Insights and Aspects to the Modeling of the Molten Core Method for Optical Fiber Fabrication
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Fiber Drawing and General Insights
- (i)
- The first fiber segment of the draw already exhibits a graded-index profile with a lower concentration of SiO2 at its core center relative to later segments. This concentration increases over time. As opposed to this, the initial precursor composition decreases (not presented here) as it is diluted by the SiO2.
- (ii)
- For a constant fiber cladding diameter, the fiber core diameter decreases as the fiber is being drawn and, therefore, the initial inner diameter / outer diameter (ID/OD) ratio is not preserved during the draw. Moreover, it is worth noting that the initial segments collected generally present a larger ID/OD ratio with respect to the initial ratio (e.g., 2/10 versus 1/10 here).
- (iii)
- The concentration profile sharpens over time, i.e., the silica composition profile is less flat at the core center as draw time increases. This concentration profile is indicative of a diffusion-controlled incorporation of silica into the molten core.
3.2. Preliminary Assumptions and Modeling Equations
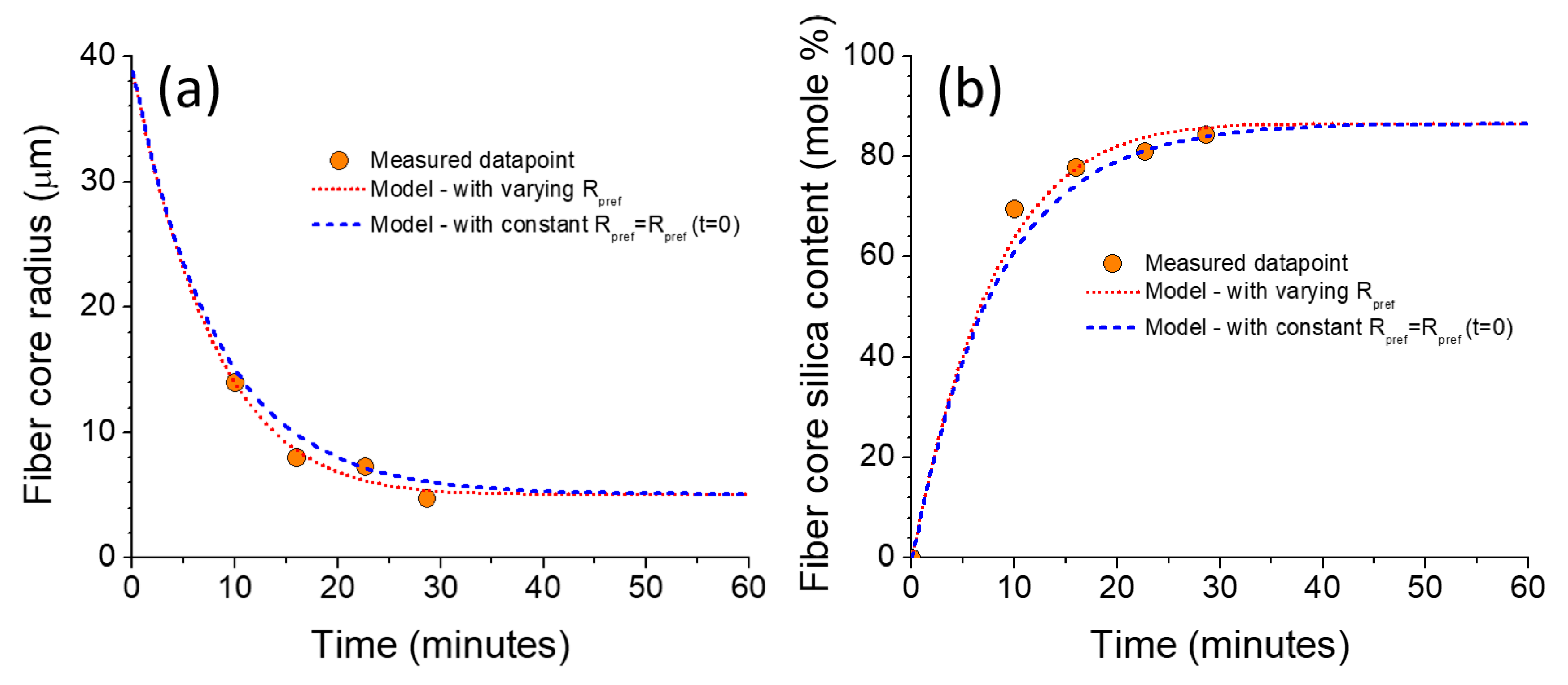
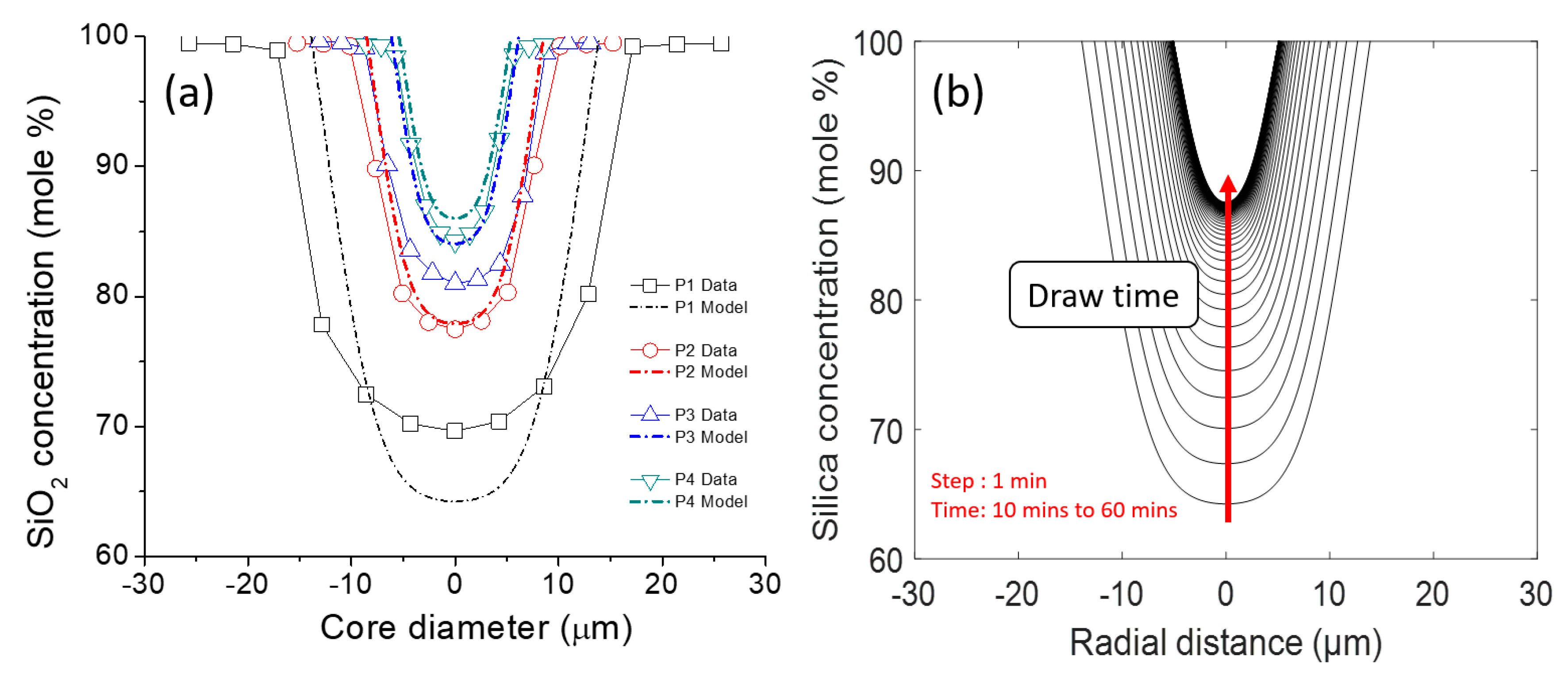
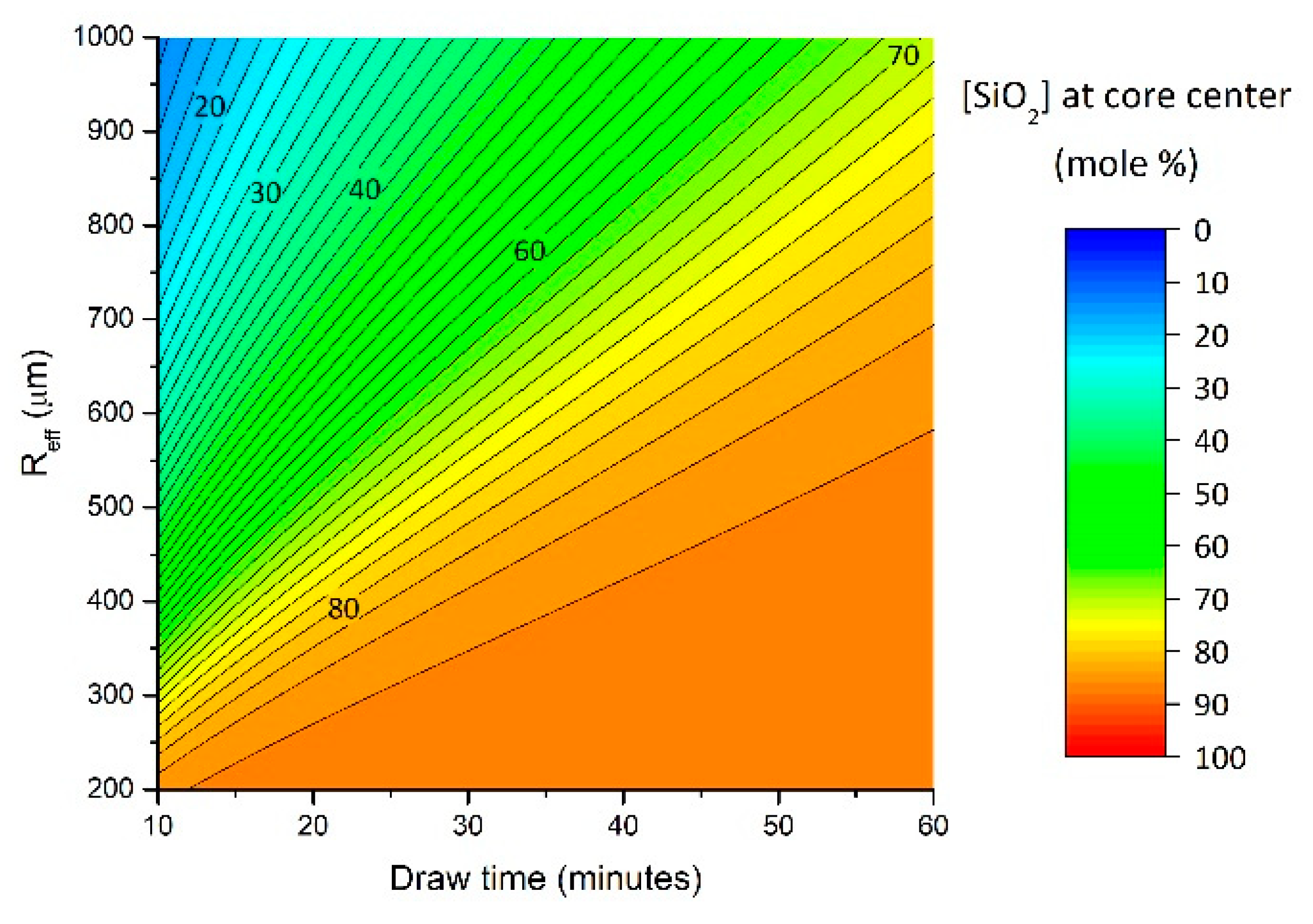
3.3. Modeling Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ballato, J.; Peacock, A.C. Perspective: Molten core optical fiber fabrication—A route to new materials and applications. APL Photonics 2018, 3, 120903. [Google Scholar] [CrossRef]
- Ballato, J.; Hawkins, T.; Foy, P.; Stolen, R.; Kokuoz, B.; Ellison, M.; Mcmillen, C.; Rao, A.M.; Daw, M.; Sharma, S.; et al. Silicon optical fiber. Opt. Express 2008, 16, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Cavillon, M.; Kucera, C.; Hawkins, T.W.; Yu, N.; Dragic, P.; Ballato, J. Ytterbium-doped multicomponent fluorosilicate optical fibers with intrinsically low optical nonlinearities. Opt. Mater. Express 2018, 8, 744–760. [Google Scholar] [CrossRef]
- Dragic, P.; Hawkins, T.; Foy, P.; Morris, S.; Ballato, J. Sapphire-derived all-glass optical fibres. Nat. Photonics 2012, 6, 627–633. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J.; Yu, C.; Zhou, Q.; Chen, D. Preparation and characterizations of Nd:YAG ceramic derived silica fibers drawn by post-feeding molten core approach. Opt. Express 2016, 24, 24248–24254. [Google Scholar] [CrossRef] [PubMed]
- Dragic, P.D.; Cavillon, M.; Ballato, A.; Ballato, J. A Unified Materials Approach to Mitigating Optical Nonlinearities in Optical Fiber. II. B. The Optical Fiber, Material Additivity and the Nonlinear Coefficients. Int. J. Appl. Glass Sci. 2018, 9, 307–318. [Google Scholar] [CrossRef]
- Dragic, P.; Law, P.-C.; Ballato, J.; Hawkins, T.; Foy, P. Brillouin spectroscopy of YAG-derived optical fibers. Opt. Express 2010, 18, 10055–10067. [Google Scholar] [CrossRef] [PubMed]
- Ballato, J.; Sanamyan, T.; Zhang, J.; Matthewson, M.J.; Foy, P.; Daw, M.; McMillen, C.; Hawkins, T.; Kokuoz, B.; Dubinskii, M.; et al. On the fabrication of all-glass optical fibers from crystals. J. Appl. Phys. 2009, 105, 053110. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.R.; Kingery, W.D. Dissolution in Ceramic Systems: I, Molecular Diffusion, Natural Convection, and Forced Convection Studies of Sapphire Dissolution in Calcium Aluminum Silicate. J. Am. Ceram. Soc. 1964, 47, 37–43. [Google Scholar] [CrossRef]
- Samaddar, B.N.; Kingery, W.D.; Cooper, A.R. Dissolution in Ceramic Systems: II, Dissolution of Alumina, Mullite, Anorthite, and Silica in a Calcium-Aluminum-Silicate Slag. J. Am. Ceram. Soc. 1964, 47, 249–254. [Google Scholar] [CrossRef]
- Oishi, Y.; Cooper, A.R.; Kingery, W.D. Dissolution in Ceramic Systems: III, Boundary Layer Concentration Gradients. J. Am. Ceram. Soc. 1965, 48, 88–95. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Brown, F.H. Kinetics of dissolution of magnesium oxide in a sodium silicate melt. Anal. Calorim. 1970, 319–337. [Google Scholar] [CrossRef]
- Hess, K.; Dingwell, D.B.; Rijssler, E. Parametrization of viscosity-temperature relations of aluminosilicate melts. Chem. Geol. 1996, 128, 155–163. [Google Scholar] [CrossRef]
- Nakamoto, M.; Lee, J.; Tanaka, T. A Model for Estimation of Viscosity of Molten Silicate Slag. Iron Steel Inst. Jpn. Int. 2005, 45, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Blomquist, R.A.; Fink, J.K.; Leibowitz, L. The Viscosity of Molten Alumina; Argonne National Laboratory: Lemont, IL, USA, 1978. [Google Scholar]
- Fratello, V.J.; Brandle, C.D. Physical properties of a Y3Al5O12 melt. J. Cryst. Growth 1993, 128, 1006–1010. [Google Scholar] [CrossRef]
- Trávníček, P.; Burg, P.; Krakowiak-Bal, A.; Junga, P.; Vítěz, T.; Ziemiańczyk, U. Study of rheological behaviour of wines. Int. Agrophysics 2016, 30, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.F.; Pask, J.A. Diffusion and Reaction Studies in the System Al2O3-SiO2. J. Am. Ceram. Soc. 1972, 55, 525–531. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cussler, E.L. Diffusion—Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Feichtinger, S.; Michelic, S.K.; Kang, Y.B.; Bernhard, C. In situ observation of the dissolution of SiO2 particles in CaO-Al2O3-SiO2 slags and mathematical analysis of its dissolution pattern. J. Am. Ceram. Soc. 2014, 97, 316–325. [Google Scholar] [CrossRef]
- Maroufi, S.; Ciezki, G.; Jahanshahi, S.; Sun, S.; Ostrovski, O. Dissolution of silica in slag in silicomanganese production. In Proceedings of the Fourteenth International Ferroalloys Congress, Kiev, Ukraine, 31 May–4 June 2015; pp. 479–487. [Google Scholar]
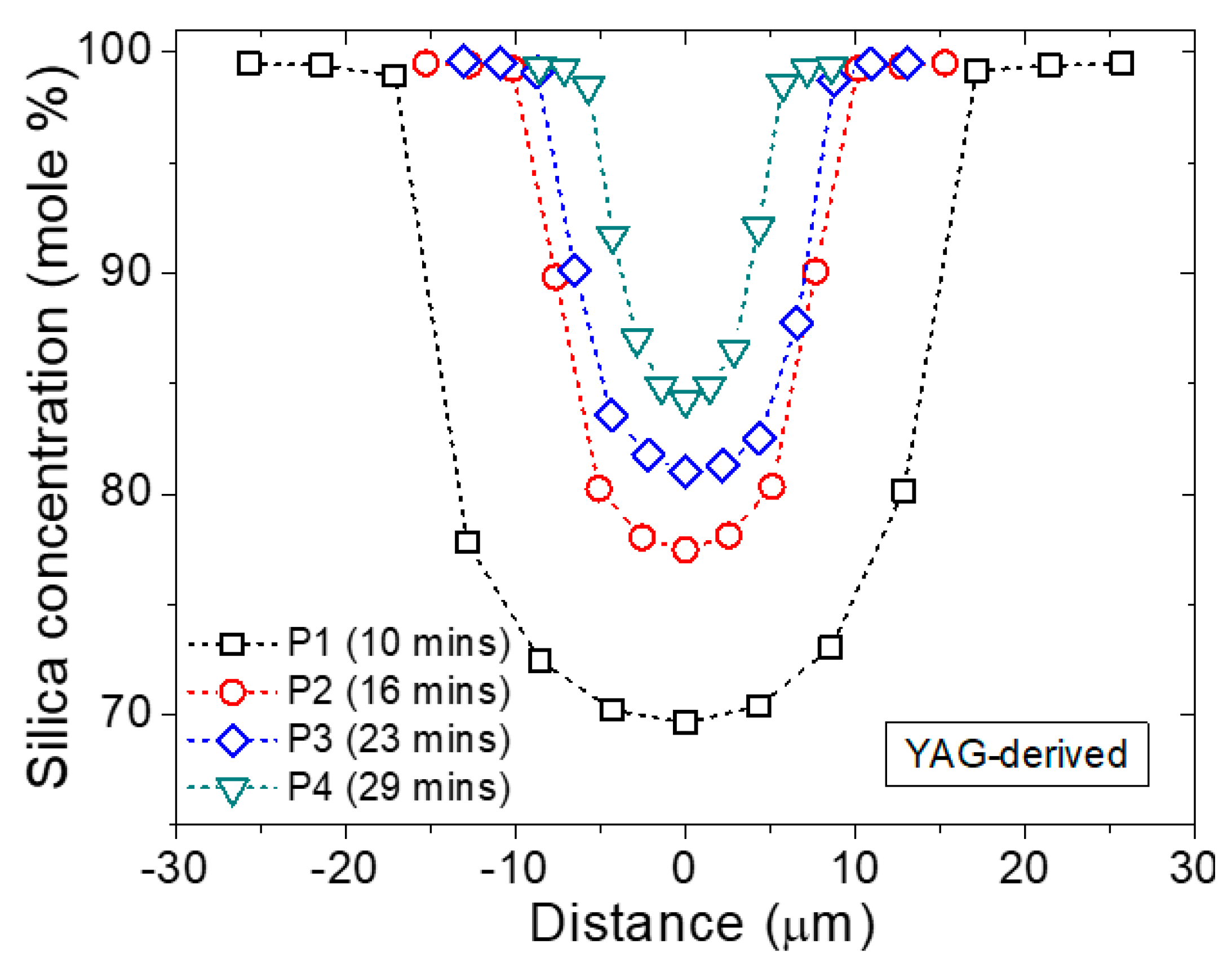
| Segment | Position during Draw (m) | Time during Draw (min) | Core/Cladding Diameters | SiO2 | Al2O3 | Y2O3 | Er2O3 |
|---|---|---|---|---|---|---|---|
| P1 | 0 | 10.0 | 28/136 | 69.6 | 19.1 | 11.1 | 0.2 |
| P2 | 180 | 16.0 | 16/123 | 77.5 | 13.2 | 9.1 | 0.2 |
| P3 | 380 | 22.7 | 14.5/128 | 81.0 | 10.9 | 7.9 | 0.2 |
| P4 | 560 | 28.7 | 9.5/128 | 84.3 | 9.1 | 6.5 | 0.1 |
| Material | Viscosity (Pa.s) | References |
|---|---|---|
| SiO2 (2000 °C) | ~45,000 a | [14] |
| SiO2 (2100 °C) | ~15,000 a | [14] |
| 1Al2O3-3SiO2 (2000 °C) | ~15,000 b | [14] |
| 1Al2O3-1SiO2 (2000 °C) | ~0.5 b | [14] |
| 3Al2O3-1SiO2 (2000 °C) | ~0.1 b | [14] |
| Al2O3 (2120–2220 °C) | ~0.03–0.025 | [15] |
| YAG (1970–2070 °C) | ~0.04–0.045 | [16] |
| Pinot Blanc (12 °C) | ~0.003 | [17] |
| Parameter Symbol | Description/Definition |
|---|---|
| R | Fiber core radius at time t. |
| R0 | Initial fiber core radius (at time t = 0 s) |
| Rs | Fiber core radius at saturation, i.e., over long length |
| Reff | Effective preform radius in the active region, Reff = deff/2 |
| k | SiO2 dissolution constant, , in·s−1 |
| S | Surface of the active region, |
| D | Diffusion coefficient, in cm2·s−1 |
| V | Volume of the active region, |
| L | Heat-zone length (40 mm) |
| cm | Silica concentration (in mol %) at the fiber core center |
| cs | Silica concentration (in mol %) at saturation, i.e., over long length |
| r | Radial distance from center (r = 0) to the effective preform radius (r = Reff) |
| cR | Silica concentration (in mol %) along the radial profile |
| csurf | Silica concentration (in mol %) at the core/cladding interface, and set to 100% |
| tx | Time for the preform to go through the active region |
| J0, J1, αn | Bessel functions of the first kind of zeroth order (J0) and first order (J1); αn is the n-th zero of J0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavillon, M.; Dragic, P.; Faugas, B.; Hawkins, T.W.; Ballato, J. Insights and Aspects to the Modeling of the Molten Core Method for Optical Fiber Fabrication. Materials 2019, 12, 2898. https://doi.org/10.3390/ma12182898
Cavillon M, Dragic P, Faugas B, Hawkins TW, Ballato J. Insights and Aspects to the Modeling of the Molten Core Method for Optical Fiber Fabrication. Materials. 2019; 12(18):2898. https://doi.org/10.3390/ma12182898
Chicago/Turabian StyleCavillon, Maxime, Peter Dragic, Benoit Faugas, Thomas W. Hawkins, and John Ballato. 2019. "Insights and Aspects to the Modeling of the Molten Core Method for Optical Fiber Fabrication" Materials 12, no. 18: 2898. https://doi.org/10.3390/ma12182898
APA StyleCavillon, M., Dragic, P., Faugas, B., Hawkins, T. W., & Ballato, J. (2019). Insights and Aspects to the Modeling of the Molten Core Method for Optical Fiber Fabrication. Materials, 12(18), 2898. https://doi.org/10.3390/ma12182898





