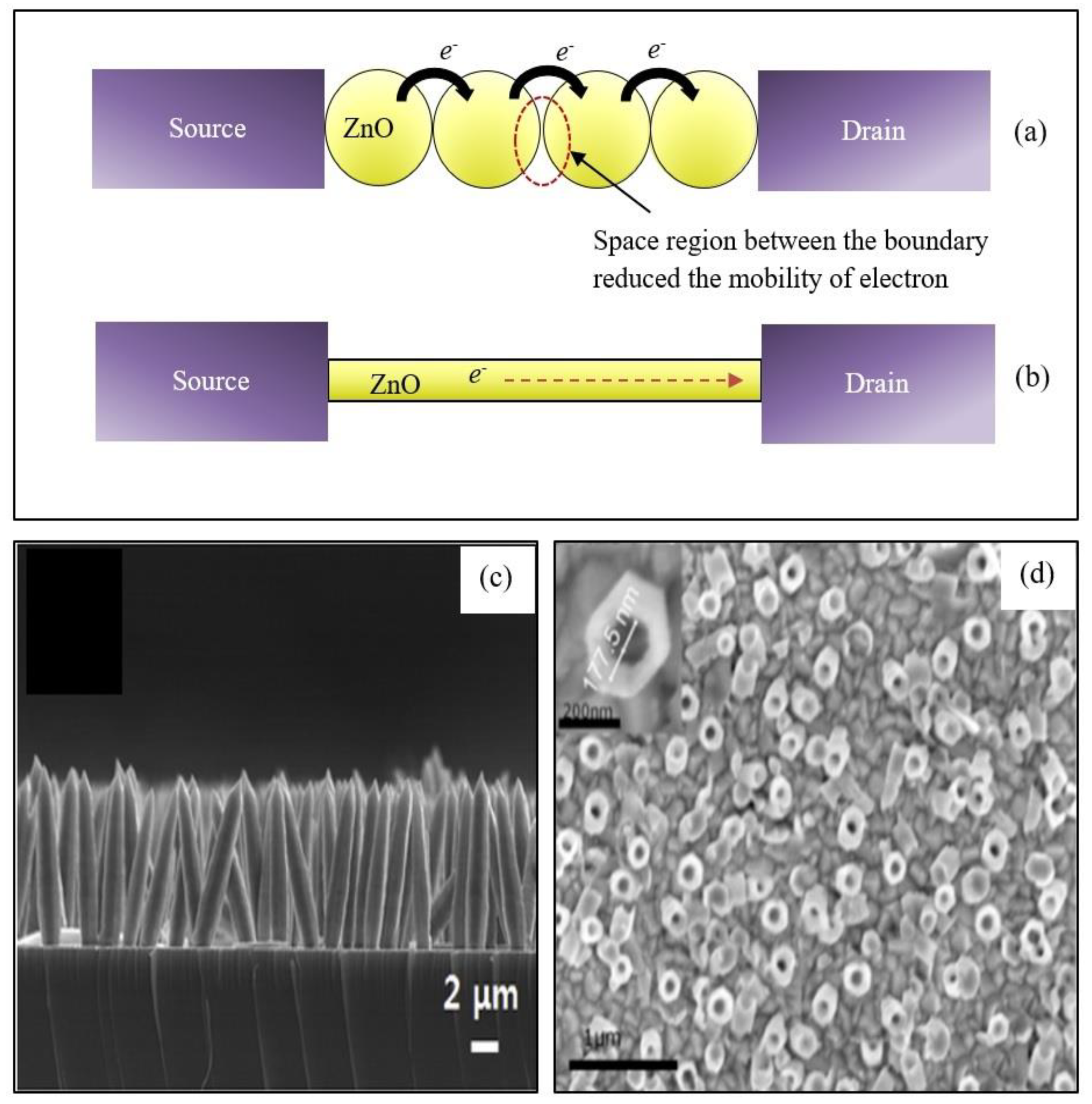
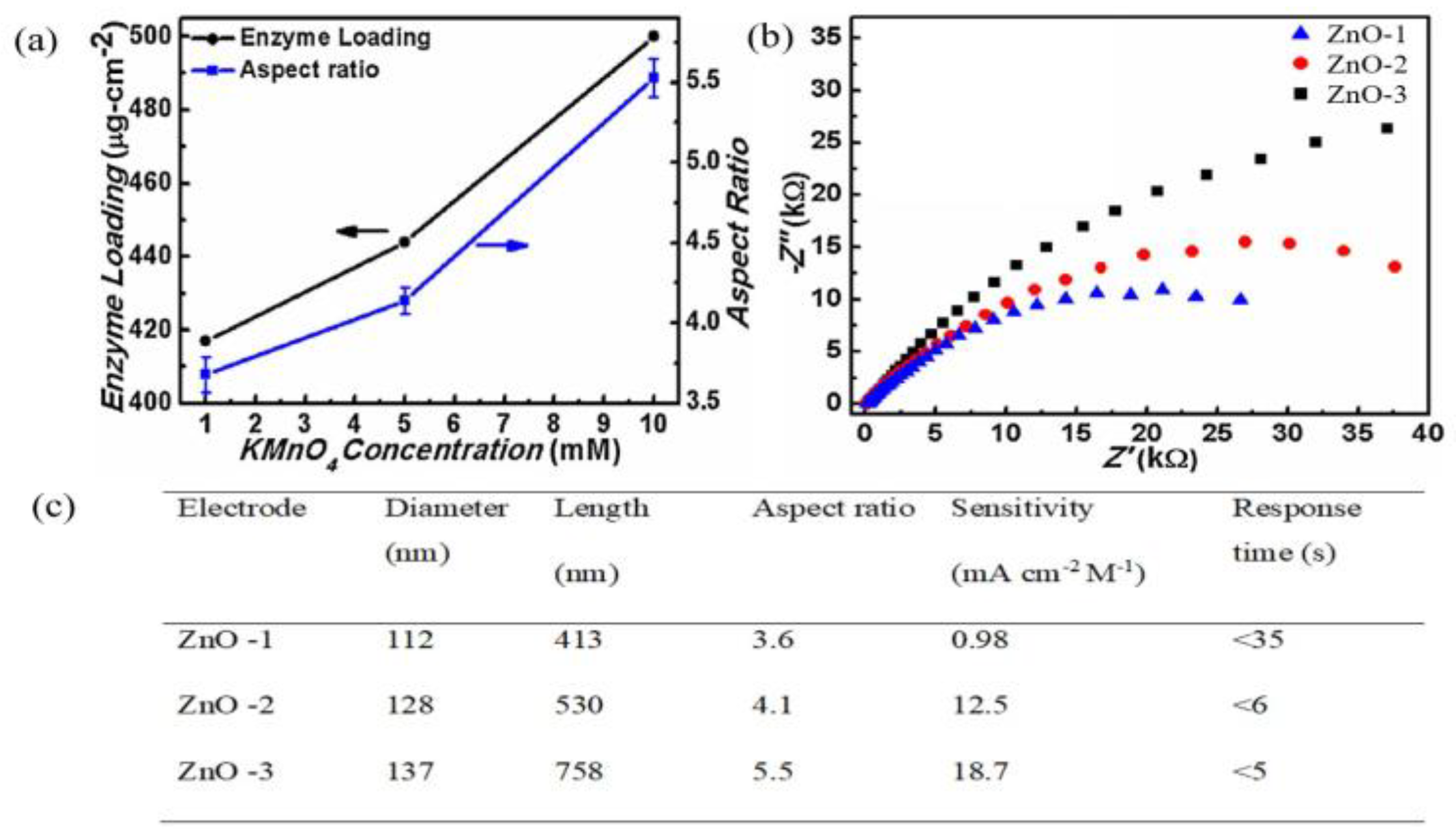
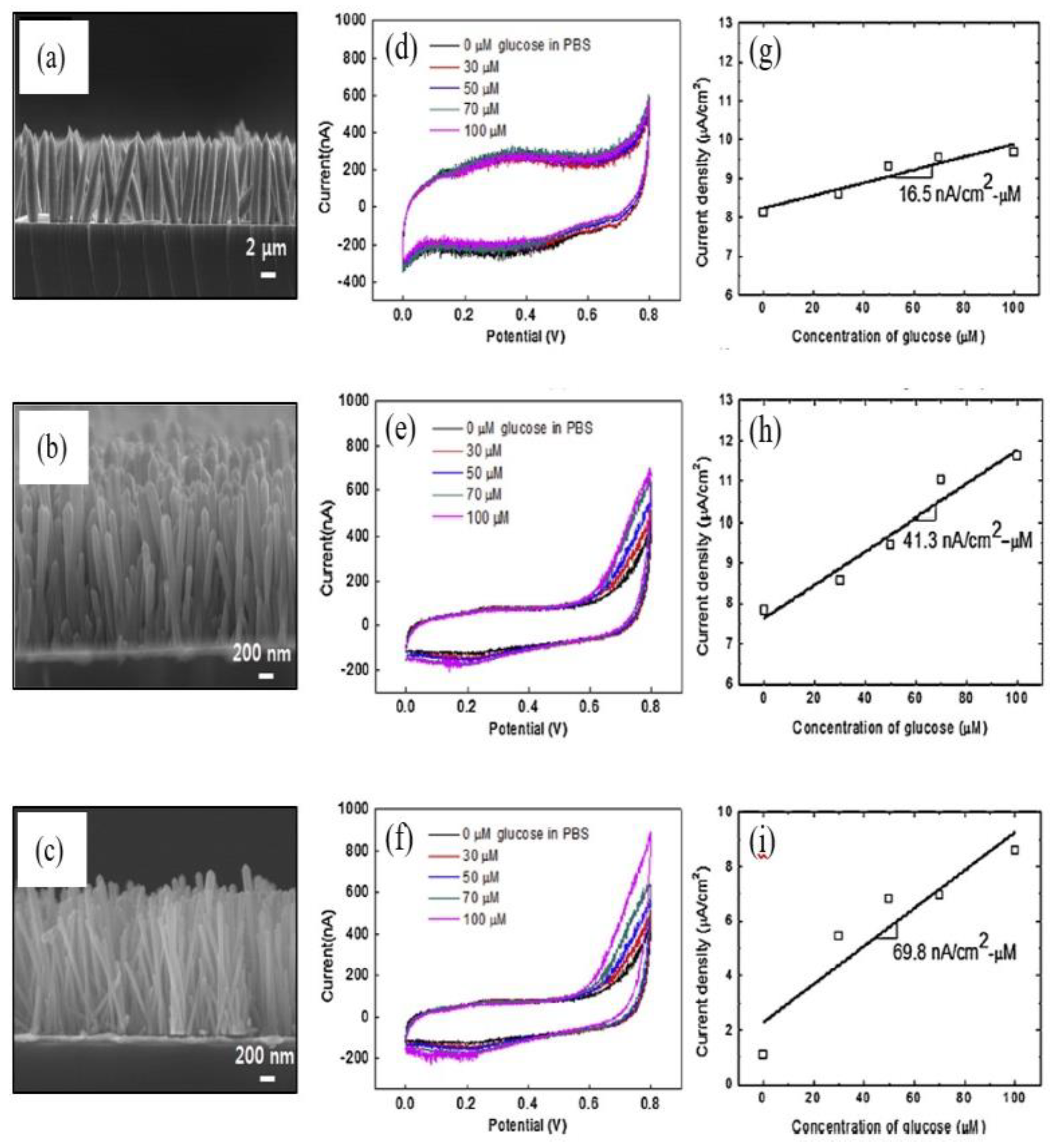
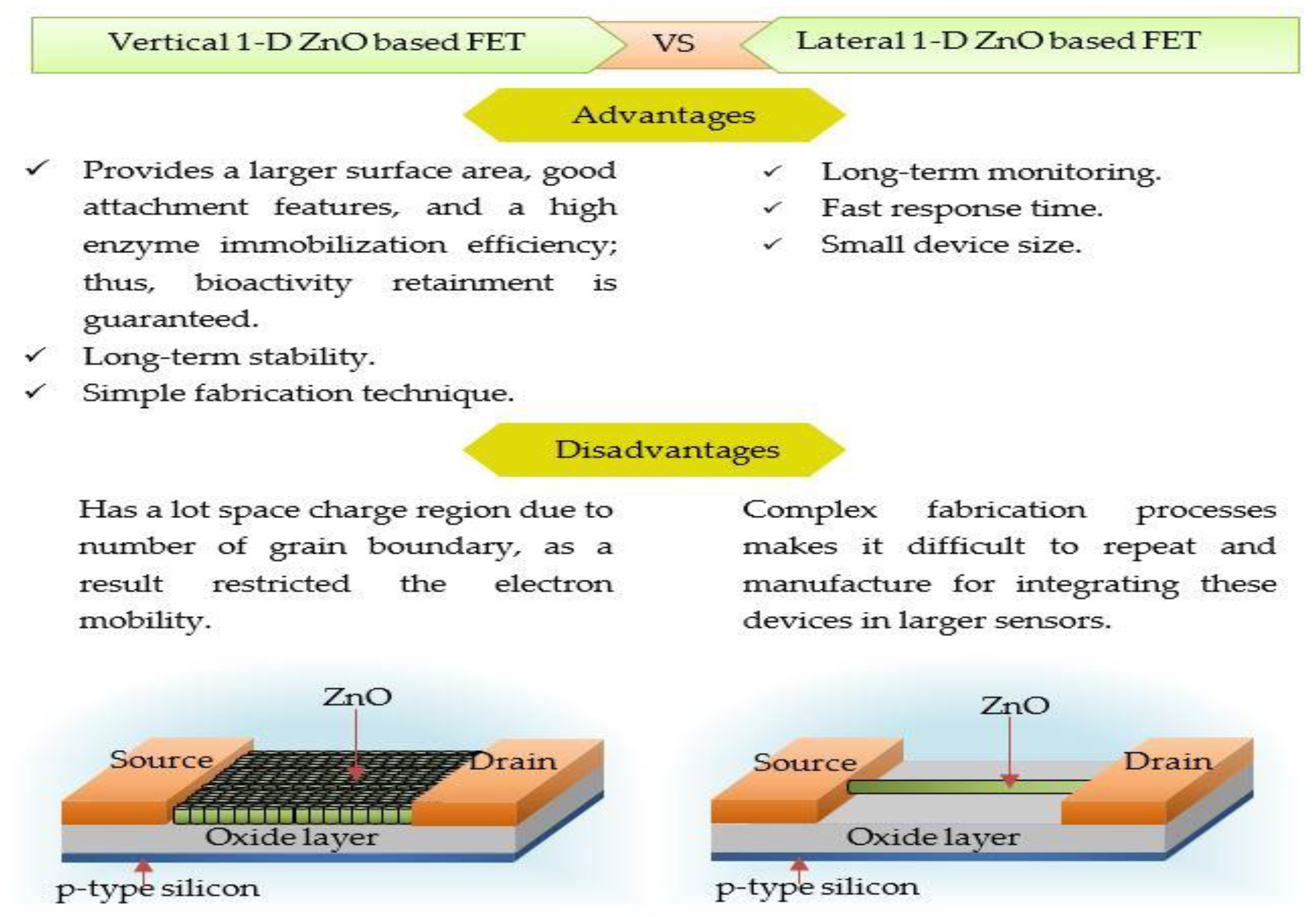
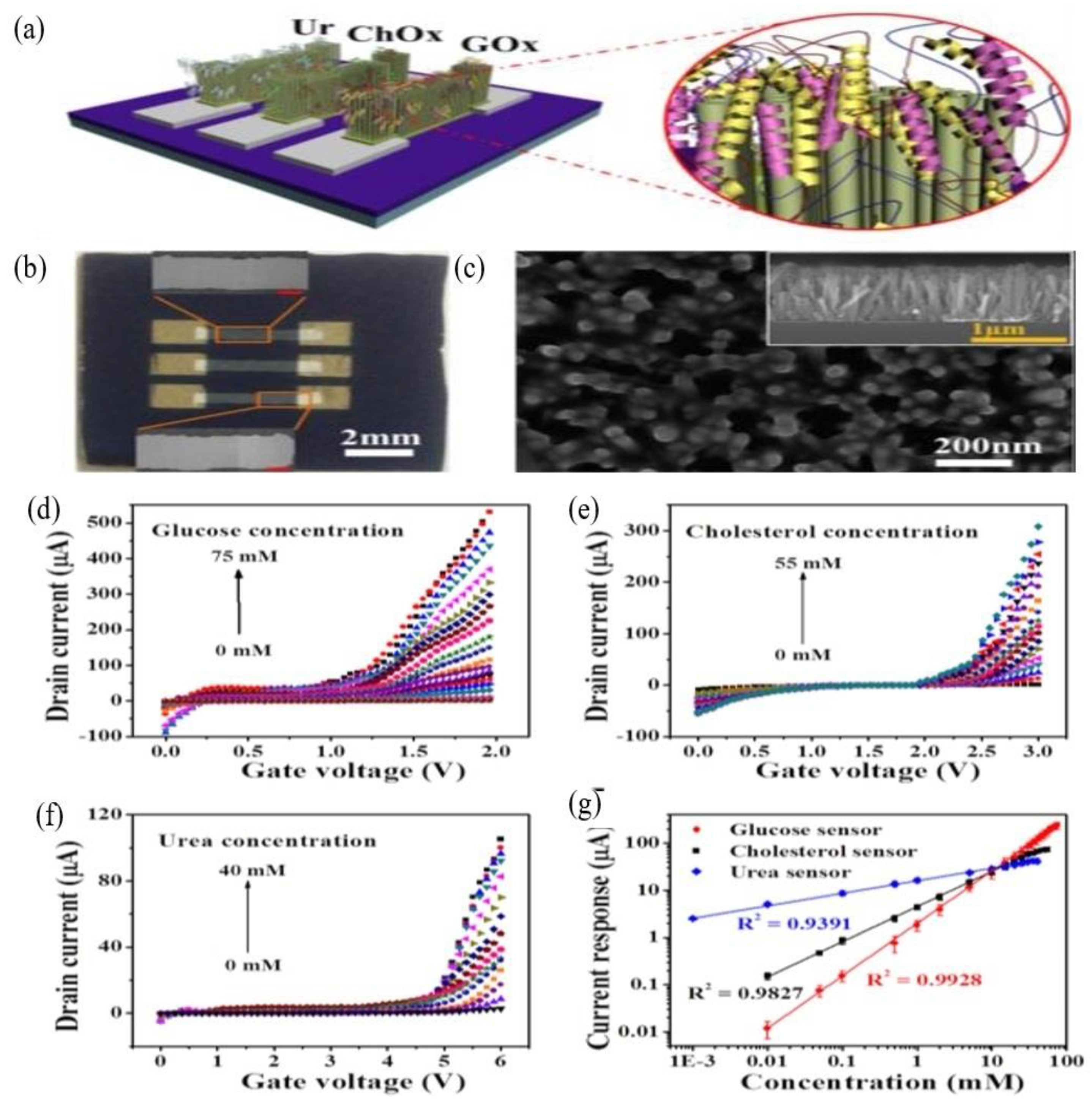
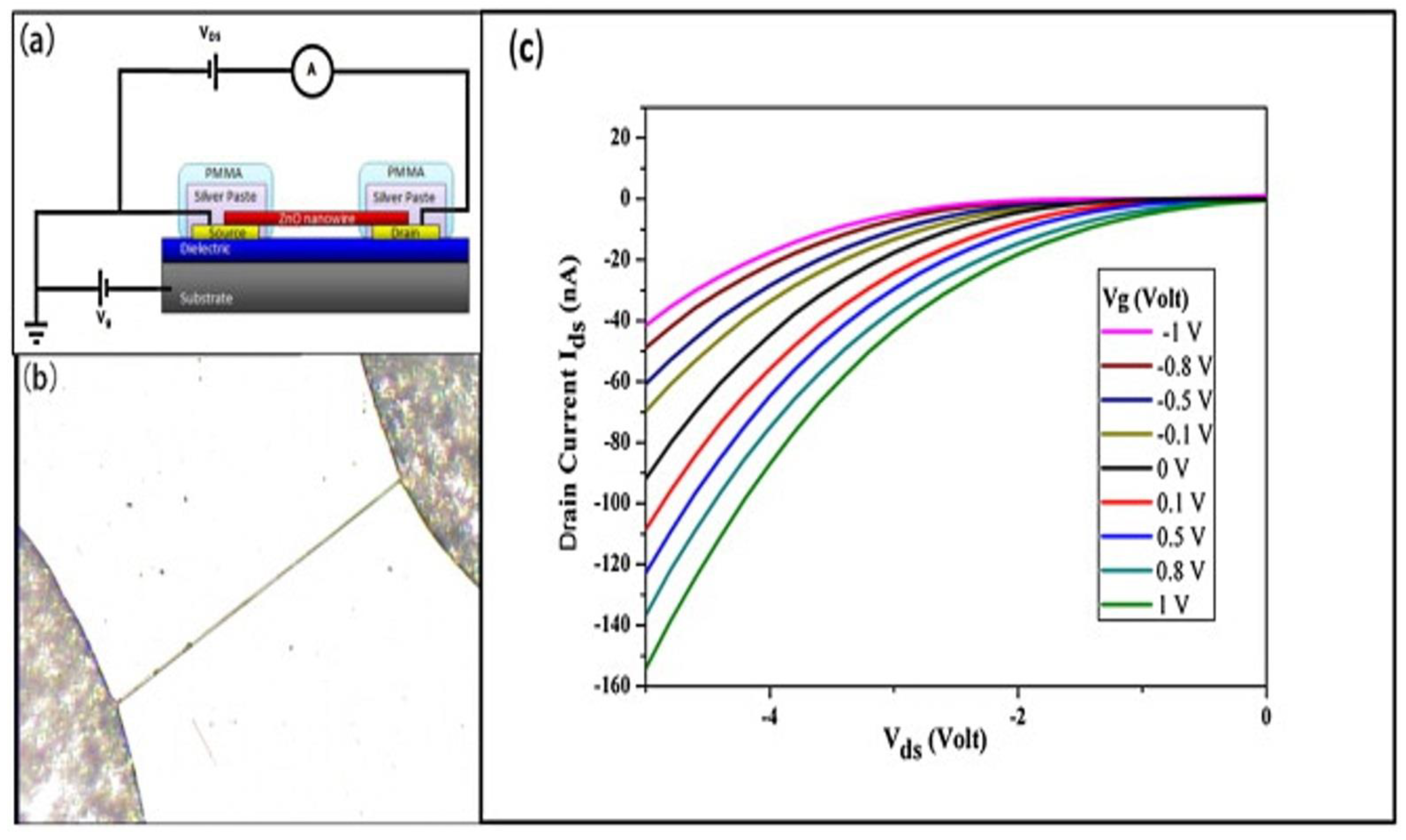
2.2. One-Dimensional ZnO Nanostructures (1-D ZnO)
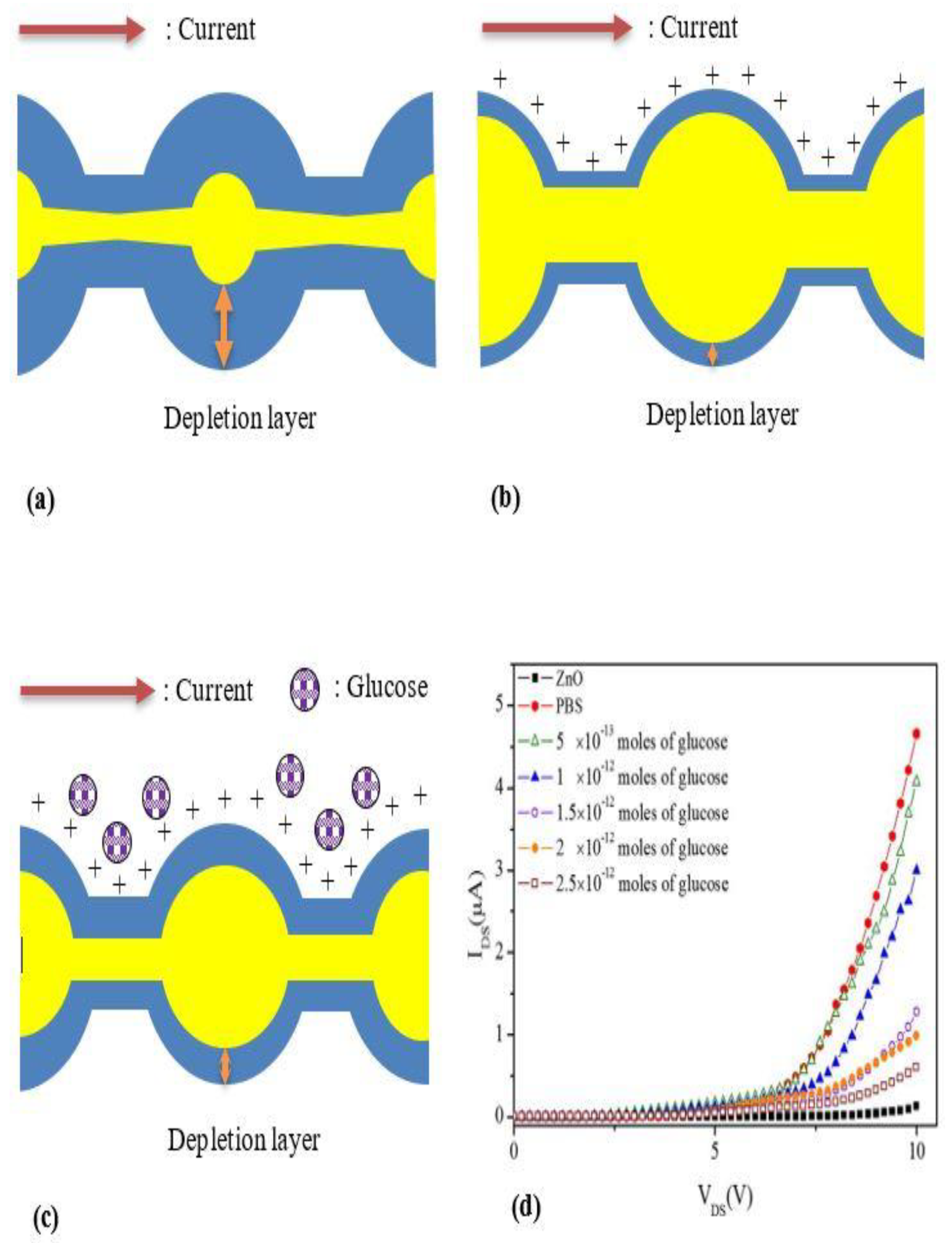
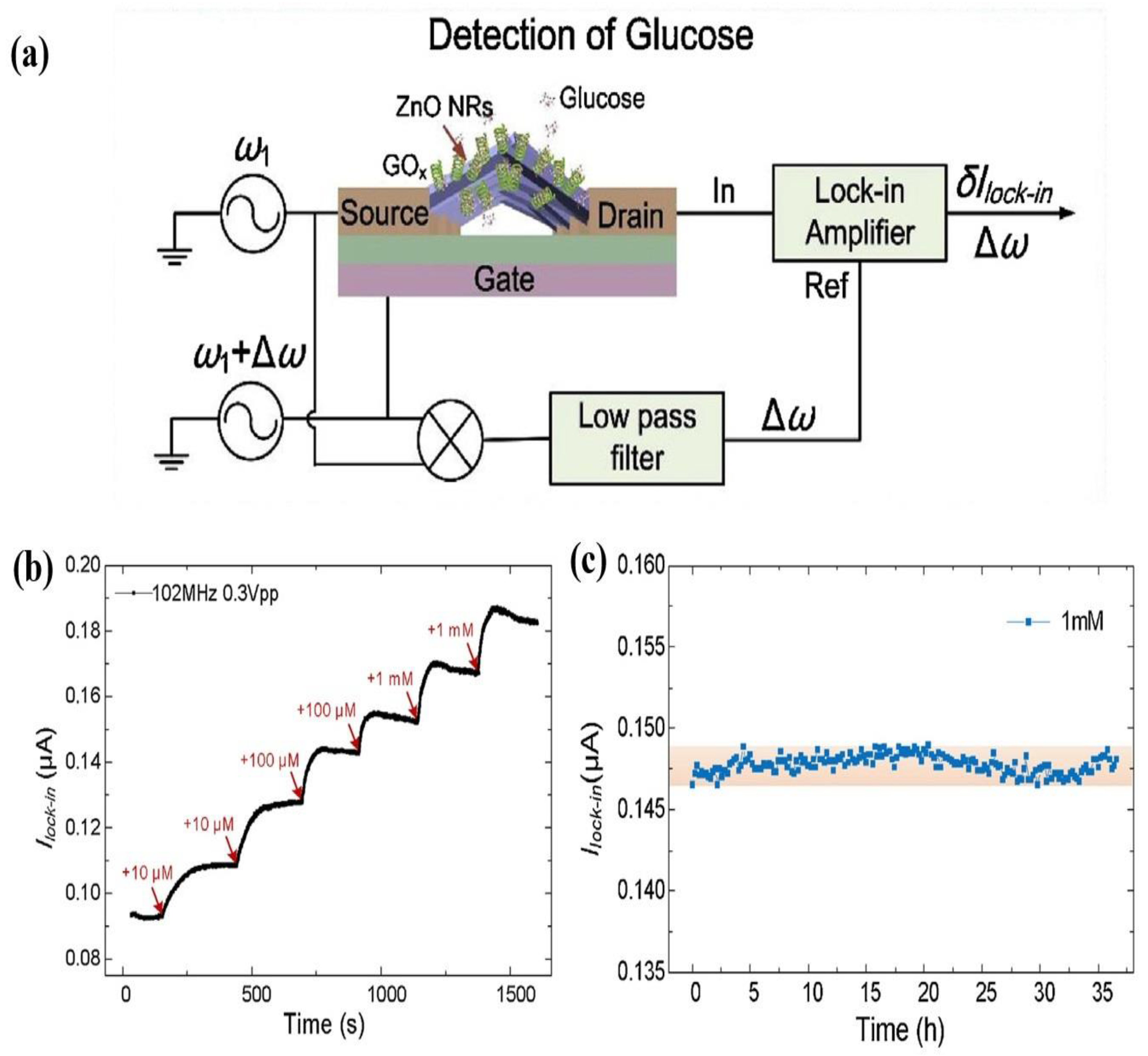
1-D ZnO-Based Field Effect Transistor (FET) Biosensors
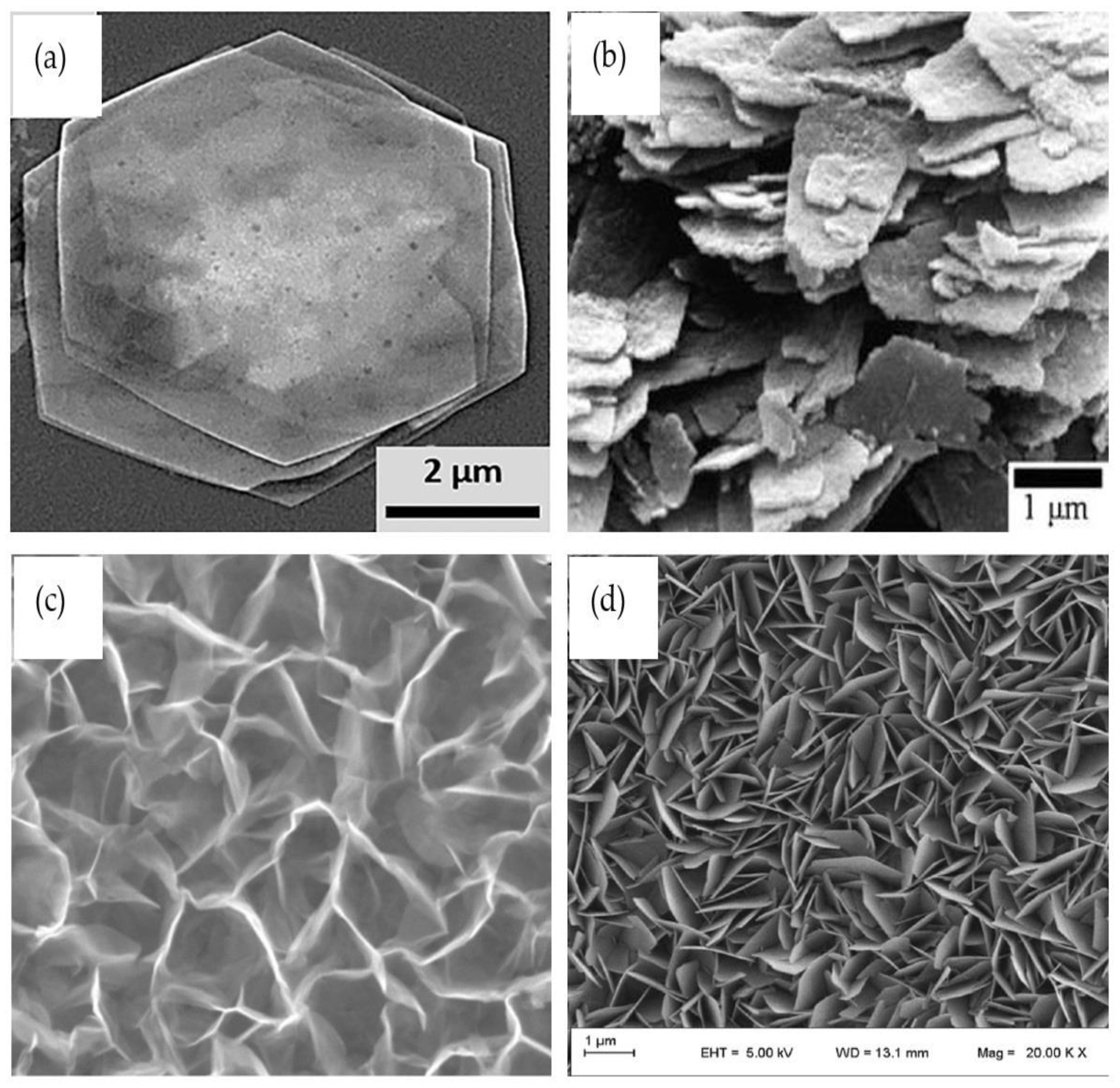
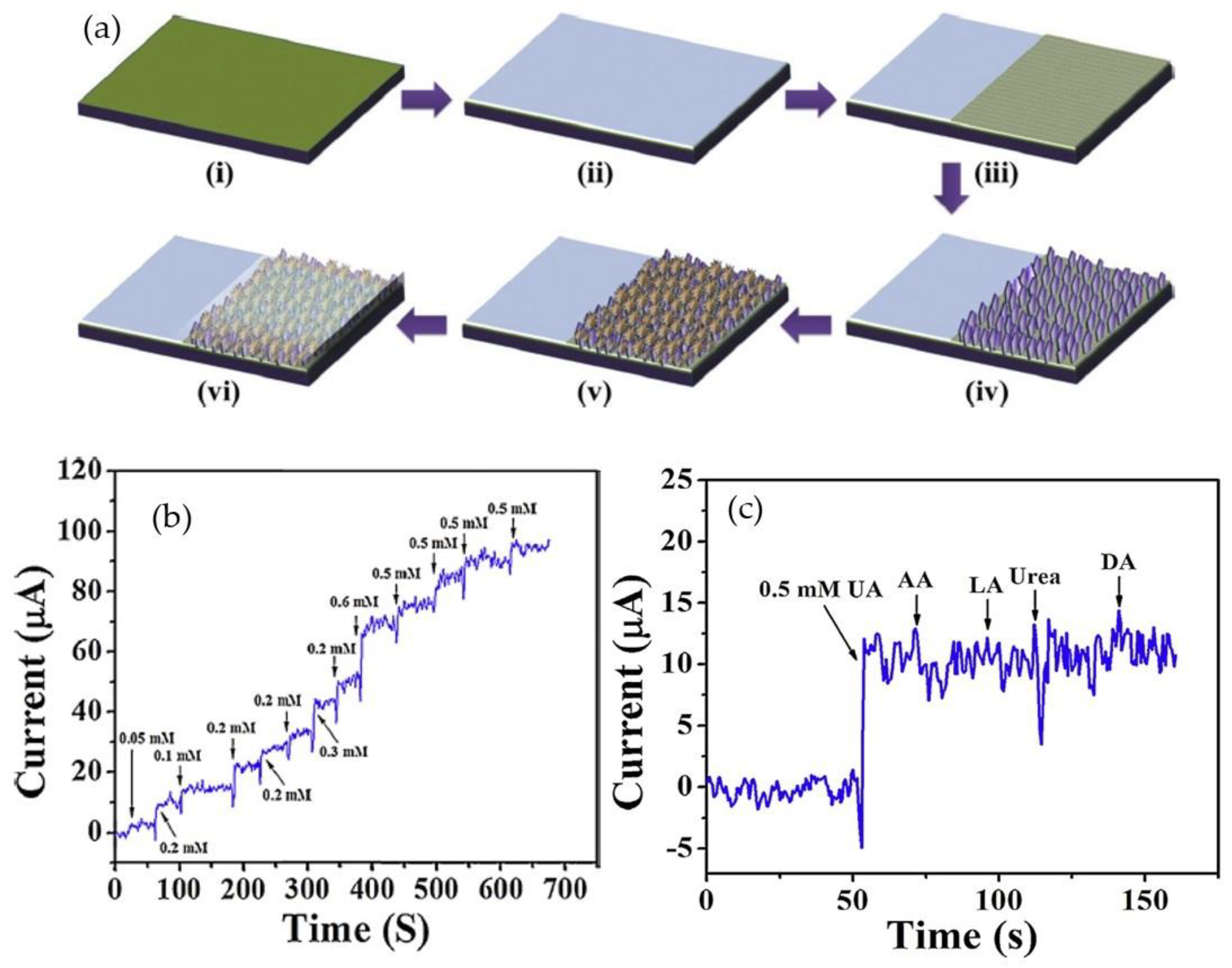
2.3. Two-Dimensional ZnO Nanostructures (2-D ZnO)
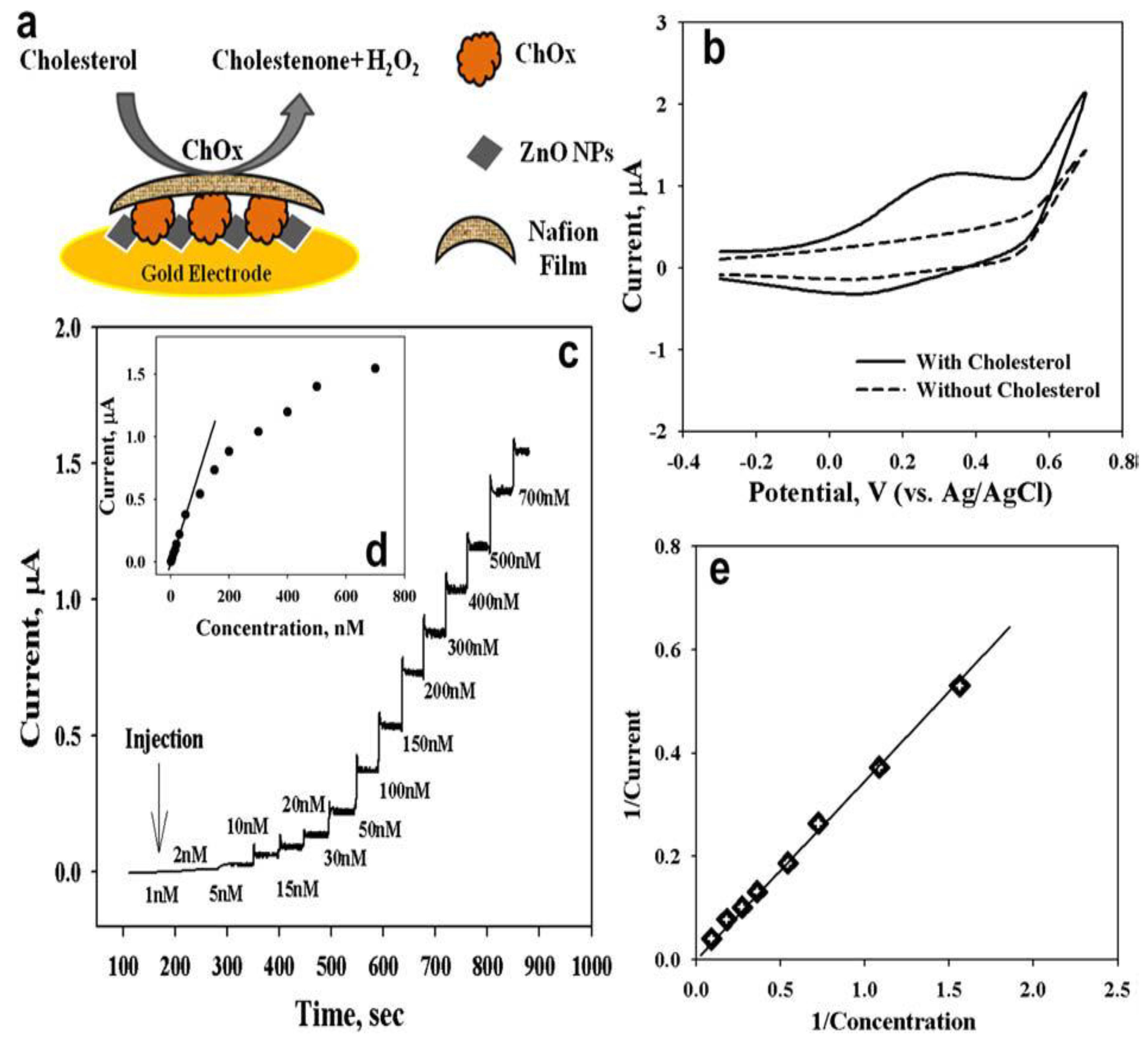
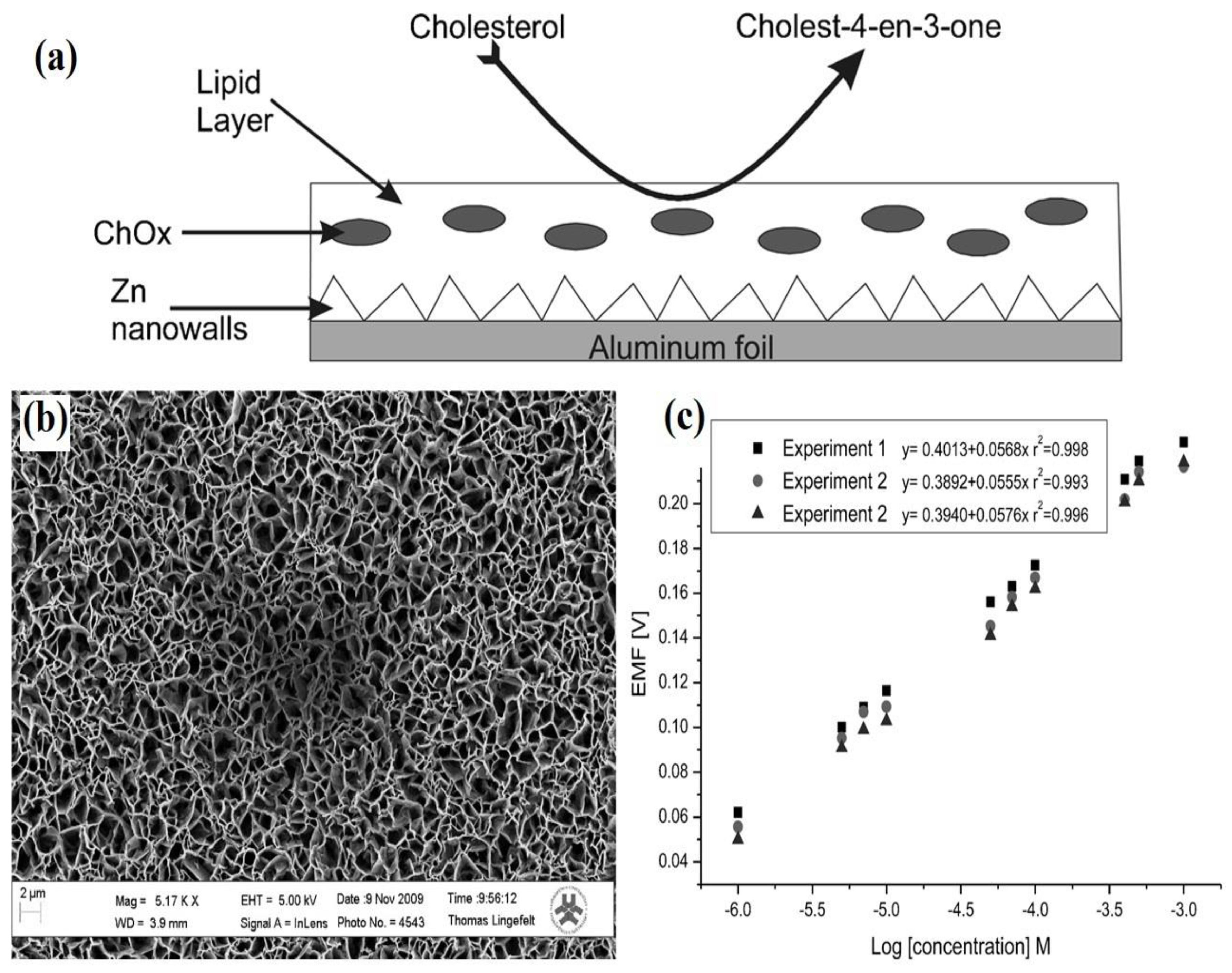
2.4. Three-Dimensional ZnO Nanostructures (3-D ZnO)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhat, S.S.; Qurashi, A.; Khanday, F.A. ZnO nanostructures based biosensors for cancer and infectious disease applications: Perspectives, prospects and promises. Trends Anal. Chem. 2017, 86, 1–13. [Google Scholar] [CrossRef]
- Radha Shanmugam, N.; Muthukumar, S.; Chaudhry, S.; Anguiano, J.; Prasad, S. Ultrasensitive nanostructure sensor arrays on flexible substrates for multiplexed and simultaneous electrochemical detection of a panel of cardiac biomarkers. Biosens. Bioelectron. 2017, 89, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Izyumskaya, N.; Tahira, A.; Ibupoto, Z.H.; Lewinski, N.; Avrutin, V.; Özgür, Ü.; Topsakal, E.; Willander, M.; Morkoç, H. Review—Electrochemical Biosensors Based on ZnO Nanostructures. ECS J. Solid State Sci. Technol. 2017, 6, Q84–Q100. [Google Scholar] [CrossRef]
- Sang, C.H.; Chou, S.J.; Pan, F.M.; Sheu, J.T. Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An Electrochemical Glucose Sensor Based on Zinc Oxide Nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Lin, J.H.; Hsu, D.X.; Wang, S.H.; Lin, S.Y.; Hsueh, T.J. Enhanced non-enzymatic glucose biosensor of ZnO nanowires via decorated Pt nanoparticles and illuminated with UV/green light emitting diodes. Sens. Actuators B Chem. 2017, 238, 150–159. [Google Scholar] [CrossRef]
- Zhou, F.; Jing, W.; Wu, Q.; Gao, W.; Jiang, Z.; Shi, J.; Cui, Q. Effects of the surface morphologies of ZnO nanotube arrays on the performance of amperometric glucose sensors. Mater. Sci Semicond. Process. 2016, 56, 137–144. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U.; Gopinath, S.C.; Haarindraprasad, R.; Foo, K.; Balakrishnan, S.; Poopalan, P. Spotted Nanoflowers: Gold-seeded Zinc Oxide Nanohybrid for Selective Bio-capture. Sci. Rep. 2015, 5, 12231. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Khalilzadeh, M.A. Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples. Food Anal. Methods 2013, 6, 1639–1647. [Google Scholar] [CrossRef]
- Gasparotto, G.; Costa, J.P.C.; Costa, P.I.; Zaghete, M.A.; Mazon, T. Electrochemical immunosensor based on ZnO nanorods-Au nanoparticles nanohybrids for ovarian cancer antigen CA-125 detection. Mater. Sci. Eng. C 2017, 76, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric Field Effect Transistor and Nanoforce Sensor Based on a Single ZnO Nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Tak, M.; Gupta, V.; Tomar, M. Flower-like ZnO nanostructure based electrochemical DNA biosensor for bacterial meningitis detection. Biosens. Bioelectron. 2014, 59, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17. [Google Scholar] [CrossRef]
- Sadeghi, R.; Karimi-Maleh, H.; Bahari, A.; Taghavi, M. A novel biosensor based on ZnO nanoparticle/1,3-dipropylimidazolium bromide ionic liquid-modified carbon paste electrode for square-wave voltammetric determination of epinephrine. Phys. Chem. Liq. 2013, 51, 704–714. [Google Scholar] [CrossRef]
- Gu, B.; Xu, C.; Yang, C.; Liu, S.; Wang, M. ZnO quantum dot labeled immunosensor for carbohydrate antigen 19–9. Biosens. Bioelectron. 2011, 26, 2720–2723. [Google Scholar] [CrossRef]
- Zhou, F.; Jing, W.; Liu, P.; Han, D.; Jiang, Z.; Wei, Z. Doping Ag in ZnO Nanorods to Improve the Performance of Related Enzymatic Glucose Sensors. Sensors 2017, 17, 2214. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, X.J.; Li, W.T.; Zhou, W.H.; Guo, X.C.; Kang, W.Y.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Tian, Q.W. ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 2018, 176, 573–581. [Google Scholar] [CrossRef]
- Sultan, S.M.; de Planque, M.R.R.; Ashburn, P.; Chong, H.M.H. Effect of Phosphate Buffered Saline Solutions on Top-Down Fabricated ZnO Nanowire Field Effect Transistor. J. Nanomater. 2017, 2017, 7. [Google Scholar] [CrossRef]
- Aydin, C. Synthesis of Pd: ZnO nanofibers and their optical characterization dependent on modified morphological properties. J. Alloy. Compd. 2019, 777, 145–151. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Aslam, N.; Kashif, M.; Cao, S.; Rashad, M.; Zhang, Y.; Zeng, W.; Javed, M.S. Polymer-assisted co-axial multi-layered circular ZnO nanodisks. Mater. Lett. 2015, 152, 260–263. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Church, J.; Jung, S.; Son, Y.; Lee, W.H.; Cho, H.J. ZnO nanoflakes as a template for in-situ electrodeposition of nanostructured cobalt electrodes as amperometric phosphate sensors. Mater. Lett. 2017, 192, 107–110. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Ma, L.; Dong, G. Noble metal-free modified electrode of exfoliated graphitic carbon nitride/ZnO nanosheets for highly efficient hydrogen peroxide sensing. Electrochim. Acta 2017, 247, 787–794. [Google Scholar] [CrossRef]
- Yu, L.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.; Qi, L.; Fan, X. Both oxygen vacancies defects and porosity facilitated NO2 gas sensing response in 2D ZnO nanowalls at room temperature. J. Alloy. Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, H.; Huangfu, C.; Lu, Z.; Wang, X.; Zeng, X.; He, H.; Rao, H. Research of protein adsorption on the different surface topography of the zinc oxide. Surf. Interface Anal. 2015, 47, 245–252. [Google Scholar] [CrossRef]
- Hahm, J.I. Fundamental properties of one-dimensional zinc oxide nanomaterials and implementations in various detection modes of enhanced biosensing. Annu. Rev. Phys. Chem. 2016, 67, 691–717. [Google Scholar] [CrossRef]
- Kim, K.E.; Kim, T.G.; Sung, Y.M. Enzyme-conjugated ZnO nanocrystals for collisional quenching-based glucose sensing. CrystEngComm 2012, 14, 2859–2865. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Khan, R.; Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Pandey, M.K.; Malhotra, B.D. Zinc oxide nanoparticles-chitosan composite film for cholesterol biosensor. Anal. Chim. Acta 2008, 616, 207–213. [Google Scholar] [CrossRef]
- Aljawfi, R.N.; Rahman, F.; Batoo, D.K.M. Effect of grain size and grain boundary defects on electrical and magnetic properties of Cr doped ZnO nanoparticles. J. Mol. Struct. 2014, 1065, 199–204. [Google Scholar] [CrossRef]
- Li, Q.; Kumar, V.; Li, Y.; Zhang, H.; Marks, T.J.; Chang, R.P.H. Fabrication of ZnO Nanorods and Nanotubes in Aqueous Solutions. Chem. Mater. 2005, 17, 1001–1006. [Google Scholar] [CrossRef]
- Choi, A.; Kim, K.; Jung, H.I.; Lee, S.Y. ZnO nanowire biosensors for detection of biomolecular interactions in enhancement mode. Sens. Actuators B Chem. 2010, 148, 577–582. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Kaushik, A.; Pokhrel, N.; Bhansali, S.; Pala, N. Electrochemical cortisol immunosensors based on sonochemically synthesized zinc oxide 1D nanorods and 2D nanoflakes. Biosens. Bioelectron. 2015, 63, 124–130. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, L.; Hu, Q.; Huang, F.; Lin, Z. ZnO nanoflower-based photoelectrochemical DNA zyme sensor for the detection of Pb2+. Biosens. Bioelectron. 2014, 56, 243–249. [Google Scholar] [CrossRef]
- Guo, W. One-pot synthesis of urchin-like ZnO nanostructure and its enhanced acetone gas sensing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 963–972. [Google Scholar] [CrossRef]
- Yazdi, M.A.P.; Martin, N.; Monsifrot, E.; Briois, P.; Billard, A. ZnO nano-tree active layer as heavy hydrocarbon sensor: From material synthesis to electrical and gas sensing properties. Thin Solid Films 2015, 596, 128–134. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell. Nano Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef]
- Breedon, M.; Rahmani, M.B.; Keshmiri, S.H.; Wlodarski, W.; Kalantar-zadeh, K. Aqueous synthesis of interconnected ZnO nanowires using spray pyrolysis deposited seed layers. Mater. Lett. 2010, 64, 291–294. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 13001. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.G.; Liu, F.; Navaraj, W.T.; Christou, A.; Shakthivel, D.; Dahiya, R. Heterogeneous integration of contact-printed semiconductor nanowires for high-performance devices on large areas. Microsyst. Nanoeng. 2018, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, J.; Kumar, G.S.; Venkatesh, M.; Maadeswaran, P.; Girija, E.K. Morphology and size controlled synthesis of zinc oxide nanostructures and their optical properties. J. Mater. Sci. Mater. Electron. 2018, 29, 9339–9346. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, L.; Zhang, H.; Sun, Z.; Zhang, Z.; Zhang, G.J. Fabrication of Ultrasensitive Field-Effect Transistor DNA Biosensors by a Directional Transfer Technique Based on CVD-Grown Graphene. ACS Appl. Mater. Interfaces 2015, 7, 16953–16959. [Google Scholar] [CrossRef]
- Arya, S.K.; Wong, C.C.; Jeon, Y.J.; Bansal, T.; Park, M.K. Advances in Complementary-Metal–Oxide–Semiconductor-Based Integrated Biosensor Arrays. Chem. Rev. 2015, 115, 5116–5158. [Google Scholar] [CrossRef]
- Han, D.; Chand, R.; Kim, Y.S. Microscale loop-mediated isothermal amplification of viral DNA with real-time monitoring on solution-gated graphene FET microchip. Biosens. Bioelectron. 2017, 93, 220–225. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Ahmad, R.; Mahmoudi, T.; Ahn, M.S.; Hahn, Y.B. Recent advances in nanowires-based field-effect transistors for biological sensor applications. Biosens. Bioelectron. 2018, 100, 312–325. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H. Hierarchical structures of ZnO spherical particles synthesized solvothermally. Sci. Technol. Adv. Mater. 2012, 12, 64707. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Rajendar, V.; Park, S.H. Novel synthesis and structural analysis of zinc oxide nanoparticles for the non enzymatic glucose biosensor. Mater. Sci. Eng. C 2017, 75, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Saha, S.; Ramirez, V.J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Das, P.; Mondal, B.; Mukherjee, K. Effects of doping, morphology and film-thickness of photo-anode materials for dye sensitized solar cell application–A review. Renew. Sustain. Energy Rev. 2016, 60, 356–376. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Meng, X.; Tang, F.; Hou, X.; Han, D.; Zhang, L. Zinc oxide nanoparticles/glucose oxidase photoelectrochemical system for the fabrication of biosensor. J. Colloid Interface Sci. 2009, 334, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Fidal, V.; Inguva, S.; Krishnamurthy, S.; Marsili, E.; Mosnier, J.P.; Chandra, T. Mediator-free interaction of glucose oxidase, as model enzyme for immobilization, with Al-doped and undoped ZnO thin films laser-deposited on polycarbonate supports. Enzym. Microb. Technol. 2017, 96, 67–74. [Google Scholar]
- Wang, Y.T.; Yu, L.; Zhu, Z.Q.; Zhang, J.; Zhu, J.Z.; Fan, C.H. Improved enzyme immobilization for enhanced bioelectrocatalytic activity of glucose sensor. Sens. Actuators B Chem. 2009, 136, 332–337. [Google Scholar] [CrossRef]
- Aini, B.N.; Siddiquee, S.; Ampon, K.; Rodrigues, K.F.; Suryani, S. Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane. Sens. Biosens. Res. 2015, 4, 46–56. [Google Scholar] [CrossRef]
- Yatsui, T.; Jeong, H.; Ohtsu, M. Controlling the energy transfer between near-field optically coupled ZnO quantum dots. Appl. Phys. A 2008, 93, 199–202. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X. Enhanced fluorescence imaging performance of hydrophobic colloidal ZnO nanoparticles by a facile method. J. Alloy. Compd. 2015, 619, 98–101. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci 2010, 5, 1246–1301. [Google Scholar]
- Chung, R.J.; Wang, A.N.; Liao, Q.L.; Chuang, K.Y. Non-enzymatic glucose sensor composed of carbon-coated nano-zinc oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, K.; Kim, S. Enzyme-free glucose sensors with channels composed of necked ZnO nanoparticles on plastic. Microelectron. Eng. 2011, 88, 2611–2613. [Google Scholar] [CrossRef]
- Mahmoud, A.; Echabaane, M.; Omri, K.; El Mir, L.; Chaabane, R.B. Development of an impedimetric non enzymatic sensor based on ZnO and Cu doped ZnO nanoparticles for the detection of glucose. J. Alloy. Compd. 2019, 786, 960–968. [Google Scholar] [CrossRef]
- Watanabe, E.; Spidle, R.; Caudle, S.; Manani, G.; Wanekaya, A.K.; Mugweru, A. Electrochemical method for analysis of cholesterol based on in situ synthesized graphene decorated with zinc oxide nanoparticles. ECS Solid State Lett. 2014, 3, M5–M9. [Google Scholar] [CrossRef]
- Nayak, P.; Anbarasan, B.; Ramaprabhu, S. Fabrication of Organophosphorus Biosensor Using ZnO Nanoparticle-Decorated Carbon Nanotube–Graphene Hybrid Composite Prepared by a Novel Green Technique. J. Phys. Chem. C 2013, 117, 13202–13209. [Google Scholar] [CrossRef]
- Hayat, A.; Haider, W.; Raza, Y.; Marty, J.L. Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 2015, 143, 157–161. [Google Scholar] [CrossRef]
- Umar, A.; Rahman, M.M.; Vaseem, M.; Hahn, Y.B. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochem. Commun. 2009, 11, 118–121. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.M.; Kwak, C.H.; Hwang, S.K.; Huh, Y.S.; Han, Y.K. Immobilization of hemoglobin on functionalized multi-walled carbon nanotubes-poly-l-histidine-zinc oxide nanocomposites toward the detection of bromate and H2O2. Sens. Actuators B Chem. 2016, 224, 607–617. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.C.; Ma, L.X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Rahmanian, R.; Mozaffari, S.A. Electrochemical fabrication of ZnO-polyvinyl alcohol nanostructured hybrid film for application to urea biosensor. Sens. Actuators B Chem. 2015, 207, 772–781. [Google Scholar] [CrossRef]
- Norouzi, P.; Gupta, V.K.; Faridbod, F.; Pirali-Hamedani, M.; Larijani, B.; Ganjali, M.R. Carcinoembryonic Antigen Admittance Biosensor Based on Au and ZnO Nanoparticles Using FFT Admittance Voltammetry. Anal. Chem. 2011, 83, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tan, X.; Zhou, Q.; Liu, Y.; Wang, M.; Yang, L. Synthesis of highly dispersed zinc oxide nanoparticles on carboxylic graphene for development a sensitive acetylcholinesterase biosensor. Sens. Actuators B Chem. 2014, 190, 730–736. [Google Scholar] [CrossRef]
- Haghighi, B.; Bozorgzadeh, S. Fabrication of a highly sensitive electrochemiluminescence lactate biosensor using ZnO nanoparticles decorated multiwalled carbon nanotubes. Talanta 2011, 85, 2189–2193. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, R.; Chai, Y.; Niu, H.; Cao, Y.; Liu, H.; Bai, L.; Yuan, Y. Highly sensitive luminol electrochemiluminescence immunosensor based on ZnO nanoparticles and glucose oxidase decorated graphene for cancer biomarker detection. Anal. Chim. Acta 2012, 745, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Muthuchamy, N.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J. Electroanal. Chem. 2018, 816, 195–204. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene–carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Kafrashi, F.; Madrakian, T. Electrochemical determination of levodopa in the presence of ascorbic acid by polyglycine/ZnO nanoparticles/multi-walled carbon nanotubes-modified carbon paste electrode. Ionics 2015, 21, 2937–2947. [Google Scholar] [CrossRef]
- Singhal, C.; Pundir, C.S.; Narang, J. A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Batra, N.; Sharma, A.; Tomar, M.; Gupta, V. Efficient detection of total cholesterol using (ChEt–ChOx/ZnO/Pt/Si) bioelectrode based on ZnO matrix. Thin Solid Films 2014, 562, 612–620. [Google Scholar] [CrossRef]
- Ghaedi, H.; Afkhami, A.; Madrakian, T.; Soltani-Felehgari, F. Construction of novel sensitive electrochemical sensor for electro-oxidation and determination of citalopram based on zinc oxide nanoparticles and multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 59, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Pilehvar, S.; De Wael, K. A graphene oxide amplification platform tagged with tyrosinase–zinc oxide quantum dot hybrids for the electrochemical sensing of hydroxylated polychlorobiphenyls. Sens. Actuators B Chem. 2014, 190, 612–620. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M.; Li, Y.; Fan, S.; Ding, L.; Liang, J.; Chen, S. Synthesis of reduced graphene oxide intercalated ZnO quantum dots nanoballs for selective biosensing detection. Appl. Surf. Sci. 2016, 376, 133–137. [Google Scholar] [CrossRef]
- Pabbi, M.; Mittal, S.K. An electrochemical algal biosensor based on silica coated ZnO quantum dots for selective determination of acephate. Anal. Methods 2017, 9, 1672–1680. [Google Scholar] [CrossRef]
- Ali, M.; Shah, I.; Kim, S.W.; Sajid, M.; Lim, J.H.; Choi, K.H. Quantitative detection of uric acid through ZnO quantum dots based highly sensitive electrochemical biosensor. Sens. Actuators A Phys. 2018, 283, 282–290. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; Ahmad, M.K. Effect of post annealing treatment on electrical and structural properties of zinc oxide nanostructures Nanotech Malaysia Universiti Teknologi Malaysia, Malaysia. Mater. Today Proc. 2018, 7, 710–714. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, X.L.; Liu, W.W.; Zhong, X.H.; Wang, J.N.; Pyrah, L.; Sanderson, K.D.; Ramsey, P.M.; Hirata, M.; Tsuri, K. Influence of preferred orientation on the electrical conductivity of fluorine-doped tin oxide films. Sci. Rep. 2014, 4, 3679. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Cui, H.; Wang, X.; Xie, X.; Wang, M.; Zhang, L.; Tian, J. Hierarchical assembly of In2O3 nanoparticles on ZnO hollow nanotubes using carbon fibers as templates: Enhanced photocatalytic and gas-sensing properties. J.Colloid Interface Sci. 2017, 498, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jo, S.Y.; Sun, G.J.; Katoch, A.; Choi, S.W.; Kim, S.S. Tailoring the surface area of ZnO nanorods for improved performance in glucose sensors. Sens. Actuators B Chem. 2014, 192, 216–220. [Google Scholar] [CrossRef]
- Demes, T.; Ternon, C.; Morisot, F.; Riassetto, D.; Legallais, M.; Roussel, H.; Langlet, M. Mechanisms involved in the hydrothermal growth of ultra-thin and high aspect ratio ZnO nanowires. Appl. Surf. Sci. 2017, 410, 423–431. [Google Scholar] [CrossRef]
- Shukla, M.; Dixit, T.; Prakash, R.; Palani, I.; Singh, V. Influence of aspect ratio and surface defect density on hydrothermally grown ZnO nanorods towards amperometric glucose biosensing applications. Appl. Surf. Sci. 2017, 422, 798–808. [Google Scholar] [CrossRef]
- Ekanayake, E.M.; Preethichandra, D.M.; Kaneto, K. Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens. Bioelectron. 2007, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Kim, S.H.; Umar, A.; Al-Hajry, A.; Hahn, Y.B. High performance cholesterol sensor based on ZnO nanotubes grown on Si/Ag electrodes. Electrochem. Commun. 2014, 38, 4–7. [Google Scholar] [CrossRef]
- Brince Paul, K.; Kumar, S.; Tripathy, S.; Vanjari, S.R.K.; Singh, V.; Singh, S.G. A highly sensitive self assembled monolayer modified copper doped zinc oxide nanofiber interface for detection of Plasmodium falciparum histidine-rich protein-2: Targeted towards rapid, early diagnosis of malaria. Biosens. Bioelectron. 2016, 80, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Park, J.H.; Hahn, Y.B. A comprehensive biosensor integrated with a ZnO nanorod FET array for selective detection of glucose, cholesterol and urea. Chem. Commun. 2015, 51, 11968–11971. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Liao, Q.; Liu, H.; Huang, Y.; Song, Y.; Zhao, Y.; Zhang, Y. Enzymatic lactic acid sensing by In-doped ZnO nanowires functionalized AlGaAs/GaAs high electron mobility transistor. Sens. Actuators B Chem. 2015, 212, 41–46. [Google Scholar] [CrossRef]
- Lee, C.T.; Chiu, Y.S. Photoelectrochemical passivated ZnO-based nanorod structured glucose biosensors using gate-recessed AlGaN/GaN ion-sensitive field-effect-transistors. Sens. Actuators B Chem. 2015, 210, 756–761. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; To, S.; You, L.; Sun, Y. Effect of Nanowire Number, Diameter, and Doping Density on Nano-FET Biosensor Sensitivity. ACS Nano 2011, 5, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, P.; Yan, X.; Kang, Z.; Zhao, Y.; Lei, Y.; Li, C.; Du, H.; Zhang, Y. Enzyme-coated single ZnO nanowire FET biosensor for detection of uric acid. Sens. Actuators B Chem. 2013, 176, 22–27. [Google Scholar] [CrossRef]
- Zong, X.; Zhu, R. ZnO nanorod-based FET biosensor for continuous glucose monitoring. Sens Actuators B Chem. 2018, 255, 2448–2453. [Google Scholar] [CrossRef]
- Wei, A.; Sun, X.W.; Wang, J.; Lei, Y.; Cai, X.; Li, C.M.; Dong, Z.; Huang, W. Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl. Phys. Lett. 2006, 89, 123902. [Google Scholar] [CrossRef]
- Anh, T.T.N.; Lan, H.; Tam, L.T.; Pham, V.H.; Tam, P.D. Highly Sensitive Nonenzymatic Cholesterol Sensor Based on Zinc Oxide Nanorods. J. Electron. Mater. 2018, 47, 6701–6708. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Cui, F.; Qiu, Q.; Chen, X.; Xu, J. Au nanoparticles-ZnO composite nanotubes using natural silk fibroin fiber as template for electrochemical non-enzymatic sensing of hydrogen peroxide. Anal. Biochem. 2018, 554, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Li, K.; Gu, S.; Zhang, L.; Lu, J. Ni foam-supported ZnO nanowires and Co3O4/NiCo2O4 double-shelled nanocages for efficient hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 262, 828–836. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, X.; Kang, Z.; Fang, X.; Zheng, X.; Zhao, L.; Du, H.; Zhang, Y. Zinc oxide nanowires-based electrochemical biosensor for L-lactic acid amperometric detection. J. Nanopart. Res. 2014, 16, 2398. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Huang, S.; Lin, X.; Gao, X.P.A.; Wang, B.; Yao, L.; Wang, W.; Guo, E. Synthesis of graphene/ZnO nanowire arrays/graphene foam and its application for determination of folic acid. J. Electroanal. Chem. 2018, 808, 189–194. [Google Scholar] [CrossRef]
- Yue, H.Y.; Zhang, H.; Huang, S.; Lin, X.Y.; Gao, X.; Chang, J.; Yao, L.H.; Guo, E.J. Synthesis of ZnO nanowire arrays/3D graphene foam and application for determination of levodopa in the presence of uric acid. Biosens. Bioelectron. 2017, 89, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Deng, B.; Zhang, Z. An electrochemical sensor based on reduced graphene oxide and ZnO nanorods-modified glassy carbon electrode for uric acid detection. Arab. J. Sci. Eng. 2016, 41, 135–141. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Hahn, Y.B. Solution Process Synthesis of High Aspect Ratio ZnO Nanorods on Electrode Surface for Sensitive Electrochemical Detection of Uric Acid. Sci. Rep. 2017, 7, 46475. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Hahn, Y.B. Highly stable urea sensor based on ZnO nanorods directly grown on Ag/glass electrodes. Sensor. Actuators. B Chem. 2014, 194, 290–295. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.Y.; Kwon, D.W.; Yang, H.Y.; Hahn, Y.B. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; He, Q.; Huang, L.; Li, S.; Wang, F. A Nonenzymatic Glucose Sensor Based on a Copper Nanoparticle–Zinc Oxide Nanorod Array. Anal. Lett. 2014, 47, 1147–1161. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.S.; Hahn, Y.B. Fabrication of a non-enzymatic glucose sensor field-effect transistor based on vertically-oriented ZnO nanorods modified with Fe2O3. Electrochem. Commun. 2017, 77, 107–111. [Google Scholar] [CrossRef]
- Jung, D.U.J.; Ahmad, R.; Hahn, Y.B. Nonenzymatic flexible field-effect transistor based glucose sensor fabricated using NiO quantum dots modified ZnO nanorods. J. Colloid Interface Sci. 2018, 512, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Ghosh, S.; Das, N.; RoyChaudhuri, C. Liquid gated ZnO nanorod FET sensor for ultrasensitive detection of Hepatitis B surface antigen with vertical electrode configuration. Biosens. Bioelectron. 2018, 122, 58–67. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Alshammari, A.S.; Alzanki, T.H.; Jarowski, P.; Henley, S.J.; Silva, S.R.P. ZnO nanodisk based UV detectors with printed electrodes. Langmuir 2014, 30, 3913–3921. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Wang, J.; Pan, M.; Yang, W.; Yang, J.; Jiang, W. Synthesis and photocatalytic performance of ZnO hollow spheres and porous nanosheets. J. Mater. Sci. Mater. Electron. 2016, 27, 203–209. [Google Scholar] [CrossRef]
- Psychoyios, V.N.; Nikoleli, G.P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Israr, M.Q.; Willander, M. Potentiometric cholesterol biosensor based on ZnO nanowalls and stabilized polymerized lipid film. Electroanal. 2013, 25, 367–372. [Google Scholar] [CrossRef]
- Du, J.; Wang, H.; Zhang, R.; Wang, X.; Du, X.; Lu, X. Oriented ZnO nanoflakes on nickel-titanium alloy fibers for solid-phase microextraction of polychlorinated biphenyls and polycyclic aromatic hydrocarbons. Microchim. Acta 2018, 185, 441. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.U.; Ibupoto, Z.H.; Kashif, M.; Hashim, U.; Willander, M. A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase. Sensors 2012, 12, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Fulati, A.; Ali, S.M.U.; Asif, M.H.; Willander, M.; Brännmark, C.; Strålfors, P.; Börjesson, S.I.; Elinder, F.; Danielsson, B. An intracellular glucose biosensor based on nanoflake ZnO. Sens. Actuat. B Chem. 2010, 150, 673–680. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Rui, Q.; Komori, K.; Tian, Y.; Liu, H.; Luo, Y.; Sakai, Y. Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets. Anal. Chim. Acta 2010, 670, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, M.; Kong, Q.; Luo, X.; Jiao, K. Toward DNA electrochemical sensing by free-standing ZnO nanosheets grown on 2D thin-layered MoS2. Biosens. Bioelectron. 2017, 89, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, W.; Nan, H.; Wang, G. Ultrathin Zinc Oxide Nanofilm on Zinc Substrate for High Performance Electrochemical Sensors. Electrochim. Acta 2014, 144, 186–193. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Rahmanian, R.; Abedi, M.; Amoli, H.S. Urea impedimetric biosensor based on reactive RF magnetron sputtered zinc oxide nanoporous transducer. Electrochim. Acta 2014, 146, 538–547. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1D and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; No, Y.S.; Kim, S.; Park, H.G.; Park, W.I. Strong interactive growth behaviours in solution-phase synthesis of three-dimensional metal oxide nanostructures. Nat. Commun. 2015, 6, 6325. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Park, S.; Son, M.K.; Kim, H.J. Ammonia treated ZnO nanoflowers based CdS/CdSe quantum dot sensitized solar cell. Electrochim. Acta 2015, 151, 531–536. [Google Scholar] [CrossRef]
- Yue, H.Y.; Wang, B.; Huang, S.; Gao, X.; Song, S.S.; Guan, E.H.; Zhang, H.J.; Wu, P.F.; Guo, X.R. Synthesis of graphene/ZnO nanoflowers and electrochemical determination of levodopa in the presence of uric acid. J. Mater. Sci. Mater. Electron. 2018, 29, 14918–14926. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Mani, G.K.; Elayaraja, R.; Durgadevi, E.; Navaneethan, M.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Hayakawa, Y. ZnO hierarchical 3D-flower like architectures and their gas sensing properties at room temperature. Appl. Surf. Sci. 2018, 449, 314–321. [Google Scholar] [CrossRef]
- Rani, B.J.; Anusiya, A.; Praveenkumar, M.; Ravichandran, S.; Guduru, R.K.; Ravi, G.; Yuvakkumar, R. Ag implanted ZnO hierarchical nanoflowers for photoelectrochemical water-splitting applications. J. Mater. Sci. Mater. Electron. 2018, 30, 1–15. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, K.; Chen, X.; Zhao, F.; Xie, T.; Wang, D.; Lin, Y. Porous Ce-doped ZnO hollow sphere with enhanced photodegradation activity for artificial waste water. J. Alloy. Compd. 2017, 699, 907–913. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X. Functional semiconductor nanowires via vapor deposition. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2011, 29, 60801. [Google Scholar] [CrossRef][Green Version]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.S.; Khang, G.; Hahn, Y.B. Hierarchically assembled ZnO nanosheets microspheres for enhanced glucose sensing performances. Ceram. Int. 2016, 42, 13464–13469. [Google Scholar] [CrossRef]
- Chu, X.; Zhu, X.; Dong, Y.; Chen, T.; Ye, M.; Sun, W. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the platinum electrode modified with NiO doped ZnO nanorods. J. Electroanal. Chem. 2012, 676, 20–26. [Google Scholar] [CrossRef]
- Ahmad, M.; Pan, C.; Luo, Z.; Zhu, J. A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor. J. Phys. Chem. C 2010, 114, 9308–9313. [Google Scholar] [CrossRef]
- Fang, L.; Liu, B.; Liu, L.; Li, Y.; Huang, K.; Zhang, Q. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sens. Actuators B Chem. 2016, 222, 1096–1102. [Google Scholar] [CrossRef]
- Fang, L.; Huang, K.; Zhang, B.; Liu, B.; Liu, Y.; Zhang, Q. Nanosheet-based 3D hierarchical ZnO structure decorated with Au nanoparticles for enhanced electrochemical detection of dopamine. RSC Adv. 2014, 4, 48986–48993. [Google Scholar] [CrossRef]
- Hussain, M.; Sun, H.; Karim, S.; Nisar, A.; Khan, M.; Ul Haq, A.; Iqbal, M.; Ahmad, M. Noble metal nanoparticle-functionalized ZnO nanoflowers for photocatalytic degradation of RhB dye and electrochemical sensing of hydrogen peroxide. J. Nanopart. Res. 2016, 18, 95. [Google Scholar] [CrossRef]
- Yue, H.Y.; Wang, B.; Huang, S.; Gao, X.; Lin, X.Y.; Yao, L.H.; Guan, E.H.; Zhang, H.J.; Song, S.S. Determination of levodopa in the presence of uric acid using a ZnO nanoflower-modified indium tin oxide glass electrode. Ionics 2017, 23, 3479–3486. [Google Scholar] [CrossRef]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid growth of zinc oxide nanotube–nanowire hybrid architectures and their use in breast cancer-related volatile organics detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, S.; Zhu, T.; Sun, W.; Li, Q.; Wei, G.; Su, Z. Hydrothermal synthesis of zinc oxide-reduced graphene oxide nanocomposites for an electrochemical hydrazine sensor. RSC Adv. 2015, 5, 22935–22942. [Google Scholar] [CrossRef]
- Yue, H.Y.; Song, S.S.; Guo, X.R.; Huang, S.; Gao, X.; Wang, Z.; Wang, W.Q.; Zhang, H.J.; Wu, P.F. Three-dimensional ZnO nanosheet spheres/graphene foam for electrochemical determination of levodopa in the presence of uric acid. J. Electroanal. Chem. 2019, 838, 142–147. [Google Scholar] [CrossRef]

| Morphology | Technique | Bio-Receptor | Doping/Hybrid | Analyte | Sensing Performance: Sensitivity (S), Limit of Detection (LOD), Linear Range (LR), Time Response (T) |
|---|---|---|---|---|---|
| Nanoparticle [66] | Impedimetric | Nonenzymatic | Cu-doped | Glucose | S: N/A LOD: 10−9 M LR: 10−9–10−5 M T: N/A |
| Nanoparticle [52] | Amperometric | Nonenzymatic | N/A | Glucose | S: 631.30 µA mM−1 cm−2 LOD: 0.043 µM LR: 1–8.6 mM T: 4 s |
| Nanoparticle [81] | Amperometric | GOx | Nitrogen-doped carbon sheets hybrid | Glucose | S: 231.7 µA mM−1 cm−2 LOD: 6.3 µM LR: 0.2–12 mM T: 3 s |
| Nanoparticle [82] | Voltammetric | GOx | Graphene–carbon nanotube (CNT) hybrid | Glucose | S: 5.362 µA mM−1 cm−2 LOD: 4.5 µM LR: 10 µM to 6.5 mM T: N/A |
| Nanoparticle [83] | Voltammetric | Enzymatic | Multiwall CNT hybrid | Levodopa | S: N/A LOD: 0.08 µmol L−1 LR: 5.0–500.0 µmol L−1 T: N/A |
| Nanoparticle [84] | Voltammetric | Probe DNA | Platinum and palladium hybrid | Dengue | S: N/A LOD: 43 µM LR: 1 µM–100 µM T: N/A |
| Nanoparticle [85] | Amperometric | ChEt & ChOx | N/A | Cholesterol | S: 190 µA mM−1 cm−2 LOD: N/A LR: 0.5–12 mM T: 5 s |
| Nanoparticle [86] | Voltammetric | Nonenzymatic | Multiwall CNT hybrid | Citalopram | S: N/A LOD: 0.005 µM LR: 0.012–1.54 µM T: N/A |
| Quantum dot [87] | Amperometric | Nonenzymatic | Graphene oxide/tyrosinase hybrid | Hydroxylated polychlorobiphenyls | S: N/A LOD: 0.15 µM LR: N/A T: N/A |
| Quantum dot [88] | Amperometric | Nonenzymatic | Reduced graphene oxide and multiwall CNT hybrid | Ascorbic acid | S: 36.12 µA mM−1 cm−2 LOD: 3.42 µM LR: 10–600 µM T: 2 s |
| Quantum dot [89] | Voltammetric | Starved algal cells | Coated by SiO2 | Acephate | S: N/A LOD: 1.0 × 10−12 M LR: 10−11–10−3 M T: N/A |
| Quantum dot [90] | Voltammetric | Enzymatic | Coated by nafion layer | Uric acid | S: 4.0 µA mM−1 cm−2 LOD: 22.97 µM LR: 1 mM–10 mM T: N/A |
| Morphology | Technique | Bio-Receptor | Doping/Hybrid | Analyte | Sensing Performance Sensitivity (S), Limit of Detection (LOD), Linear Range (LR), Time Response (T) |
|---|---|---|---|---|---|
| Nanorod [107] | Impedimetric | Nonenzymatic | N/A | Cholesterol | S: 4.2 µm mM−1 cm−2 LOD: 1.78 mM LR: 1–9 mM T: N/A |
| Nanotube [98] | Amperometric | Enzymatic ChOx | N/A | Cholesterol | S: 79.40 µA mM−1cm−2 LOD: 0.5 nM (S/N = 3) LR: 1.0 µm–13.0 mM T: 2 s |
| Nanotube [108] | Amperometric | Nonenzymatic | Au nanoparticles hybrid | Hydrogen peroxide | S: 1336.7 µm mM−1 cm−2 LOD: N/A LR: 1 µM–3.0 mM T: N/A |
| Nanowire [109] | Amperometric | Nonenzymatic | CO3O4/NiCo2O4/Ni foam hybrid | Hydrogen peroxide | S: 0.388 mA mM−1 cm−2 LOD: 0.163 µM (S/N = 3) LR: 0.2 µM–2.4 mM T: 5 s |
| Nanowire [110] | Amperometric | Enzymatic | N/A | L-lactic acid | S: 15.6 µA mM−1 cm−2 LOD: 12 µM LR: 12 µM–1.2 mM T: N/A |
| Nanowire [111] | Voltammetric | Nonenzymatic | Graphene/graphene foam | Folic acid | S: 0.18 µm mM−1 LOD: 1 µM LR: 0–60 µM T: N/A |
| Nanowire [112] | Voltammetric | Nonenzymatic | Graphene foam hybrid | Levodopa | S: 3.15 µA µM−1 LOD: 50 nM LR: 0.05–20 µM T: N/A |
| Nanorod [113] | Potentiometric | N/A | Reduced graphene oxide hybrid | Uric acid | LOD: 0.312 µM (S/N = 3) LR: 1–800 µM |
| Nanorod [114] | Amperometric | Uricase | N/A | Uric acid | S: 239.67 µA mM−1 cm−2 LOD: 5 nM LR: 0.01–4.56 mM T: 3 s |
| Nanorod [115] | Voltammetric | Urease | Ag hybrid | Urea | S: 41.64 µA mM−1 cm−2 LOD: 10 µM LR: 0.001–24.0 mM |
| Nanotube [8] | Amperometric | GOx | N/A | Glucose | S: 2.63 µA mM−1 cm−2 LOD: 8 µM (S/N = 3) LR: 0–6.5 mM |
| Nanorod [116] | Amperometric | Nonenzymatic | CuO hybrid | Glucose | S: 2961.7 µA mM−1 cm−2 LOD: 0.40 µM LR: Up to 8.45 mM T: <2 s |
| Nanorod [117] | Voltammetric | Nonenzymatic | Copper nanoparticles hybrid | Glucose | S: 609.6 µA mM−1 LOD: 0.03 µM (S/N = 3) LR: 5 µM–1.1 mM |
| Nanorod [6] | Amperometric | GOx | N/A | Glucose | S: 10.911 mA mM−1 cm−2 LOD: 0.22 µM LR: 0.6–1.4 mM T: 3 s |
| Nanorod [118] | FET | Nonenzymatic | Fe2O3 hybrid | Glucose | S: 105.75 µm mM−1 cm−2 LOD: 12 µM (S/N = 3) LR: 0.05–18 mM |
| Nanorod [119] | FET | Nonenzymatic | NiO quantum dots hybrid | Glucose | S: 13.14 µA mM−1 cm−2 LOD: 26 µM LR: 0.00–10 mM |
| Nanorod [105] | FET | GOx | N/A | Glucose | S: 1.6 mA mM−1 cm−2 LOD: 1 µM |
| Nanorod [120] | Liquid gate FET | Antibody | N/A | Hepatitis B | LOD: 20 aM LR: 20 aM to 1 pM |
| Morphology | Technique | Bio-Receptor | Doping/Hybrid | Analyte | Sensing Performance Sensitivity (S), Limit of Detection (LOD), Linear Range (LR), Time Response (T) |
|---|---|---|---|---|---|
| Nanowall [130] | Amperometric | Nonenzymatic | N/A | Glucose | S: 700.6 1 µm mM−1 cm−2 LOD: 1 µm (S/N = 3) LR: 1 µm to 19.2 mM T: N/A |
| Hydrazine | S: 660.2 µm mM−1 cm−2 LOD: 0.5 µM (S/N = 3) LR: 0.5 µM to 14.2 mM T: N/A | ||||
| Nanosheet [128] | Amperometric | Uricase | Ag hybrid | Uric acid | S: 129.81 µA mM−1 cm−2 LOD: 0.019 µM LR: 0.05–2.0 mM T: 5 s |
| Nanosheet [24] | Amperometric | Nonenzymatic | Graphitic carbon nitride | Hydrogen peroxide | S: 540.8 µA mM−1 cm−2 LOD: 1.7 µM LR: 0.05–14.15 mM T: N/A |
| Nanosheet [129] | Voltammetric | DNA | MoS2 hybrid | PMA/RARA fusion gene | S: N/A LOD: 6.6 10−16 M LR: 1.0 10−15 M–1.0 10−6 M T: N/A |
| Nanoflake [34] | Amperometric | Anti-cortisol antibody | Bovine serum albumin and Au for immobilizing and hybrid, respectively | Cortisol | S: 7.74 µA/M LOD: 1 pM LR: N/A T: N/A |
| Nanoporous [131] | Impedimetric | Urease | N/A | Urea | S: 0.637 kΩ mM−1 LOD: 0.40 mM LR: 0.83–23.2 mM T: N/A |
| Morphology | Technique | Bio-Receptor | Doping/Hybrid | Analyte | Sensing Performance: Sensitivity (S), Limit of Detection (LOD), Linear Range (LR), Time Response (T) |
|---|---|---|---|---|---|
| Spherical lamellar [143] | Voltammetric | GOx | Au nanoparticles hybrid | Glucose | S: 1.409 µA mM−1 (S/N = 3) LOD: 0.02 mM LR: 1–20 mM |
| Spherical nanosheet [144] | Amperometric | Nonenzymatic | Au nanoparticles hybrid | Dopamine | LOD: 0.02 µM LR: 0.1–300 µM |
| Nanoflower [148] | Voltammetric | Nonenzymatic | Polyaniline/reduced graphene oxide hybrid | Dopamine | LOD: 0.8 nM (S/N = 3) LR: 0.001–1 µM and 1–1000 µM |
| Uric acid | LOD: 0.042 µM (S/N = 3) LR: 0.1–100 µM and 100–1000 µM | ||||
| Spherical nanosheet [150] | Voltammetric | Nonenzymatic | Graphene foam hybrid | Levodopa | S: 0.66 µA µM−1 LOD: 1 µM LR: 1–75 µM |
| Nanoflower [135] | Voltammetric | Nonenzymatic | Graphene hybrid | Levodopa | S: 0.32 µA µM−1 LOD: 1 µM LR: 1–60 µM |
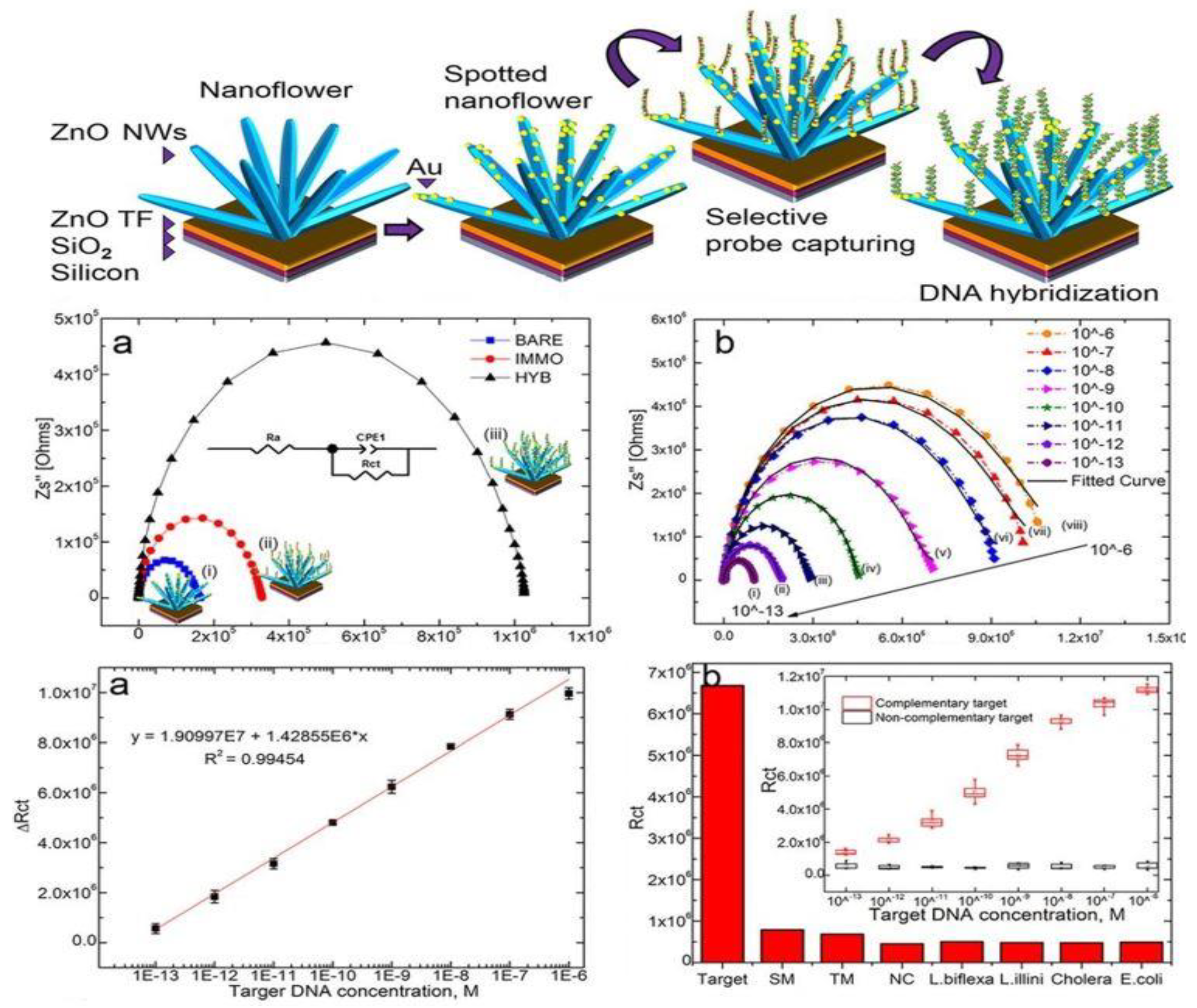
| Nanoflower [10] | Impedimetric | DNA | Au nanoparticles hybrid | Leptospira | LOD: 100 fM LR: 10−6–10−13 M |
| Nanoflower [14] | Voltammetric and impedimetric | DNA | N/A | Bacterial meningitis | S: 168.64 µA ng−1 µL cm−2 LOD: 5 ng µL−1 LR: 5–240 ng µL−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. https://doi.org/10.3390/ma12182985
Napi MLM, Sultan SM, Ismail R, How KW, Ahmad MK. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials. 2019; 12(18):2985. https://doi.org/10.3390/ma12182985
Chicago/Turabian StyleNapi, Muhammad Luqman Mohd, Suhana Mohamed Sultan, Razali Ismail, Khoo Wei How, and Mohd Khairul Ahmad. 2019. "Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review" Materials 12, no. 18: 2985. https://doi.org/10.3390/ma12182985
APA StyleNapi, M. L. M., Sultan, S. M., Ismail, R., How, K. W., & Ahmad, M. K. (2019). Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials, 12(18), 2985. https://doi.org/10.3390/ma12182985





