Thermodynamics Analysis of Multiple Microelements’ Coupling Behavior in High Fatigue Resistance 50CrVA Spring Steel with Nanoparticles
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Fe–V–Ti–N–C System Thermodynamics Calculation Methods
2.2.1. Wagner’s Formalism
2.2.2. Mass Conservation
2.3. Tests
3. Results and Discussion
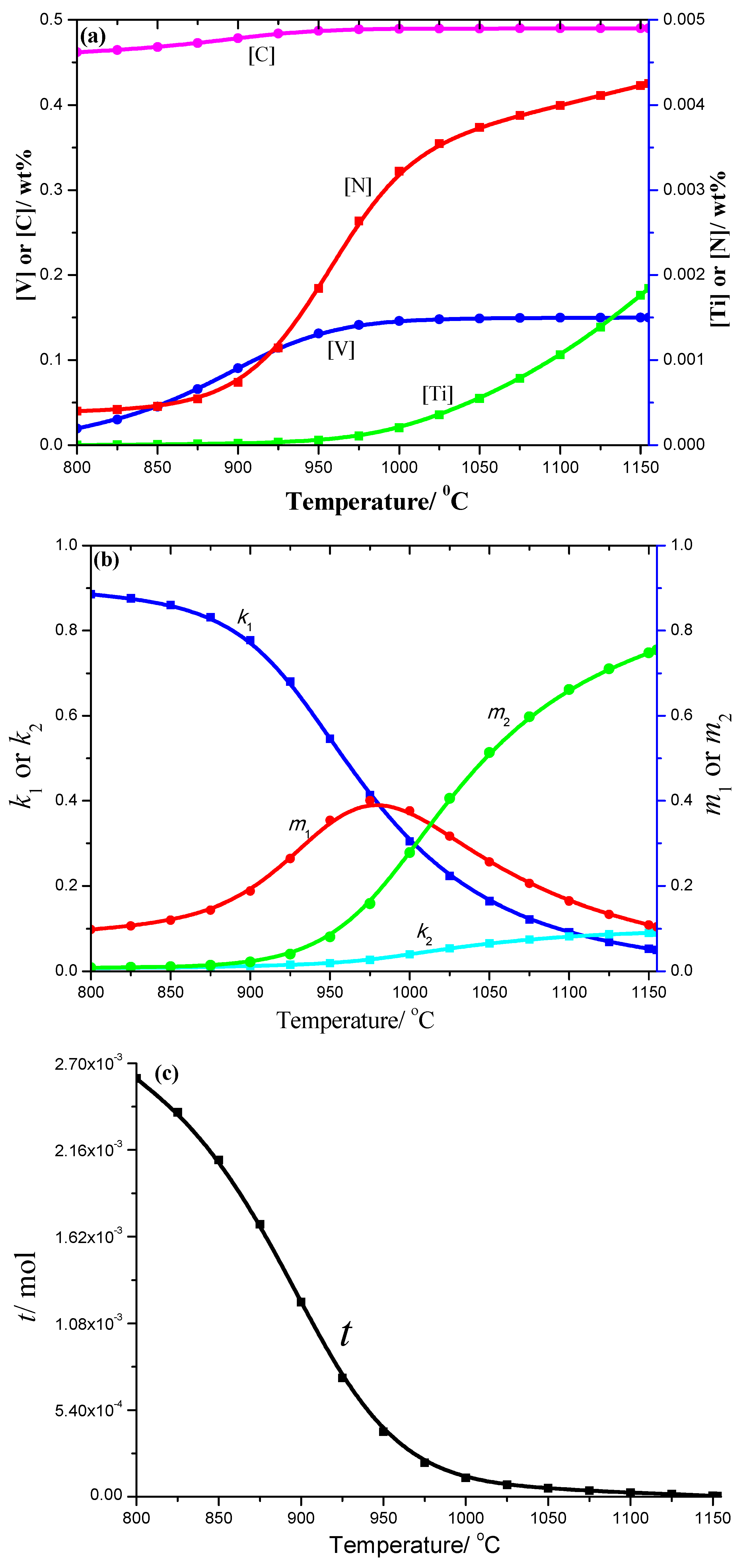
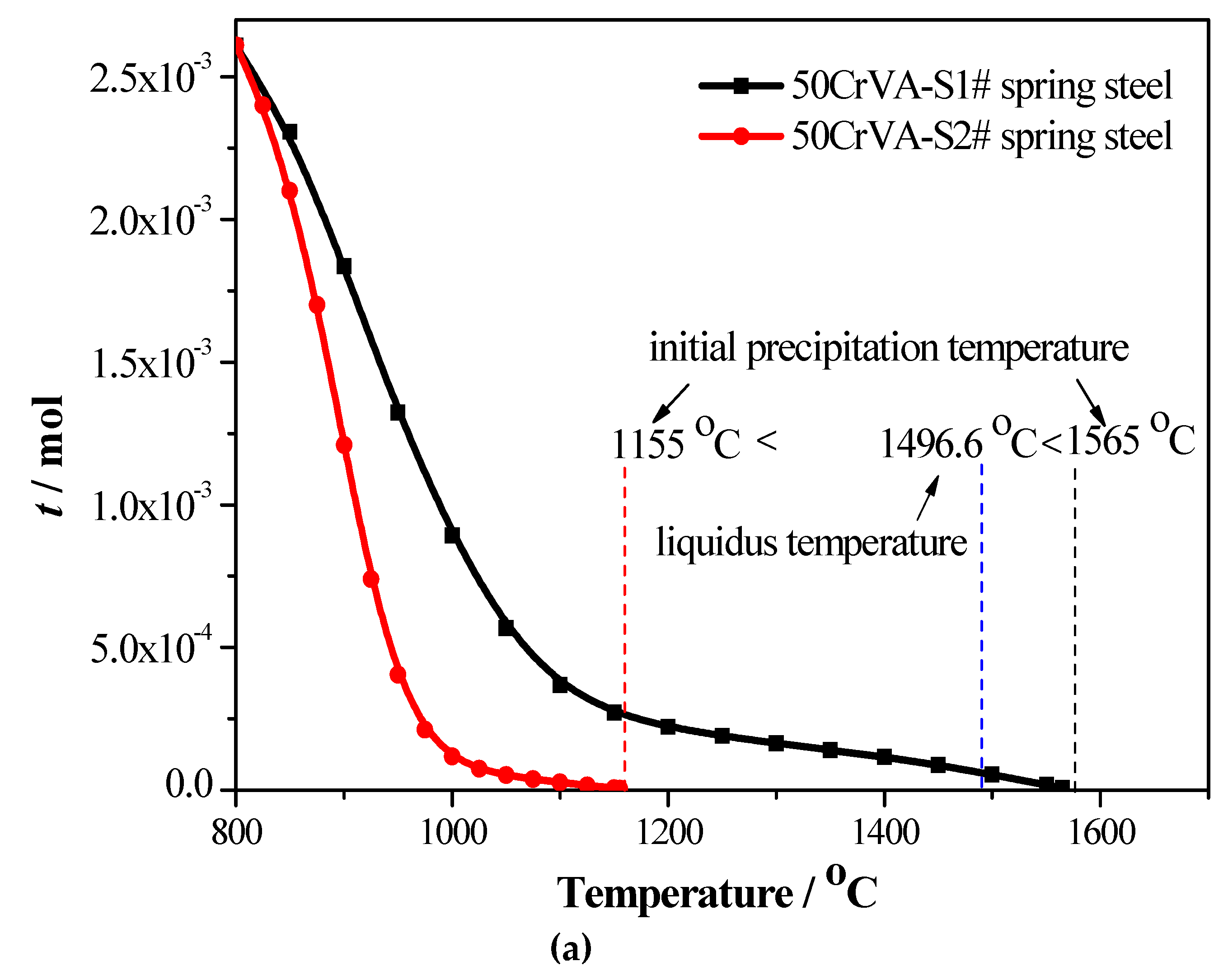
3.1. Thermodynamics Analysis Results
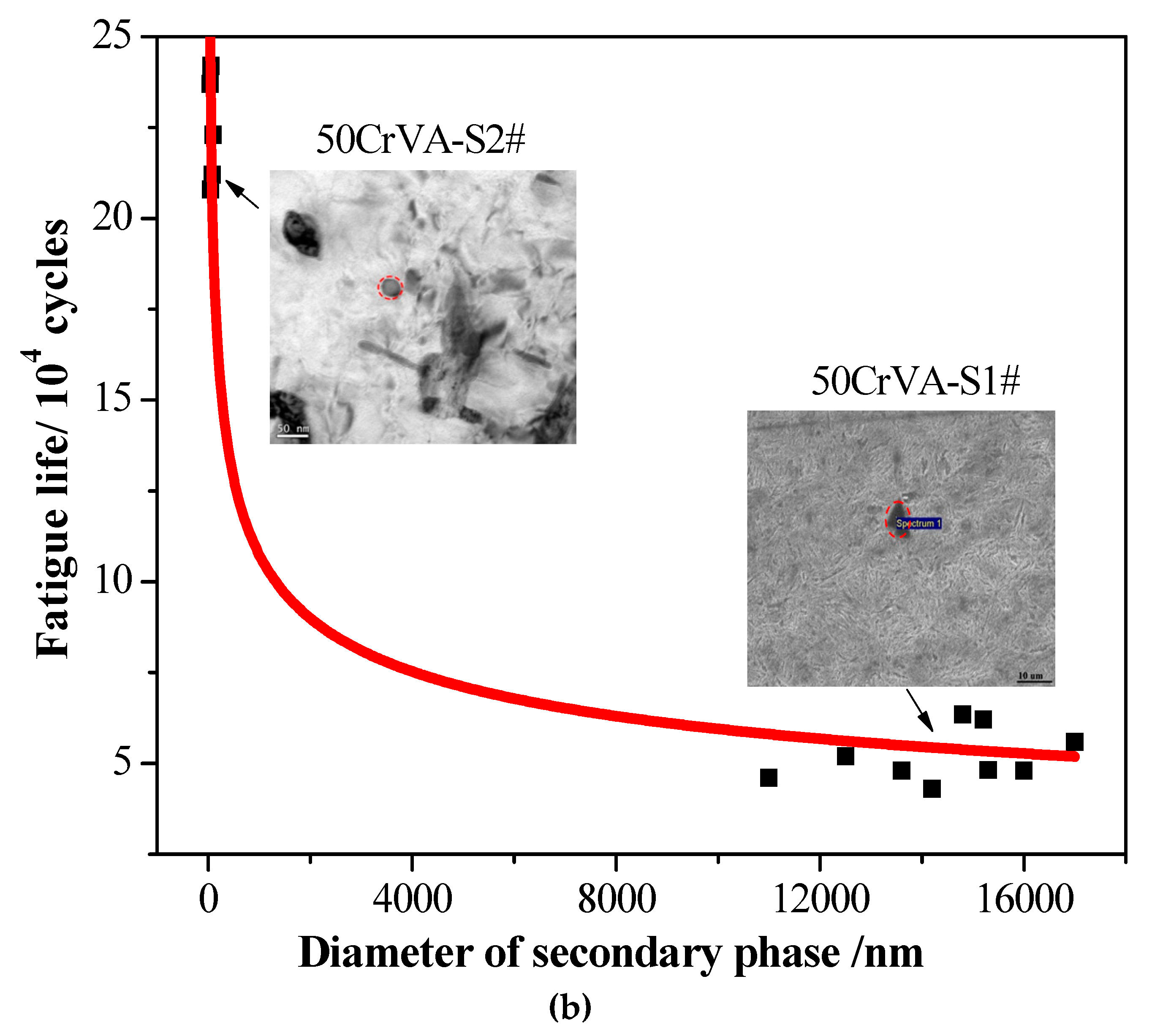
3.2. Secondary Phase Microstructure and Fatigue Life
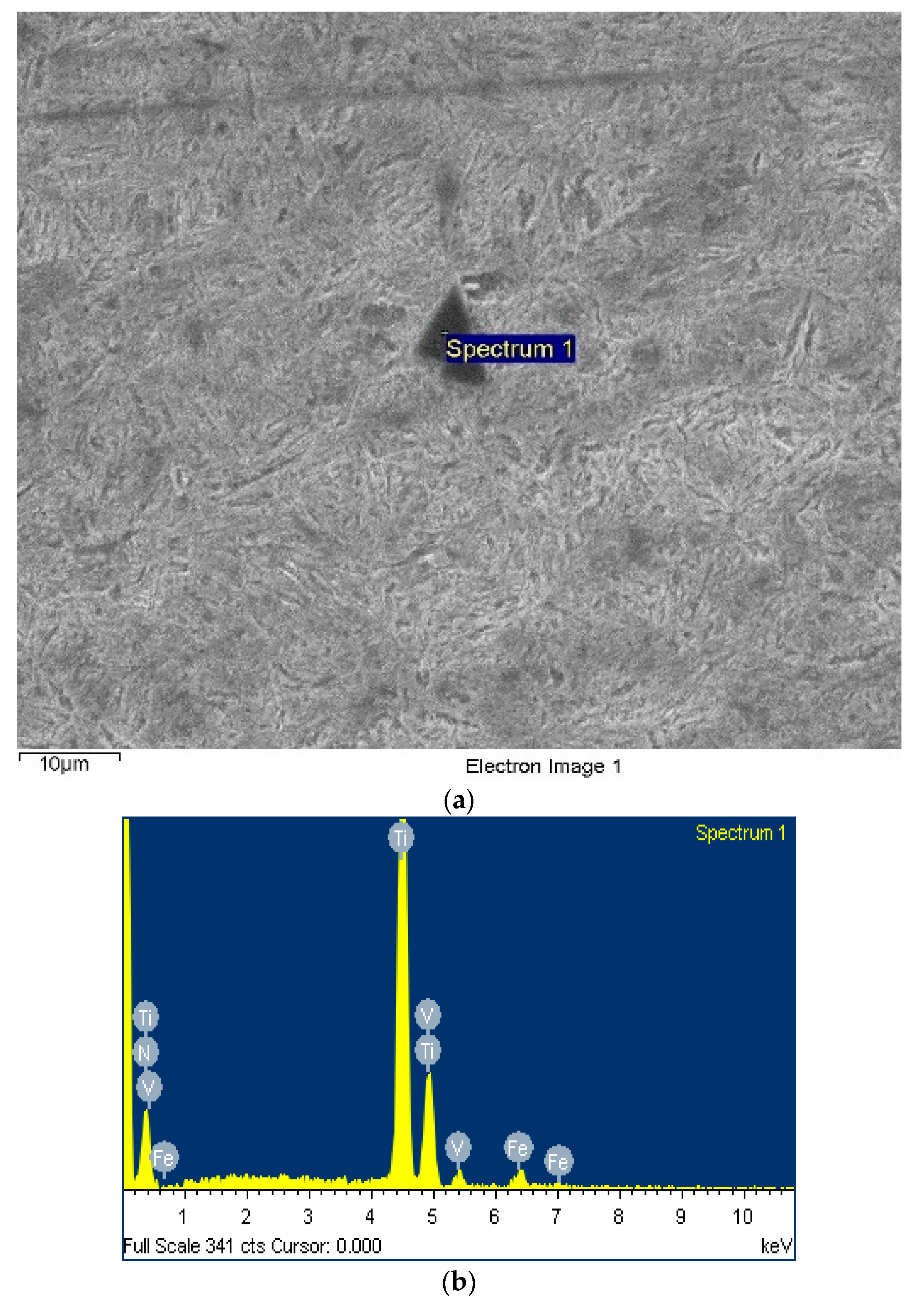
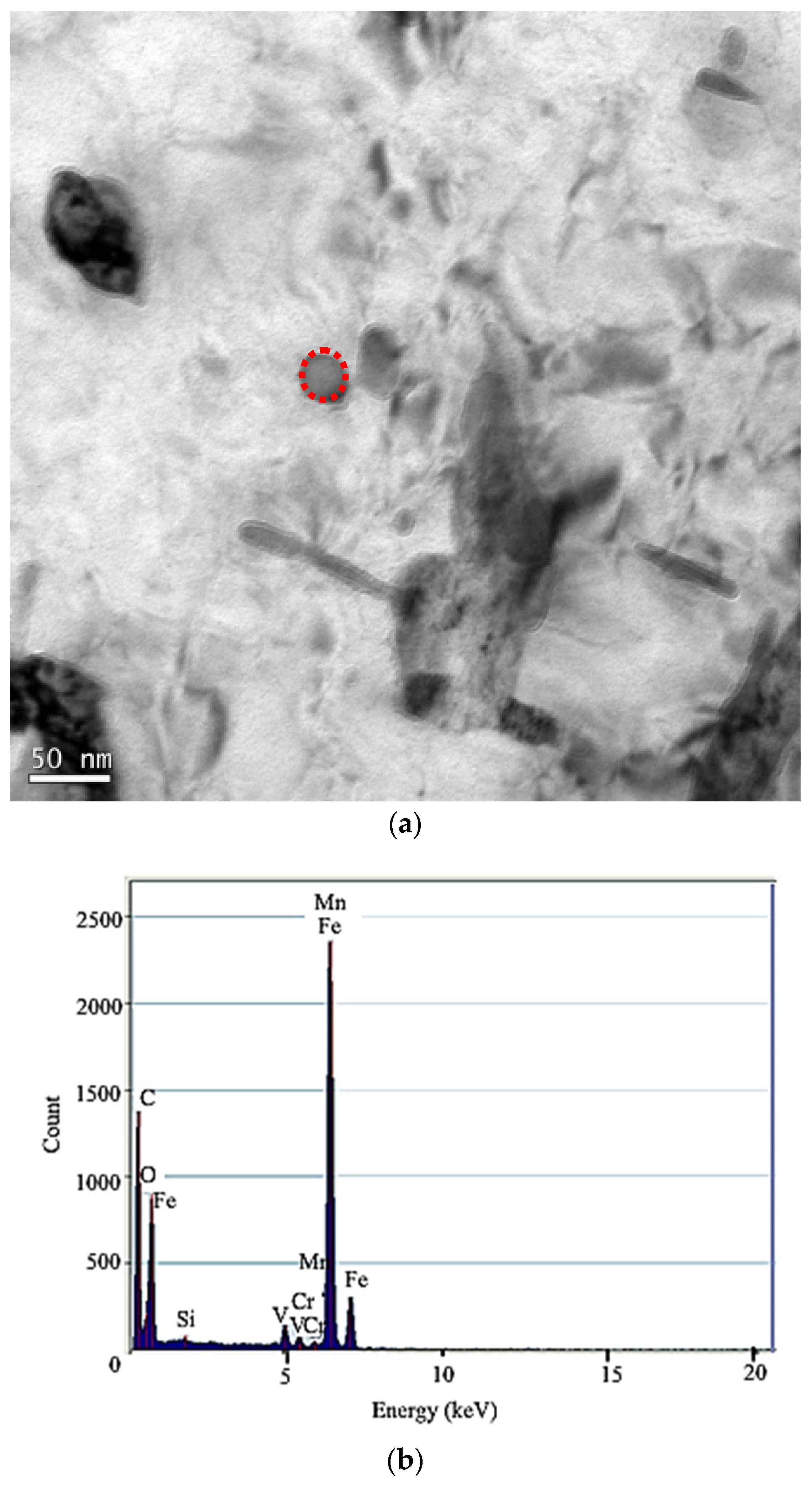
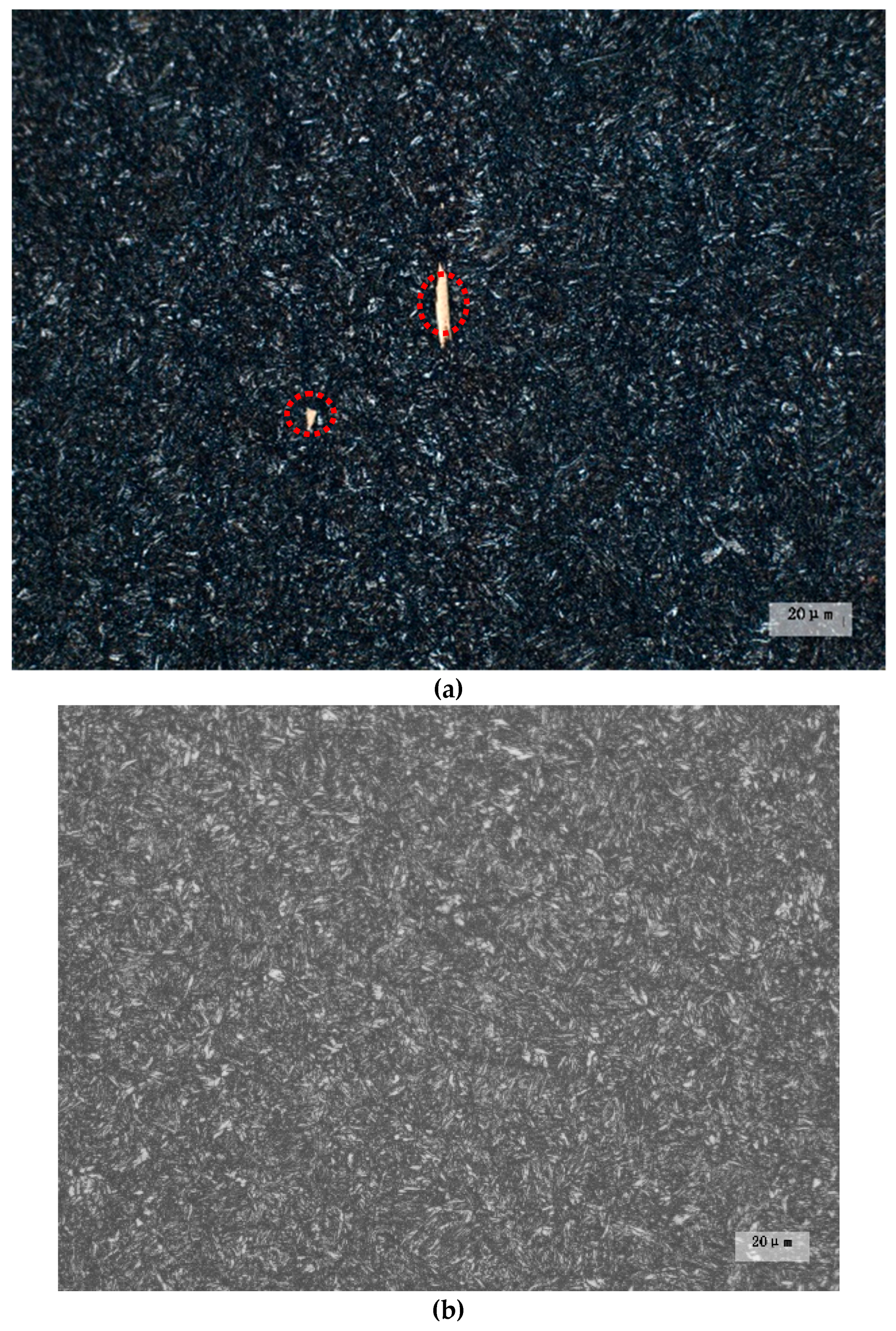
3.2.1. Secondary Phase Microstructure
3.2.2. Fatigue Life
4. Conclusions
- (1)
- Thermodynamics calculation results show that the solid solution contents of different elements, such as Ti, V, N, and C, decrease as the temperature decreases, especially the change of N and Ti. The change of k1, k2, m1, and m2 coefficients with temperature is more complex, and the total moles amount (t) of corresponding compounds increases gradually as the temperature decreases in both spring steels;
- (2)
- For the 50CrVA-S1#, its initial precipitation temperature was 1565 °C, higher than the corresponding liquidus temperature. Carbonitrides constitutional liquation occurred, and the compound was a microparticle. For the 50CrVA-S2#, its initial precipitation temperature was 1155 °C, lower than the liquidus temperature, so no carbonitride constitutional liquation occurred, and the compound was nanoparticle;
- (3)
- Experimental results indicate that the fatigue life reduces by about an order of magnitude when the secondary phase size changes from nanometer scale to micron level. For 50CrVA-S1#, the secondary phase size range detected in fracture place was 10.5–17 µm, and the corresponding fatigue life range was 43–63.5 × 103 cycles. For the 50CrVA-S2#, the secondary phase size range detected in fracture place was 10–100 nm, and the corresponding fatigue life range was 208–242 × 103 cycles.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.L.; Zhuo, L.C.; Chen, M.W.; Wang, Z.D. Thermodynamic model for precipitation of carbonitrides in microalloyed steels and its application on Ti-V-C-N system. Rare Met. 2016, 35, 735–741. [Google Scholar] [CrossRef]
- Zhang, C.L.; Liu, Y.Z.; Jiang, C. Effects of niobium and vanadium on hydrogen-induced delayed fracture in high strength spring steel. J. Iron Steel Res. Int. 2011, 18, 49–53. [Google Scholar] [CrossRef]
- Htun, M.S.; Kyaw, S.T.; Lwin, K.T. Effect of heat treatment on microstructures and mechanical properties of spring steel. J. Met. Mater. Miner. 2008, 18, 191–197. [Google Scholar]
- Yoneguchi, A.; Schaad, J.; Kurebayashi, Y. Development of High Strength Spring Steel and Its Application to Automotive Coil Spring; Society of Automotive Engineers Inc.: Warrendale, PA, USA, 2000; pp. 101–107. [Google Scholar]
- Wu, H.Z.; Gao, D.P.; Guo, H.D. Generalization of life characteristic investigation for probabilistic fatigue failure. J. Hubei Polytech. Univ. 2002, 17, 18–22. [Google Scholar]
- Zhang, L.N.; Wang, P.; Dong, J.X.; Zhang, M.C. Microstructures’ effects on high temperature fatigue failure behavior of typical superalloys. Mater. Sci. Eng. A 2013, 587, 168–178. [Google Scholar] [CrossRef]
- Sun, C.Q.; Lei, Z.Q.; Xie, J.J.; Hong, Y.S. Effects of inclusion size and stress ratio on fatigue strength for high-strength steels with fish-eye mode failure. Int. J. Fatigue 2013, 48, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.Q.; Hong, Y.S.; Xie, J.J.; Sun, C.Q.; Zhao, A.G. Effects of inclusion size and location on very-high-cycle fatigue behavior for high strength steels. Mater. Sci. Eng. A 2012, 558, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.Y.; Bathias, C.; Kawagoishi, N.; Chen, Q. Effect of inclusion on subsurface crack initiation and giga-cycle fatigue strength. Int. J. Fatigue 2002, 24, 1269–1274. [Google Scholar] [CrossRef]
- Hattori, C.S.; Couto, A.A.; Vatavuk, J.; deLima, N.B.; Reis, D.A.P. Evaluation of fatigue behavior of SAE 9254 steel suspension springs manufactured by two different processes: Hot and cold winding. In Experimental and Numerical Investigation of Advanced Materials and Structures; Springer: Cham, Switzerland, 2013; pp. 91–105. [Google Scholar]
- Costa, L.V.; Carneiro, J.R.G.; Catalão, R.P.C.; Rouhaud, E. Residual stress gradients in AISI 9254 steel springs submitted to shot peening and heat treatment for increased fatigue resistance. Adv. Mater. Res. 2014, 996, 749–754. [Google Scholar] [CrossRef]
- Jiang, S.H.; Wang, H.; Wu, Y.; Liu, X.J.; Chen, H.H.; Yao, M.J.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Liu, C.T. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Acta Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Lu, K. Stabilizing nanostructures in metals using grain and twin boundary architectures. Nat. Rev. Mater. 2016, 1, 16019. [Google Scholar] [CrossRef]
- Cao, Y.B.; Xiao, F.R.; Qiao, G.Y.; Liao, B. Quantitative research on dissolving of Nb in high Nb microalloyed steels during reheating. J. Iron Steel Res. Int. 2014, 21, 596–599. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, M.; Pang, X.; Chen, X.; Wang, Z.; Volinsky, A.; Tang, H. Applications and Thermodynamic Analysis of Equilibrium Solution for Secondary Phases in Ti-N-C Gear Steel System with Nano-Particles. Metals 2017, 7, 110. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, J.S.; Zhao, H.M.; Chen, Y.F. Influence factors on solid-solution of carbonitride of niobium in steel. J. Univ. Sci. Technol. B 2009, 31 (Suppl. 1), 194–198. [Google Scholar]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.F.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 62, 273–312. [Google Scholar]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Potazhevska, O.A.; Velikanova, T.Y. Thermodynamic modelling of the ternary B-Mo-Ti system with refined B-Mo description. J. Alloy. Compd. 2016, 65, 336–352. [Google Scholar] [CrossRef]
- Drápala, J.; Kostiuková, G.; Smetana, B.; Madaj, M.; Kroupa, A. Thermodynamic and experimental study of Tin-Zinc-Aluminum ternary system. Adv. Sci. Eng. Med. 2015, 7, 291–295. [Google Scholar] [CrossRef]
- Uhm, S.; Moon, J.; Lee, C.; Yoon, J.; Lee, B. Prediction model for the austenite grain size in the coarse grained heat affected zone of Fe-C-Mn steels: Considering the effect of in itial grain size on isothermal growth behavior. ISIJ Int. 2004, 4, 1230–1237. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels; Metallurgical Industry Press: Beijing, China, 2006; pp. 123–124. [Google Scholar]
- Wang, Y.L.; Zhou, M.; Pang, X.L.; Chen, X.H.; Wang, Z.D.; Volinsky, A.A. Thermodynamic analysis of Ti3O5 nanoparticles formed in melt and their effects on ferritic steel microstructure. Materials 2018, 11, 1343. [Google Scholar] [CrossRef]
- Wagner, C. Thermodynamic of Alloys; Addision-Wesley Press: Cambridge, MA, USA, 1952; pp. 47–51. [Google Scholar]
- The Japanese Society for the Promotion of Science. Steelmaking Data Sourcebook. The 19th Committee on Steelmaking (Revised Edition); Gordon and Breach Science Publishers: New York, NY, USA, 1988; pp. 45–169. [Google Scholar]
- Morita, Z.; Kunisada, K. Solubility of nitrogen and equilibrium of Ti-nitride forming reaction in liquid Fe-Ti alloys. ISIJ 1977, 63, 1663–1671. [Google Scholar]
- Lee, S.H.; Lee, K.S.; Lee, K.J. Evaluation of wagner interaction parameter in Fe-Mn-Si-Nb-Ti-V-C system. Mater. Sci. Forum 2005, 475, 3327–3330. [Google Scholar] [CrossRef]
- Chen, J.X. Steelmaking Common Data Charts Manual; Metallurgical Industry Press: Beijing, China, 2010; pp. 511–520. [Google Scholar]
- Morita, Z.; Tanaka, T.; Yanai, T. Equilibria of nitride forming reactions in liquid iron alloys. Metall. Trans. B 1987, 18, 195–202. [Google Scholar]
- Turkdogan, E.T. Fundamental of Steelmaking; The Institute of Materials: London, UK, 1996; p. 128. [Google Scholar]
- Evans, D.B.; Pehlke, R.D. Equilibria of nitrogen with the refractory metals titanium, zirconium, columbium, vanadium and tantalum in liquid iron. Trans. Met. Soc. AIME 1965, 233, 1620–1624. [Google Scholar]
- Sigworth, G.K.; Elliott, J.F. The thermodynamics of liquid dilute iron alloys. Met. Sci. 1974, 8, 298–310. [Google Scholar] [CrossRef]
- Cha, W.Y.; Miki, T.; Sasaki, Y.; Hino, M. Temperature dependence of Ti deoxidation equilibrium of liquid iron in coexistence with Ti3O5 and Ti2O3. ISIJ Int. 2008, 48, 729–738. [Google Scholar] [CrossRef]
- Pak, J.J.; Yoo, J.T.; Jeong, Y.S.; Tae, S.J.; Seo, S.M.; Kim, D.S.; Lee, Y.D. Thermodynamics of titanium and nitrogen in Fe-Si met. ISIJ Int. 2005, 45, 23–29. [Google Scholar] [CrossRef]
- Gan, Y. Practical Manual of Modern Continuous Casting Steel; Metallurgical Industry Press: Beijing, China, 2010; pp. 52–53. [Google Scholar]
- Kimura, Y.; Inoue, T.; Yin, F.; Tsuzaki, K. Inverse temperatured ependence of toughness in an ultrafine grain-structure steel. Science 2008, 320, 1057–1060. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Wang, J.J. Effect of V on intragranular ferrite nucleation of high Ti bearing steel. Scr. Mater. 2013, 68, 953–956. [Google Scholar] [CrossRef]
- Song, R.; Ponge, D.; Raabe, D. Mechanical properties of an ultrafine grained C-Mn steel processed by warm deformation and annealing. Acta Mater. 2005, 53, 4881–4892. [Google Scholar] [CrossRef]
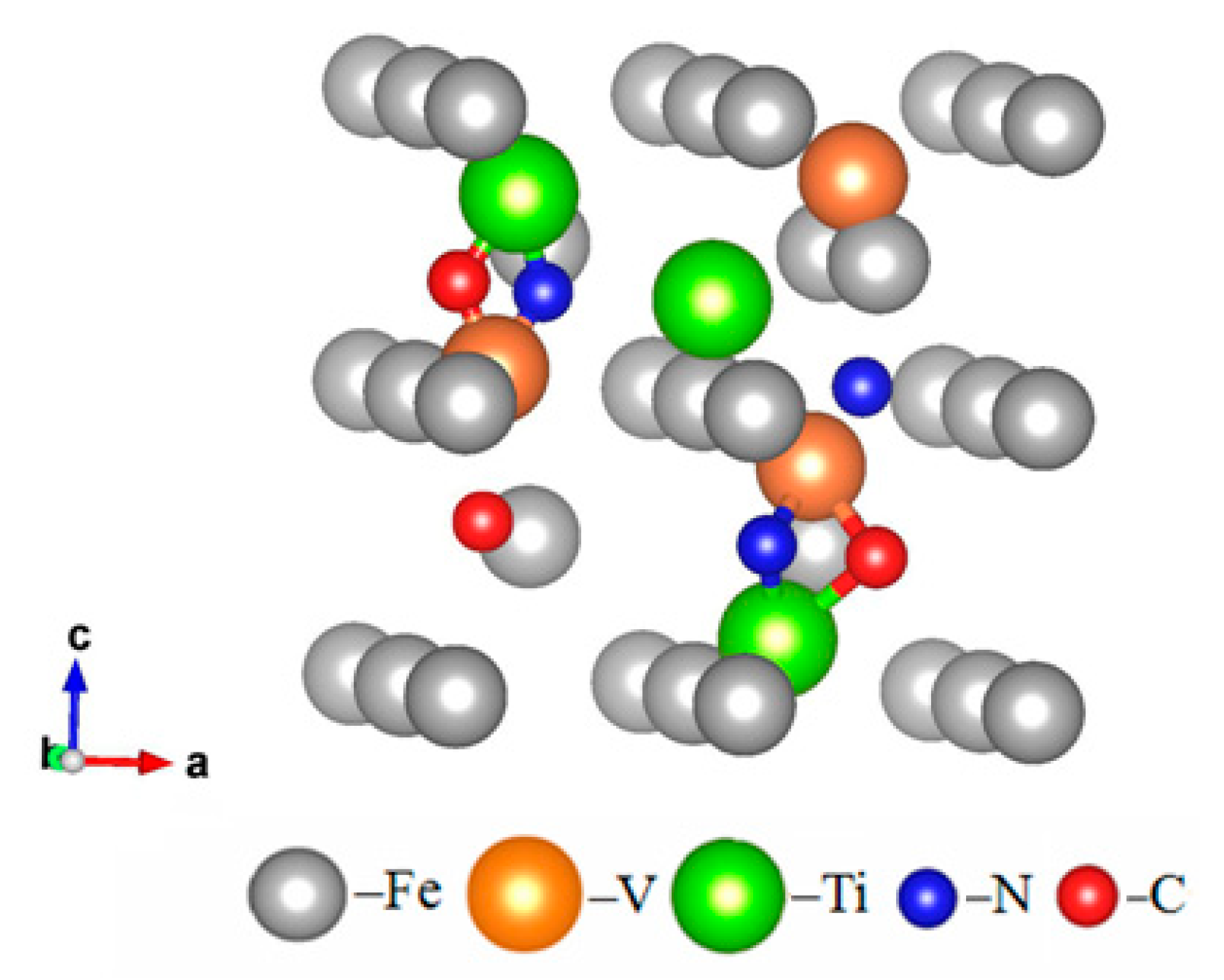


| Steel | Number | C | Si | Mn | Cr | V | Ti | N | Cu | Sn | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50CrVA | S1# | 0.5 | 0.22 | 0.74 | 1.02 | 0.136 | 0.009 | 0.0172 | 0.03 | 0.0016 | 0.011 | 0.005 |
| S2# | 0.49 | 0.26 | 0.75 | 1.01 | 0.15 | 0.002 | 0.0043 | 0.03 | 0.0018 | 0.008 | 0.004 |
| Element j | ||||
|---|---|---|---|---|
| C | −221/T − 0.072 [25] | −571.75/T + 0.0644 [26,27] | 0.06 [28] | 8890/T [22] |
| N | −19500/T + 8.37 [29] | −1270/T + 0.33 [27] | 6294/T [22] | 5790/T [22] |
| Mn | −0.043 [25] | 6.2427/T + 0.000146 [26,27] | −8336/T − 27.8 + 3.652In T [22] | −5070/T [22] |
| Cr | −0.016 [30] | * | −65150/T + 24.1 [28] | −21880/T + 7.02 [22] |
| S | −0.27 [25] | −29.968/T [28] | 0.007 [30] | 0.046 [28] |
| P | −74.92/T [27] | −43.079/T [27] | 167/T − 0.038 [27] | 1190/T − 0.608 [27] |
| Si | 177.5/T − 0.12 [31] | 162.74/T − 0.0385 [26,27] | −286/T + 0.202 [31] | 162/T − 0.008 [30] |
| Ti | 212/T − 0.0640 [32] | 30.196/T + 0.00313 [26,27] | −5700/T + 2.45 [29] | −55/T − 0.015 [26] |
| V | 28.416/T + 0.0032 [26,27] | 470/T − 0.22 [33] | −356/T + 0.0973 [34] | −134.79/T + 0.0185 [26,27] |
| Steels | Hardness (HRC) | Mean Hardness (HRC) | Standard Deviation | Matrix Microstructure |
|---|---|---|---|---|
| S1#-1 | 42 | 40.7 | 1.53 | Tempered martensite |
| S1#-2 | 39 | |||
| S1#-3 | 41 | |||
| S2#-1 | 40.5 | 40.3 | 0.76 | Tempered martensite |
| S2#-2 | 41 | |||
| S2#-3 | 39.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fu, L.; Zhou, M.; Zhou, Z.; Pang, X.; Zhong, S.; Volinsky, A.A. Thermodynamics Analysis of Multiple Microelements’ Coupling Behavior in High Fatigue Resistance 50CrVA Spring Steel with Nanoparticles. Materials 2019, 12, 2952. https://doi.org/10.3390/ma12182952
Wang Y, Fu L, Zhou M, Zhou Z, Pang X, Zhong S, Volinsky AA. Thermodynamics Analysis of Multiple Microelements’ Coupling Behavior in High Fatigue Resistance 50CrVA Spring Steel with Nanoparticles. Materials. 2019; 12(18):2952. https://doi.org/10.3390/ma12182952
Chicago/Turabian StyleWang, Yanlin, Lihua Fu, Meng Zhou, Zirong Zhou, Xiaolu Pang, Shouyan Zhong, and Alex A. Volinsky. 2019. "Thermodynamics Analysis of Multiple Microelements’ Coupling Behavior in High Fatigue Resistance 50CrVA Spring Steel with Nanoparticles" Materials 12, no. 18: 2952. https://doi.org/10.3390/ma12182952
APA StyleWang, Y., Fu, L., Zhou, M., Zhou, Z., Pang, X., Zhong, S., & Volinsky, A. A. (2019). Thermodynamics Analysis of Multiple Microelements’ Coupling Behavior in High Fatigue Resistance 50CrVA Spring Steel with Nanoparticles. Materials, 12(18), 2952. https://doi.org/10.3390/ma12182952







