Nanosilicon-Based Composites for (Bio)sensing Applications: Current Status, Advantages, and Perspectives
Abstract
1. Introduction
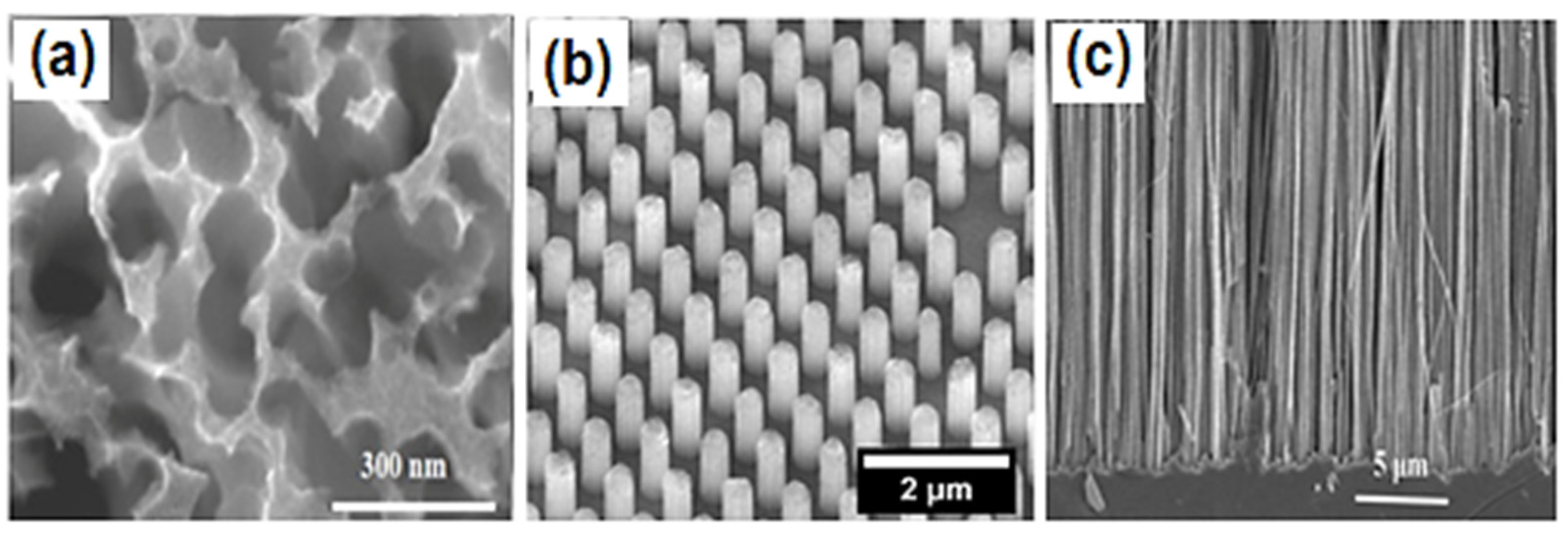
2. Types of Nano-Si Morphology and Methods of Fabrication
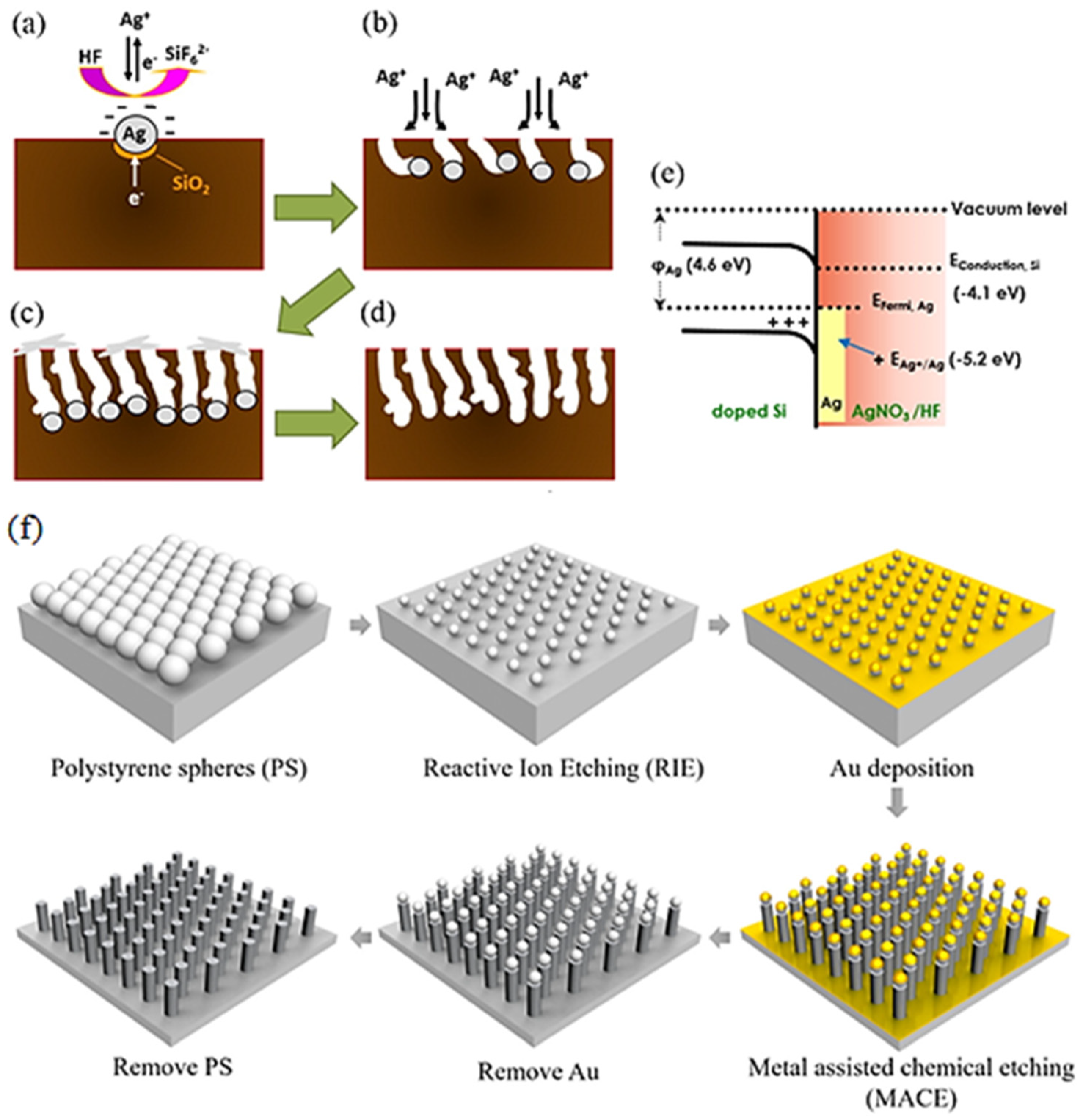
2.1. Porous Silicon (PSi)
2.2. Silicon Nanowires (SiNWs)
2.3. Silicon Nanopillars (SiNPs)
3. (Bio)sensors Based on PSi, SiNWs, SiNPs and Their Composites with Polymers
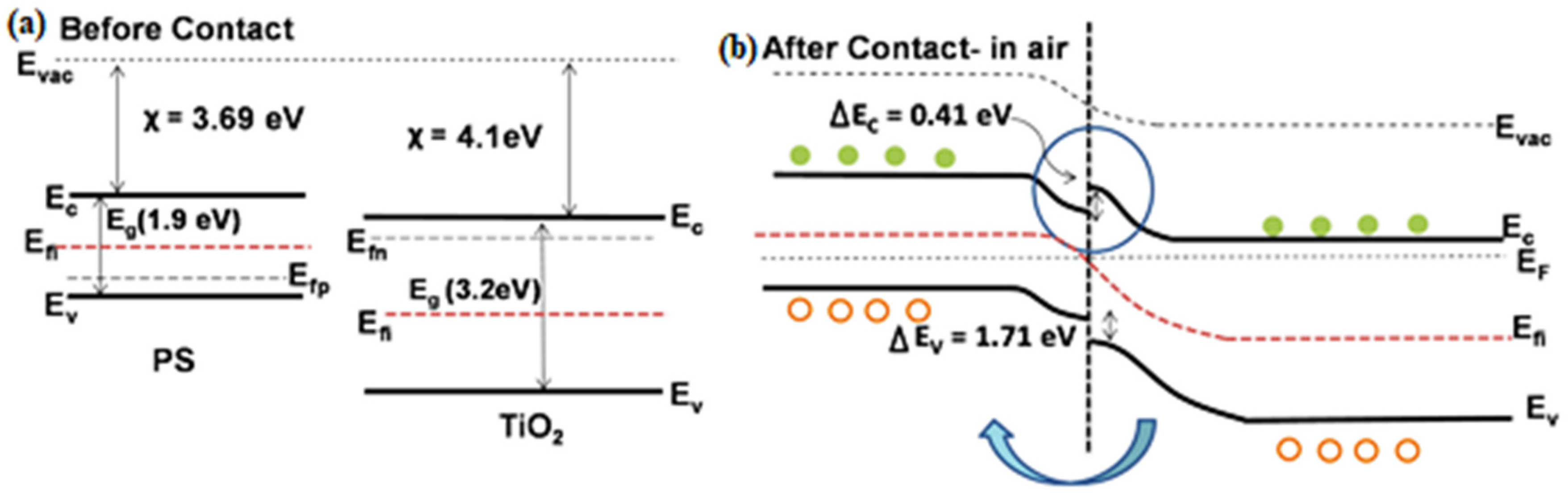
4. (Bio)sensors Based on Nano-Si and Metals Oxides Nanocomposites
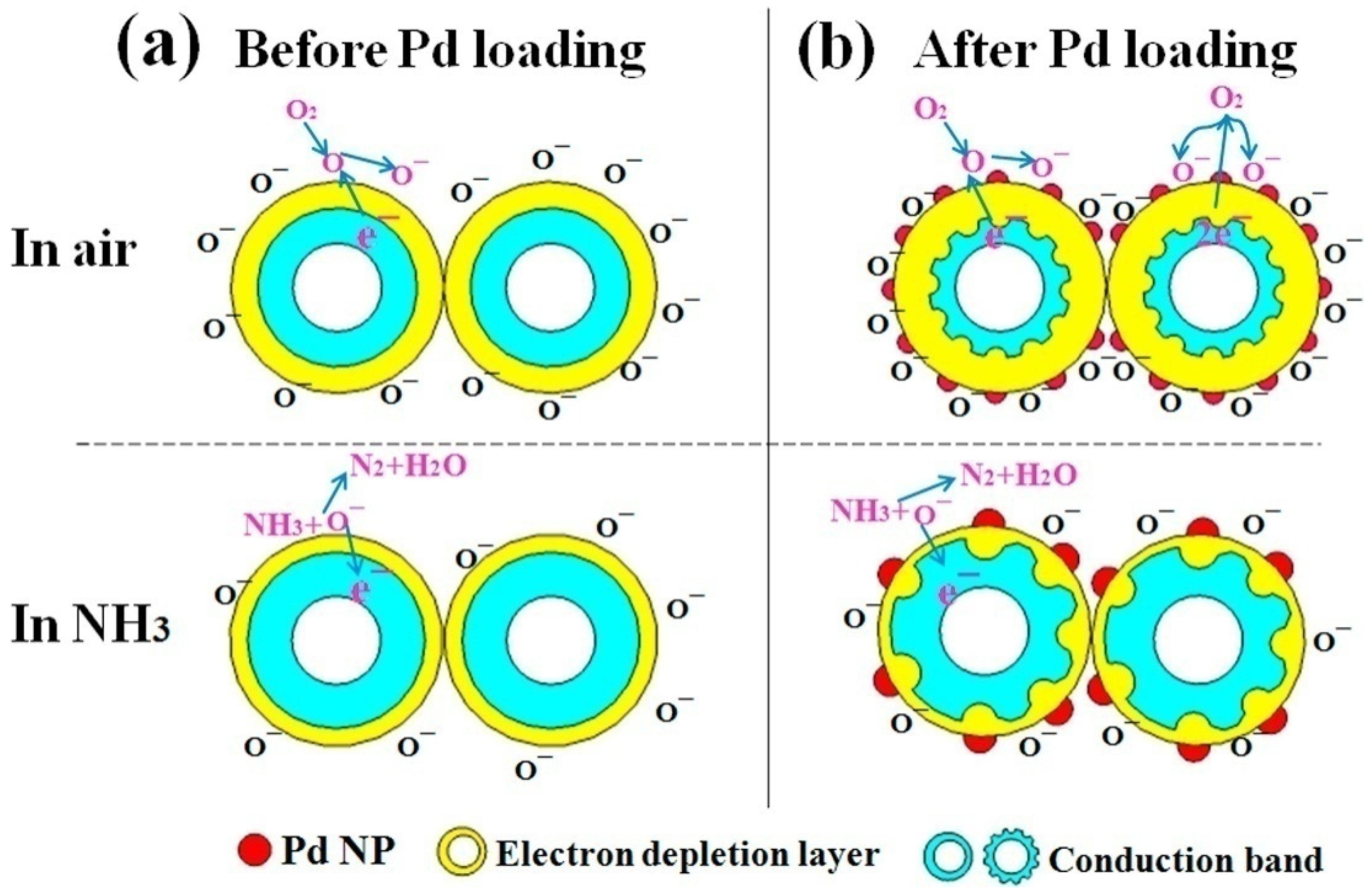
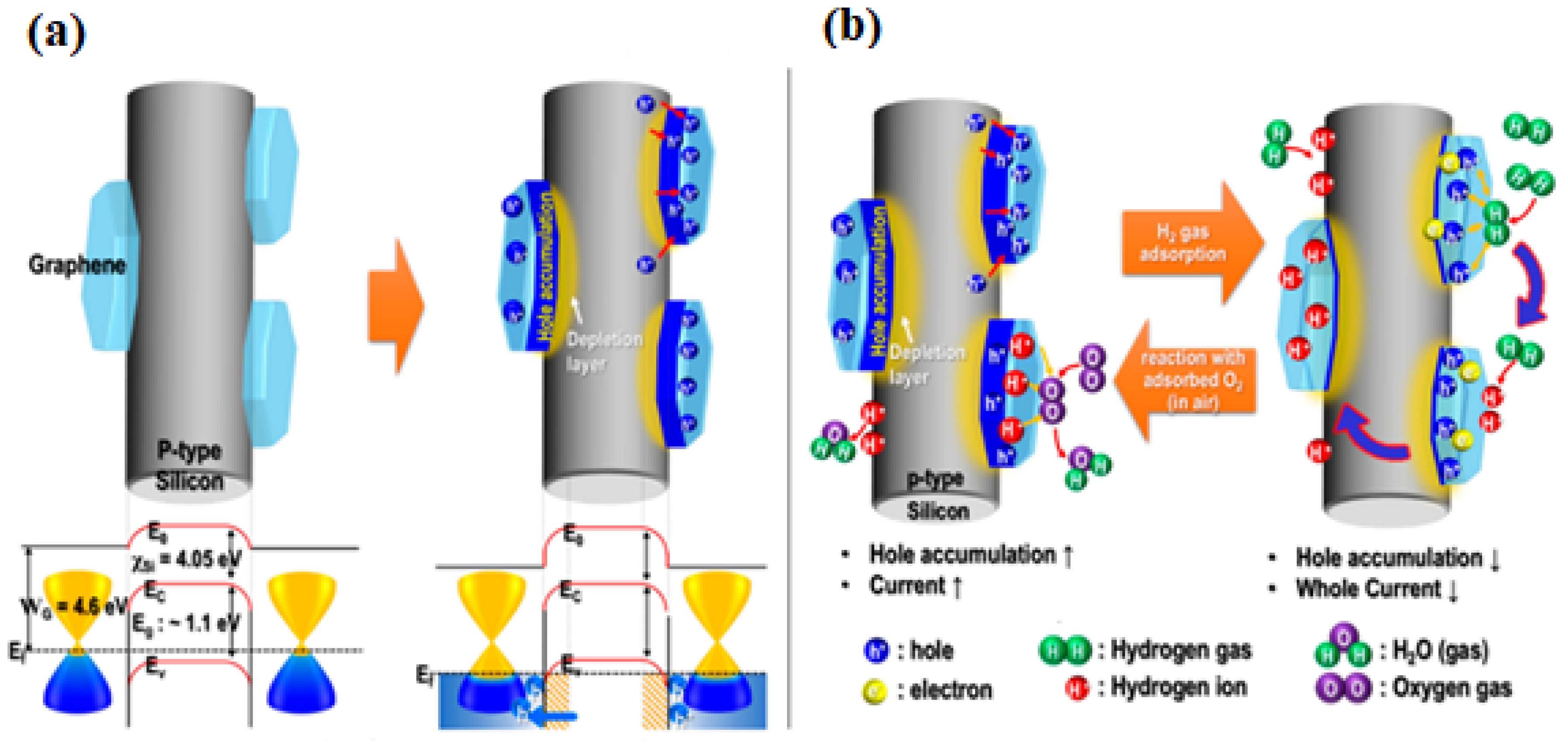
- In the case of the PSi/WO3 nanocomposite, the sensing mechanism directly depends on the heterojunction parameters and efficiency of O2 absorption-desorption;
- PSi/WO3 substrates decorated with Pd NPs would possess enhanced catalytic activity that will lead to enhanced dissociation of oxygen molecules O2 and absorption of oxygen ions O− on the PSi/WO3/Pd surface. More ion absorbed oxygen on the surface would provide more sensing sites, leading to enhanced gas response and reaction rate.
- Additionally, the work function of Pd was larger than that of WO3, therefore the electrons from WO3 will transfer to Pd, causing the generation of the Schottky barrier at the interface between Pd and WO3. By these reasons, the conduction band of PSi/WO3/Pd will become much narrower when compared with WO3 and the concentration of the conduction electrons will be reduced. As a consequence, the interaction of NH3 molecules with the PSi/WO3/Pd substrate will lead to more significant resistance variation and higher sensor response.
5. (Bio)sensors Based on Nano-silicon and Metals Nanoparticles
6. (Bio)sensors Based on Nano-Si and Carbon-based Nanomaterials
7. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Uhlir, A. Electrolytic Shaping of Germanium and Silicon. Bell Syst. Tech. J. 1956, 35, 333–347. [Google Scholar] [CrossRef]
- Cullis, A.G.; Canham, L.T. Visible light emission due to quantum size effects in highly porous crystalline silicon. Nature 1991, 353, 335–338. [Google Scholar] [CrossRef]
- Myndrul, V.; Viter, R.; Savchuk, M.; Shpyrka, N.; Erts, D.; Jevdokimovs, D.; Silamiķelis, V.; Smyntyna, V.; Ramanavicius, A.; Iatsunskyi, I. Porous silicon based photoluminescence immunosensor for rapid and highly-sensitive detection of Ochratoxin A. Biosens. Bioelectron. 2018, 102, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Iatsunskyi, I.; Nowaczyk, G.; Jurga, S.; Fedorenko, V.; Pavlenko, M.; Smyntyna, V. One and two-phonon Raman scattering from nanostructured silicon. Optik 2015, 126, 1650–1655. [Google Scholar] [CrossRef]
- Brytavskyi, I.; Hušeková, K.; Myndrul, V.; Pavlenko, M.; Coy, E.; Zaleski, K.; Gregušová, D.; Yate, L.; Smyntyna, V.; Iatsunskyi, I. Effect of porous silicon substrate on structural, mechanical and optical properties of MOCVD and ALD ruthenium oxide nanolayers. Appl. Surf. Sci. 2019, 471, 686–693. [Google Scholar] [CrossRef]
- Pavlenko, M.; Coy, E.L.; Jancelewicz, M.; Załęski, K.; Smyntyna, V.; Jurga, S.; Iatsunskyi, I. Enhancement of optical and mechanical properties of Si nanopillars by ALD TiO2 coating. RSC Adv. 2016, 6, 97070–97076. [Google Scholar] [CrossRef]
- Pavlenko, M.; Myndrul, V.; Iatsunskyi, I.; Jurga, S.; Smyntyna, V. Study on structural and optical properties of TiO2 ALD coated silicon nanostructures. In Proceedings of the Nanophotonics VI, Brussels, Belgium, 3–7 April 2016; Andrews, D.L., Nunzi, J.-M., Ostendorf, A., Eds.; SPIE: Bellingham, DC, USA, 2016; p. 98842H. [Google Scholar]
- Graniel, O.; Fedorenko, V.; Viter, R.; Iatsunskyi, I.; Nowaczyk, G.; Weber, M.; Załęski, K.; Jurga, S.; Smyntyna, V.; Miele, P.; et al. Optical properties of ZnO deposited by atomic layer deposition (ALD) on Si nanowires. Mater. Sci. Eng. B 2018, 236, 139–146. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef]
- Pavlenko, M.; Siuzdak, K.; Coy, E.; Jancelewicz, M.; Jurga, S.; Iatsunskyi, I. Silicon/TiO2 core-shell nanopillar photoanodes for enhanced photoelectrochemical water oxidation. Int. J. Hydrogen Energy 2017, 42, 30076–30085. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, Z.; Cui, Y. Optimal design of an antireflection coating structure for enhancing the energy-conversion efficiency of a silicon nanostructure solar cell. RSC Adv. 2018, 8, 34793–34807. [Google Scholar] [CrossRef]
- Hakonen, A.; Rindzevicius, T.; Schmidt, M.S.; Andersson, P.O.; Juhlin, L.; Svedendahl, M.; Boisen, A.; Käll, M. Detection of nerve gases using surface-enhanced Raman scattering substrates with high droplet adhesion. Nanoscale 2016, 8, 1305–1308. [Google Scholar] [PubMed]
- Myndrul, V.; Viter, R.; Savchuk, M.; Koval, M.; Starodub, N.; Silamiķelis, V.; Smyntyna, V.; Ramanavicius, A.; Iatsunskyi, I. Gold coated porous silicon nanocomposite as a substrate for photoluminescence-based immunosensor suitable for the determination of Aflatoxin B1. Talanta 2017, 175, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar]
- Qiang, X.; Hu, M.; Zhao, B.; Qin, Y.; Yang, R.; Zhou, L.; Qin, Y. Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature. Materials 2018, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Mohammadnejad Arough, J.; Yaghoobi, M.; Davoodi, H.; Sepehri, F.; Amirabadizadeh, M. A novel approach for osteocalcin detection by competitive ELISA using porous silicon as a substrate. Biotechnol. Appl. Biochem. 2017, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Shahkarami, M.M.H.; Koohsorkhi, J.; Fard, H.G. Fabrication of High Sensitive UV Photodetector Based on n-ZnO Nanowire/n-Porous-Si Heterojunction. Nano 2017, 12, 1–9. [Google Scholar]
- Syshchyk, O.; Skryshevsky, V.A.; Soldatkin, O.O.; Soldatkin, A.P. Enzyme biosensor systems based on porous silicon photoluminescence for detection of glucose, urea and heavy metals. Biosens. Bioelectron. 2015, 66, 89–94. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Myndrul, V.; Smyntyna, V.; Viter, R.; Melnyk, Y.; Pavlova, K. Porous silicon photoluminescence biosensor for rapid and sensitive detection of toxins. In Proceedings of the Organic Sensors and Bioelectronics X, San Diego, CA, USA, 6–10 August 2017; Shinar, R., Kymissis, I., Torsi, L., Eds.; SPIE: Bellingham, DC, USA, 2017; p. 28. [Google Scholar]
- Huang, J.; Ma, D.; Chen, F.; Bai, M.; Xu, K.; Zhao, Y. Ag Nanoparticles Decorated Cactus-Like Ag Dendrites/Si Nanoneedles as Highly Efficient 3D Surface-Enhanced Raman Scattering Substrates toward Sensitive Sensing. Anal. Chem. 2015, 87, 10527–10534. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Faisal, M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Organic analytes sensitivity in meso-porous silicon electrical sensor with front side and backside contacts. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Basu, D.; Sarkar, T.; Sen, K.; Hossain, S.M.; Das, J. Multi-Parametric Optical Glucose Sensor Based on Surface Functionalized Nano-Porous Silicon. IEEE Sens. J. 2018, 18, 9940–9947. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Smyntyna, V.; Pavlenko, M.; Kanevska, O.; Kirik, Y.; Myndrul, V. Ammonia detection using optical reflectance from porous silicon formed by metal-assisted chemical etching. In Proceedings of the SPIE—The International Society for Optical Engineering, Dresden, Germany, 23–26 September 2013; Zamboni, R., Kajzar, F., Szep, A.A., Burgess, D., Owen, G., Eds.; SPIE: Bellingham, DC, USA, 2013; Volume 8901, p. 89010K. [Google Scholar]
- Al-Salman, H.S.; Abdullah, M.J. Preparation of ZnO nanostructures by RF-magnetron sputtering on thermally oxidized porous silicon substrate for VOC sensing application. Meas. J. Int. Meas. Confed. 2015, 59, 248–257. [Google Scholar] [CrossRef]
- Wang, L.L.; Kang, L.P.; Wang, H.Y.; Chen, Z.P.; Li, X.J. Capacitive humidity sensitivity of SnO2: Sn thin film grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2016, 229, 513–519. [Google Scholar] [CrossRef]
- Liu, R.; Li, W.; Cai, T.; Deng, Y.; Ding, Z.; Liu, Y.; Zhu, X.; Wang, X.; Liu, J.; Liang, B.; et al. TiO2 Nanolayer-Enhanced Fluorescence for Simultaneous Multiplex Mycotoxin Detection by Aptamer Microarrays on a Porous Silicon Surface. ACS Appl. Mater. Interfaces 2018, 10, 14447–14453. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, D.; Wang, Z.; Jiang, Y. Ag nanoparticles-functionalized rough silicon nanowires array and its unique response characteristics to ultrararefied NO2. Sens. Actuators B Chem. 2018, 258, 730–738. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Jancelewicz, M.; Nowaczyk, G.; Kempiński, M.; Peplińska, B.; Jarek, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Atomic layer deposition TiO2 coated porous silicon surface: Structural characterization and morphological features. Thin Solid Films 2015, 589, 303–308. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Pavlenko, M.; Viter, R.; Jancelewicz, M.; Nowaczyk, G.; Baleviciute, I.; Załęski, K.; Jurga, S.; Ramanavicius, A.; Smyntyna, V. Tailoring the structural, optical, and photoluminescence properties of porous silicon/TiO2 nanostructures. J. Phys. Chem. C 2015, 119, 7164–7171. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Nowaczyk, G.; Jancelewicz, M.; Pavlenko, M.; Załeski, K.; Jurga, S. Structural and XPS studies of PSi/TiO2 nanocomposites prepared by ALD and Ag-assisted chemical etching. Appl. Surf. Sci. 2015, 347, 777–783. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.L.; Huang, J.A.; Zhang, Z.; Chen, X.; Zhang, W. Plasmonic nanopillar array embedded microfluidic chips: An in situ SERS monitoring platform. J. Mater. Chem. A 2015, 3, 6408–6413. [Google Scholar] [CrossRef]
- Bryche, J.F.; Bélier, B.; Bartenlian, B.; Barbillon, G. Low-cost SERS substrates composed of hybrid nanoskittles for a highly sensitive sensing of chemical molecules. Sens. Actuators B Chem. 2017, 239, 795–799. [Google Scholar] [CrossRef]
- Bandarenka, H.; Girel, K.; Zavatski, S.; Panarin, A.; Terekhov, S. Progress in the Development of SERS-Active Substrates Based on Metal-Coated Porous Silicon. Materials 2018, 11, 852. [Google Scholar] [CrossRef]
- Dwivedi, P.; Dhanekar, S.; Das, S.; Chandra, S. Effect of TiO2 Functionalization on Nano-Porous Silicon for Selective Alcohol Sensing at Room Temperature. J. Mater. Sci. Technol. 2017, 33, 516–522. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Liu, S.; Tan, M.; Li, D.; Zhu, Y. Electrochemical synthesis of ZnO nanorods/porous silicon composites and their gas-sensing properties at room temperature. J. Solid State Electrochem. 2016, 20, 459–468. [Google Scholar]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Porous silicon filled with Pd/WO3-ZnO composite thin film for enhanced H2 gas-sensing performance. RSC Adv. 2017, 7, 39666–39675. [Google Scholar] [CrossRef]
- Yan, W.; Hu, M.; Wang, D.; Li, C. Room temperature gas sensing properties of porous silicon/V2O5 nanorods composite. Appl. Surf. Sci. 2015, 346, 216–222. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Wang, G.; Hong, M. Hybrid structures of Fe3O4 and Ag nanoparticles on Si nanopillar arrays substrate for SERS applications. Mater. Chem. Phys. 2018, 214, 377–382. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Wang, G.; Liao, J.; Lv, S.; Zhu, Z. Highly enhanced response of MoS2/porous silicon nanowire heterojunctions to NO2 at room temperature. RSC Adv. 2018, 8, 11070–11077. [Google Scholar] [CrossRef]
- Feng, M.H.; Wang, W.C.; Li, X.J. Capacitive humidity sensing properties of CdS/ZnO sesame-seed-candy structure grown on silicon nanoporous pillar array. J. Alloy. Compd. 2017, 698, 94–98. [Google Scholar] [CrossRef]
- Visser, D.; Choudhury, B.D.; Krasovska, I.; Anand, S. Refractive index sensing in the visible/NIR spectrum using silicon nanopillar arrays. Opt. Express 2017, 25, 12171. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Jurga, S.; Smyntyna, V.; Pavlenko, M.; Myndrul, V.; Zaleska, A. Raman spectroscopy of nanostructured silicon fabricated by metal-assisted chemical etching. In Proceedings of the SPIE—The International Society for Optical Engineering, Brussels, Belgium, 13–17 April 2014; Gorecki, C., Asundi, A.K., Osten, W., Eds.; SPIE: Bellingham, DC, USA, 2014; Volume 9132, p. 913217. [Google Scholar]
- Lv, J.; Zhang, T.; Zhang, P.; Zhao, Y.; Li, S. Review Application of Nanostructured Black Silicon. Nanoscale Res. Lett. 2018, 13, 110. [Google Scholar] [CrossRef]
- Zhao, X.; Alizadeh, M.H.; Reinhard, B.M. Harnessing Leaky Modes for Fluorescence Enhancement in Gold-Tipped Silicon Nanowires. J. Phys. Chem. C 2016, 120, 20555–20562. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Liu, Y.; Zhang, X. KOH post-etching-induced rough silicon nanowire array for H2 gas sensing application. Nanotechnology 2016, 27, 465502. [Google Scholar] [CrossRef] [PubMed]
- Albuschies, J.; Baus, M.; Winkler, O.; Hadam, B.; Spangenberg, B.; Kurz, H. High-density silicon nanowire growth from self-assembled Au nanoparticles. Microelectron. Eng. 2006, 83, 1530–1533. [Google Scholar] [CrossRef]
- Liu, M.; Jin, P.; Xu, Z.; Hanaor, D.A.H.; Gan, Y.; Chen, C.Q. Two-dimensional modeling of the self-limiting oxidation in silicon and tungsten nanowires. Theor. Appl. Mech. Lett. 2016, 6, 195–199. [Google Scholar] [CrossRef]
- Cornago, I.; Hernández, A.L.; Casquel, R.; Holgado, M.; Laguna, M.F.; Sanza, F.J.; Bravo, J. Bulk sensing performance comparison between silicon dioxide and resonant high aspect ratio nanopillars arrays fabricated by means of interference lithography. Opt. Mater. Express 2016, 6, 2264. [Google Scholar] [CrossRef]
- Li, W.; Ding, C.; Cai, Y.; Liu, J.; Wang, L.; Ren, Q.; Xu, J. Enhanced Humidity Sensitivity with Silicon Nanopillar Array by UV Light. Sensors 2018, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Liu, Y.R.; Chen, C.Y. Highly-antireflective porous Si films prepared with metal-assisted chemical etching. Surf. Coat. Technol. 2016, 303, 232–236. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Zhu, L.S.; Zhang, J.; Xu, X.W.; Yu, Y.Z.; Wu, X.; Yang, T.; Wang, X.H. Room temperature H2 detection based on Pd/SiNWs/p-Si Schottky diode structure. Sens. Actuators B Chem. 2016, 227, 515–523. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Luo, Q.; Liu, J.; Wu, J. Bacteria detection based on its blockage effect on silicon nanopore array. Biosens. Bioelectron. 2016, 79, 715–720. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Paratore, F.; Scheper, T.; Bercovici, M.; Segal, E. On Chip Protein Pre-Concentration for Enhancing the Sensitivity of Porous Silicon Biosensors. ACS Sens. 2017, 2, 1767–1773. [Google Scholar] [CrossRef]
- Vilensky, R.; Bercovici, M.; Segal, E. Oxidized Porous Silicon Nanostructures Enabling Electrokinetic Transport for Enhanced DNA Detection. Adv. Funct. Mater. 2015, 25, 6725–6732. [Google Scholar] [CrossRef]
- Massad-Ivanir, N.; Shtenberg, G.; Raz, N.; Gazenbeek, C.; Budding, D.; Bos, M.P.; Segal, E. Porous Silicon-Based Biosensors: Towards Real-Time Optical Detection of Target Bacteria in the Food Industry. Sci. Rep. 2016, 6, 38099. [Google Scholar] [CrossRef] [PubMed]
- Urmann, K.; Reich, P.; Walter, J.G.; Beckmann, D.; Segal, E.; Scheper, T. Rapid and label-free detection of protein a by aptamer-tethered porous silicon nanostructures. J. Biotechnol. 2017, 257, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jia, Z.; Lü, X.; Liu, Y.; Ning, X.; Mo, J.; Wang, J. Spectrometer-free biological detection method using porous silicon microcavity devices. Opt. Express 2015, 23, 24626. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mao, H.; Han, X.; Xu, J.; Wang, W. Fabrication and characterization of SiO2/Si heterogeneous nanopillar arrays. Nanotechnology 2016, 27, 305301. [Google Scholar] [CrossRef] [PubMed]
- Serre, P.; Stambouli, V.; Weidenhaupt, M.; Baron, T.; Ternon, C. Silicon nanonets for biological sensing applications with enhanced optical detection ability. Biosens. Bioelectron. 2015, 68, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, G.; Massad-Ivanir, N.; Segal, E. Detection of trace heavy metal ions in water by nanostructured porous Si biosensors. Analyst 2015, 140, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gaur, G.; Retterer, S.T.; Laibinis, P.E.; Weiss, S.M. Flow-through porous silicon membranes for real-time label-free biosensing. Anal. Chem. 2016, 88, 10940–10948. [Google Scholar] [CrossRef]
- Sola-Rabada, A.; Sahare, P.; Hickman, G.J.; Vasquez, M.; Canham, L.T.; Perry, C.C.; Agarwal, V. Biogenic porous silica and silicon sourced from Mexican Giant Horsetail (Equisetum myriochaetum) and their application as supports for enzyme immobilization. Colloids Surf. B Biointerfaces 2018, 166, 195–202. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jia, Z.H.; Wang, J.J.; Lü, C.W. Biological reaction signal enhancement in porous silicon Bragg mirror based on quantum dots fluorescence. Optoelectron. Lett. 2017, 13, 172–174. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Jia, Z. Detection of Ammonia-Oxidizing Bacteria (AOB) Using a Porous Silicon Optical Biosensor Based on a Multilayered Double Bragg Mirror Structure. Sensors 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Z.; Lv, G.; Wen, H.; Li, P.; Zhang, H.; Wang, J. Detection of Echinococcus granulosus antigen by a quantum dot/porous silicon optical biosensor. Biomed. Opt. Express 2017, 8, 3458. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Robbiano, V.; Strambini, L.M.; Debrassi, A.; Egri, G.; Dähne, L.; Barillaro, G. Layer-by-layer biofunctionalization of nanostructured porous silicon for high-sensitivity and high-selectivity label-free affinity biosensing. Nat. Commun. 2018, 9, 5256. [Google Scholar] [CrossRef]
- Mariani, S.; Pino, L.; Strambini, L.M.; Tedeschi, L.; Barillaro, G. 10000-Fold Improvement in Protein Detection Using Nanostructured Porous Silicon Interferometric Aptasensors. ACS Sens. 2016, 1, 1471–1479. [Google Scholar] [CrossRef]
- Mariani, S.; Strambini, L.; Tedeschi, L.; Barillaro, G. Porous silicon interferometers for high-sensitivity label-free detection of biomolecules. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–3. [Google Scholar]
- Mariani, S.; Strambini, L.M.; Tedeschi, L.; Barillaro, G. Interferogram Average over Wavelength Spectroscopy: An Ultrasensitive Technique for Biosensing with Porous Silicon Interferometers. ECS Trans. 2017, 77, 1815–1823. [Google Scholar] [CrossRef]
- Luan, E.; Yun, H.; Laplatine, L.; Dattner, Y.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Enhanced Sensitivity of Subwavelength Multibox Waveguide Microring Resonator Label-Free Biosensors. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Ramakrishan, S.K.; Martin Fernandez, M.; Cloitre, T.; Agarwal, V.; Cuisinier, F.J.G.; Gergely, C. Porous silicon microcavities redefine colorimetric ELISA sensitivity for ultrasensitive detection of autoimmune antibodies. Sens. Actuators B Chem. 2018, 272, 211–218. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Q.; Wu, J. Label-free discrimination of membrane-translocating peptides on porous silicon microfluidic biosensors. Biomicrofluidics 2016, 10, 064113. [Google Scholar] [CrossRef]
- Piya, R.; Zhu, Y.; Soeriyadi, A.H.; Silva, S.M.; Reece, P.J.; Gooding, J.J. Micropatterning of porous silicon Bragg reflectors with poly (ethylene glycol) to fabricate cell microarrays: Towards single cell sensing. Biosens. Bioelectron. 2019, 127, 229–235. [Google Scholar] [CrossRef]
- Chhasatia, R.; Sweetman, M.J.; Harding, F.J.; Waibel, M.; Kay, T.; Thomas, H.; Loudovaris, T.; Voelcker, N.H. Non-invasive, in vitro analysis of islet insulin production enabled by an optical porous silicon biosensor. Biosens. Bioelectron. 2017, 91, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Reta, N.; Michelmore, A.; Saint, C.; Prieto-Simón, B.; Voelcker, N.H. Porous silicon membrane-modified electrodes for label-free voltammetric detection of MS2 bacteriophage. Biosens. Bioelectron. 2016, 80, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Jenie, S.N.A.; Prieto-Simon, B.; Voelcker, N.H. Development of L-lactate dehydrogenase biosensor based on porous silicon resonant microcavities as fluorescence enhancers. Biosens. Bioelectron. 2015, 74, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Tücking, K.S.; Vasani, R.B.; Cavallaro, A.A.; Voelcker, N.H.; Schönherr, H.; Prieto-Simon, B. Hyaluronic Acid–Modified Porous Silicon Films for the Electrochemical Sensing of Bacterial Hyaluronidase. Macromol. Rapid Commun. 2018, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Armenia, I.; Balzaretti, R.; Pirrone, C.; Allegretti, C.; D’Arrigo, P.; Valentino, M.; Gornati, R.; Bernardini, G.; Pollegioni, L. L-Aspartate Oxidase Magnetic Nanoparticles: Synthesis, Characterization and L-Aspartate Bioconversion. RSC Adv. 2017, 7, 21136–21143. [Google Scholar] [CrossRef]
- Nayef, U.M.; Khudhair, I.M. Study of porous silicon humidity sensor vapors by photoluminescence quenching for organic solvents. Opt. Int. J. Light Electron Opt. 2017, 135, 169–173. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Plush, S.E.; Voelcker, N.H. Singlet Oxygen Detection on a Nanostructured Porous Silicon Thin Film via Photonic Luminescence Enhancements. Langmuir 2017, 33, 8606–8613. [Google Scholar] [CrossRef]
- Kayahan, E. Porous silicon based CO2 sensors with high sensitivity. Optik (Stuttg) 2018, 164, 271–276. [Google Scholar] [CrossRef]
- Georgobiani, V.A.; Gonchar, K.A.; Zvereva, E.A.; Osminkina, L.A. Porous Silicon Nanowire Arrays for Reversible Optical Gas Sensing. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–5. [Google Scholar] [CrossRef]
- Shi, Y.M.; Rong, G.G.; Wang, D.N.; Zhang, S.L.; Zhu, Y.X. A Label-Free Biosensor Based on Nanoscale Porous Silicon Thin Film for Tuberculosis Detection. Adv. Mater. Res. 2014, 1082, 555–561. [Google Scholar] [CrossRef]
- Urmann, K.; Walter, J.G.; Scheper, T.; Segal, E. Label-free optical biosensors based on aptamer-functionalized porous silicon scaffolds. Anal. Chem. 2015, 87, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Soeriyadi, A.H.; Guan, B.; Reece, P.J.; Gooding, J.J. The analytical performance of a porous silicon Bloch surface wave biosensors as protease biosensor. Sens. Actuators B Chem. 2015, 211, 469–475. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Borbone, N.; Politi, J.; Oliviero, G.; Nici, F.; Casalino, M.; Piccialli, G.; Dardano, P.; Varra, M.; et al. Solid phase synthesis of a thrombin binding aptamer on macroporous silica for label free optical quantification of thrombin. RSC Adv. 2016, 6, 86762–86769. [Google Scholar] [CrossRef]
- Mariani, S.; Strambini, L.M.; Barillaro, G. Femtomole Detection of Proteins Using a Label-Free Nanostructured Porous Silicon Interferometer for Perspective Ultrasensitive Biosensing. Anal. Chem. 2016, 88, 8502–8509. [Google Scholar] [CrossRef]
- Urmann, K.; Arshavsky-Graham, S.; Walter, J.G.; Scheper, T.; Segal, E. Whole-cell detection of live: Lactobacillus acidophilus on aptamer-decorated porous silicon biosensors. Analyst 2016, 141, 5432–5440. [Google Scholar] [CrossRef] [PubMed]
- Azuelos, P.; Girault, P.; Lorrain, N.; Dumeige, Y.; Bodiou, L.; Poffo, L.; Guendouz, M.; Thual, M.; Charrier, J. Optimization of porous silicon waveguide design for micro-ring resonator sensing applications. J. Opt. 2018, 20, 085301. [Google Scholar] [CrossRef]
- Rahimi, F.; Fardindoost, S.; Ansari-Pour, N.; Sepehri, F.; Makiyan, F.; Shafiekhani, A.; Rezayan, A.H. Optimization of porous silicon conditions for DNA-based biosensing via reflectometric interference spectroscopy. Cell J. 2019, 20, 584–591. [Google Scholar] [PubMed]
- Mariani, S.; Strambini, L.M.; Paghi, A.; Barillaro, G. Low-Concentration Ethanol Vapor Sensing with Nanostructured Porous Silicon Interferometers Using Interferogram Average over Wavelength Reflectance Spectroscopy. IEEE Sens. J. 2018, 18, 7842–7849. [Google Scholar] [CrossRef]
- Bui, H.; Pham, V.H.; Pham, V.D.; Hoang, T.H.C.; Pham, T.B.; Do, T.C.; Ngo, Q.M.; Nguyen, T. Van Determination of low solvent concentration by nano-porous silicon photonic sensors using volatile organic compound method. Environ. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, R.; Martín Sánchez, D.; Ponce Alcántara, S.; Prats Quilez, F.; Torrijos Morán, L.; García-Rupérez, J. Real-Time and In-Flow Sensing Using a High Sensitivity Porous Silicon Microcavity-Based Sensor. Sensors 2017, 17, 2813. [Google Scholar] [CrossRef]
- Zhao, Y.; Gaur, G.; Mernaugh, R.L.; Laibinis, P.E.; Weiss, S.M. Comparative Kinetic Analysis of Closed-Ended and Open-Ended Porous Sensors. Nanoscale Res. Lett. 2016, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Strambini, L.M.; Barillaro, G. Electrical Double Layer-Induced Ion Surface Accumulation for Ultrasensitive Refractive Index Sensing with Nanostructured Porous Silicon Interferometers. ACS Sens. 2018, 3, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Politi, J.; Dardano, P.; Caliò, A.; Iodice, M.; Rea, I.; De Stefano, L. Reversible sensing of heavy metal ions using lysine modified oligopeptides on porous silicon and gold. Sens. Actuators B Chem. 2017, 244, 142–150. [Google Scholar] [CrossRef]
- Tsai, W.T.; Nguyen, M.H.; Lai, J.R.; Nguyen, H.B.; Lee, M.C.; Tseng, F.G. ppb-level heavy metal ion detection by electrochemistry-assisted nanoPorous silicon (ECA-NPS) photonic sensors. Sens. Actuators B Chem. 2018, 265, 75–83. [Google Scholar] [CrossRef]
- Kumeria, T.; Wang, J.; Chan, N.; Harris, T.J.; Sailor, M.J. Visual Sensor for Sterilization of Polymer Fixtures Using Embedded Mesoporous Silicon Photonic Crystals. ACS Sens. 2018, 3, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.A.; Hu, S.; Weiss, S.M. Porous silicon ring resonator for compact, high sensitivity biosensing applications. Opt. Express 2015, 23, 7111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rodriguez, G.A.; Graham, Y.M.; Cao, T.; Gaur, G.; Weiss, S.M. Resonant Photonic Structures in Porous Silicon for Biosensing. In Proceedings of the Frontiers in Biological Detection: From Nanosensors to Systems IX, San Francisco, CA, USA, 28 January–2 February 2017; Danielli, A., Miller, B.L., Weiss, S.M., Eds.; SPIE: Bellingham, DC, USA, 2017; Volume 10081, p. 100810D. [Google Scholar]
- Girault, P.; Azuelos, P.; Lorrain, N.; Poffo, L.; Lemaitre, J.; Pirasteh, P.; Hardy, I.; Thual, M.; Guendouz, M.; Charrier, J. Porous silicon micro-resonator implemented by standard photolithography process for sensing application. Opt. Mater. 2017, 72, 596–601. [Google Scholar] [CrossRef]
- Jiménez Vivanco, M.; García, G.; Doti, R.; Faubert, J.; Lugo Arce, J. Time-Resolved Spectroscopy of Ethanol Evaporation on Free-Standing Porous Silicon Photonic Microcavities. Materials 2018, 11, 894. [Google Scholar] [CrossRef]
- Shashaani, H.; Faramarzpour, M.; Hassanpour, M.; Namdar, N.; Alikhani, A.; Abdolahad, M. Silicon nanowire based biosensing platform for electrochemical sensing of Mebendazole drug activity on breast cancer cells. Biosens. Bioelectron. 2016, 85, 363–370. [Google Scholar] [CrossRef]
- Abbas, R.A.; Alwan, A.M.; Abdulhamied, Z.T. Synthesis and characterization of porous silicon gas sensors. J. Phys. Conf. Ser. 2018, 1003, 012087. [Google Scholar] [CrossRef]
- Li, W.; Dai, E.W.; Bai, G.; Xu, J. Depth-dependent humidity sensing properties of silicon nanopillar array. Sens. Actuators B Chem. 2016, 237, 526–533. [Google Scholar] [CrossRef]
- Li, W.; Feng, Z.; Dai, E.; Xu, J.; Bai, G. Organic vapour sensing properties of area-ordered and size-controlled silicon nanopillar. Sensors 2016, 16, 1880. [Google Scholar] [CrossRef] [PubMed]
- Karadan, P.; Parida, S.; Kumar, A.; Anappara, A.A.; Dhara, S.; Barshilia, H.C. Charge transport studies on Si nanopillars for photodetectors fabricated using vapor phase metal-assisted chemical etching. Appl. Phys. A Mater. Sci. Process. 2017, 123, 1–10. [Google Scholar] [CrossRef]
- Abdul-Hameed, A.A.; Mahdi, M.A.; Ali, B.; Selman, A.M.; Al-Taay, H.F.; Jennings, P.; Lee, W.J. Fabrication of a high sensitivity and fast response self-powered photosensor based on a core-shell silicon nanowire homojunction. Superlattices Microstruct. 2018, 116, 27–35. [Google Scholar] [CrossRef]
- Liu, J.Q.; Gao, Y.; Wu, G.A.; Tong, X.W.; Xie, C.; Luo, L.B.; Liang, L.; Wu, Y.C. Silicon/Perovskite Core-Shell Heterojunctions with Light-Trapping Effect for Sensitive Self-Driven Near-Infrared Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 27850–27857. [Google Scholar] [CrossRef] [PubMed]
- Al-Hardan, N.H.; Hamid, M.A.A.; Ahmed, N.M.; Jalar, A.; Shamsudin, R.; Othman, N.K.; Keng, L.K.; Chiu, W.; Al-Rawi, H.N. High sensitivity pH sensor based on porous silicon (PSi) extended gate field-effect transistor. Sensors 2016, 16, 839. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.R.; Ali, G.M. A Porous Silicon P-Type Interdigitated Extended-Gate Field Effect Transistor pH Sensor. Silicon 2018, 1–8. [Google Scholar] [CrossRef]
- Huang, B.R.; Hsu, C.L.; Wang, Y.K.; Yang, W.L. Core-Shell P-N Junction Si Nanowires as Rapid Response and High-Sensitivity pH Sensor. IEEE Sens. J. 2017, 17, 3967–3974. [Google Scholar] [CrossRef]
- Jalkanen, T.; Määttänen, A.; Mäkilä, E.; Tuura, J.; Kaasalainen, M.; Lehto, V.P.; Ihalainen, P.; Peltonen, J.; Salonen, J. Fabrication of Porous Silicon Based Humidity Sensing Elements on Paper. J. Sens. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, L.; Kong, D.; Yu, Z.; Wang, H.; Ma, Z.; Wang, Y.; Wang, J.; Pan, L.; Shi, Y. Fast-response and low-hysteresis flexible pressure sensor based on silicon nanowires. IEEE Electron Device Lett. 2018, 39, 1069–1072. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, Z.; Zhang, T.; Liu, D. Polypyrrole shell (nanoparticles)-functionalized silicon nanowires array with enhanced NH3-sensing response. Sens. Actuators B Chem. 2018, 258, 246–254. [Google Scholar] [CrossRef]
- Azuelos, P.; Girault, P.; Lorrain, N.; Poffo, L.; Hardy, I.; Guendouz, M.; Thual, M. Theoretical investigation of Vernier effect based sensors with hybrid porous silicon-polymer optical waveguides. J. Appl. Phys. 2017, 121, 144501. [Google Scholar] [CrossRef]
- Dwivedi, P.; Chauhan, N.; Vivekanandan, P.; Das, S.; Sakthi Kumar, D.; Dhanekar, S. Scalable fabrication of prototype sensor for selective and sub-ppm level ethanol sensing based on TiO2 nanotubes decorated porous silicon. Sens. Actuators B Chem. 2017, 249, 602–610. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Choi, S.W.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of the benzene-sensing performance of Si nanowires through the incorporation of TeO2 heterointerfaces and Pd-sensitization. Sens. Actuators B Chem. 2017, 244, 1085–1097. [Google Scholar] [CrossRef]
- Karthik, T.V.K.; Martinez, L.; Agarwal, V. Porous silicon ZnO/SnO2 structures for CO2 detection. J. Alloy. Compd. 2018, 731, 853–863. [Google Scholar] [CrossRef]
- Dalvand, R.; Mahmud, S.; Shabannia, R. Fabrication of UV photodetector using needle-shaped ZnO nanostructure arrays prepared on porous silicon substrate by a facile low-temperature method. J. Mater. Sci. Mater. Electron. 2018, 29, 4999–5008. [Google Scholar] [CrossRef]
- Liu, X.; Hu, M.; Wang, Y.; Liu, J.; Qin, Y. High sensitivity NO2 sensor based on CuO/p-porous silicon heterojunction at room temperature. J. Alloy. Compd. 2016, 685, 364–369. [Google Scholar] [CrossRef]
- Martínez, L.; Holguín-Momaca, J.T.; Karthik, T.V.K.; Olive-Méndez, S.F.; Campos-Alvarez, J.; Agarwal, V. Sputtering temperature dependent growth kinetics and CO2 sensing properties of ZnO deposited over porous silicon. Superlattices Microstruct. 2016, 98, 8–17. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, M.; Liu, X.; Wei, Y.; Li, N.; Qin, Y. Synthesis of the cactus-like silicon nanowires/tungsten oxide nanowires composite for room-temperature NO2 gas sensor. J. Alloy. Compd. 2016, 679, 391–399. [Google Scholar] [CrossRef]
- Antunez, E.E.; Salazar-Kuri, U.; Estevez, J.O.; Campos, J.; Basurto, M.A.; Jiménez Sandoval, S.; Agarwal, V. Porous silicon-VO2 based hybrids as possible optical temperature sensor: Wavelength-dependent optical switching from visible to near-infrared range. J. Appl. Phys. 2015, 118, 134503. [Google Scholar] [CrossRef]
- Keramatnejad, K.; Khorramshahi, F.; Khatami, S.; Asl-Soleimani, E. Optimizing UV detection properties of n-ZnO nanowire/p-Si heterojunction photodetectors by using a porous substrate. Opt. Quantum Electron. 2015, 47, 1739–1749. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, M.; Yan, W.; Wang, D.; Yuan, L.; Qin, Y. Hydrothermal synthesis porous silicon/tungsten oxide nanorods composites and their gas-sensing properties to NO2 at room temperature. Appl. Surf. Sci. 2015, 353, 79–86. [Google Scholar] [CrossRef]
- Miao, F.; Lu, X.; Tao, B.; Li, R.; Chu, P.K. Glucose oxidase immobilization platform based on ZnO nanowires supported by silicon nanowires for glucose biosensing. Microelectron. Eng. 2016, 149, 153–158. [Google Scholar] [CrossRef]
- Liao, J.; Li, Z.; Wang, G.; Chen, C.; Lv, S.; Li, M. ZnO nanorod/porous silicon nanowire hybrid structures as highly-sensitive NO2 gas sensors at room temperature. Phys. Chem. Chem. Phys. 2016, 18, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Nayef, U.M.; Hubeatir, K.A.; Abdulkareem, Z.J. Ultraviolet photodetector based on TiO2 nanoparticles/porous silicon hetrojunction. Optik (Stuttg) 2016, 127, 2806–2810. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, Z.J.; Luo, L.; Zhao, C.Z.; Kang, L.P.; Liu, D.W. Methanol sensing properties of honeycomb-like SnO2 grown on silicon nanoporous pillar array. J. Alloy. Compd. 2016, 682, 170–175. [Google Scholar] [CrossRef]
- Husairi, F.S.; Rouhi, J.; Eswar, K.A.; Ooi, C.H.R.; Rusop, M.; Abdullah, S. Ethanol solution sensor based on ZnO/PSi nanostructures synthesized by catalytic immersion method at different molar ratio concentrations: An electrochemical impedance analysis. Sens. Actuators A Phys. 2015, 236, 11–18. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Hu, M.; Liu, S.; Zhu, Y.; Cao, M. Electrochemical synthesis and the gas-sensing properties of the Cu2O nanofilms/porous silicon hybrid structure. Sens. Actuators B Chem. 2016, 223, 626–633. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Chen, Q.; Zhou, H.; Wu, J. Low Power Consumption Gas Sensor Created from Silicon Nanowires/TiO2 Core–Shell Heterojunctions. ACS Sens. 2017, 2, 1491–1497. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, M.; Wang, D.; Zhang, W.; Qin, Y. Room temperature NO2-sensing properties of porous silicon/tungsten oxide nanorods composite. J. Alloy. Compd. 2015, 640, 517–524. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Liu, S.; Tan, M.; Cao, M. Electrodeposited tungsten oxide films onto porous silicon for NO2 detection at room temperature. J. Alloy. Compd. 2018, 735, 718–727. [Google Scholar] [CrossRef]
- Qiang, X.; Hu, M.; Zhao, B.; Qin, Y.; Zhang, T.; Zhou, L.; Liang, J. Preparation of porous silicon/Pd-loaded WO3nanowires for enhancement of ammonia sensing properties at room temperature. Mater. Sci. Semicond. Process. 2018, 79, 113–118. [Google Scholar] [CrossRef]
- Baker, C.; Gole, J.L. Detection of Liquid Organic Solvents on Metal Oxide Nanostructure Decorated Porous Silicon Interfaces. ACS Sens. 2016, 1, 235–242. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Q.; Liu, W. Au@ZnO nanostructures on porous silicon for photocatalysis and gas-sensing: The effect of plasmonic hot-electrons driven by visible-light. Mater. Res. Express 2016, 3, 085006. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, M.; Wei, Y.; Ma, W. Enhanced NO2 sensing characteristics of Au modified porous silicon/thorn-sphere-like tungsten oxide composites. Appl. Surf. Sci. 2016, 389, 824–834. [Google Scholar] [CrossRef]
- Wang, D.-F.; Liang, J.-R.; Li, C.-Q.; Yan, W.-J.; Hu, M. Room temperature NO2 gas sensing of Au-loaded tungsten oxide nanowires/porous silicon hybrid structure. Chin. Phys. B 2016, 25, 028102. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; He, Y. Highly sensitive and reproducible silicon-based surface-enhanced Raman scattering sensors for real applications. Analyst 2016, 141, 5010–5019. [Google Scholar] [CrossRef]
- Novara, C.; Dalla Marta, S.; Virga, A.; Lamberti, A.; Angelini, A.; Chiadò, A.; Rivolo, P.; Geobaldo, F.; Sergo, V.; Bonifacio, A.; et al. SERS-active Ag nanoparticles on porous silicon and PDMS substrates: A comparative study of uniformity and Raman efficiency. J. Phys. Chem. C 2016, 120, 16946–16953. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Chauhan, S.; Gautam, Y.K.; Chandra, R. Fast and reversible hydrogen sensing properties of Pd/Mg thin film modified by hydrophobic porous silicon substrate. Sens. Actuators B Chem. 2015, 213, 252–260. [Google Scholar] [CrossRef]
- Mohaček-Grošev, V.; Gebavi, H.; Bonifacio, A.; Sergo, V.; Daković, M.; Bajuk-Bogdanović, D. Binding of p-mercaptobenzoic acid and adenine to gold-coated electroless etched silicon nanowires studied by surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Feng, W.C.; Su, F.C.; Wang, G.J. An Electrochemical Glucose Biosensor with a Silicon Nanowire Array Electrode. J. Electrochem. Soc. 2015, 162, B264–B268. [Google Scholar] [CrossRef]
- Karadan, P.; Aggarwal, S.; Anappara, A.A.; Narayana, C.; Barshilia, H.C. Tailored periodic Si nanopillar based architectures as highly sensitive universal SERS biosensing platform. Sens. Actuators B Chem. 2018, 254, 264–271. [Google Scholar] [CrossRef]
- Novara, C.; Lamberti, A.; Chiadò, A.; Virga, A.; Rivolo, P.; Geobaldo, F.; Giorgis, F. Surface-enhanced Raman spectroscopy on porous silicon membranes decorated with Ag nanoparticles integrated in elastomeric microfluidic chips. RSC Adv. 2016, 6, 21865–21870. [Google Scholar] [CrossRef]
- Bandarenka, H.V.; Girel, K.V.; Bondarenko, V.P.; Khodasevich, I.A.; Panarin, A.Y.; Terekhov, S.N. Formation Regularities of Plasmonic Silver Nanostructures on Porous Silicon for Effective Surface-Enhanced Raman Scattering. Nanoscale Res. Lett. 2016, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Yakimchuk, D.; Kaniukov, E.; Bundyukova, V.; Osminkina, L.; Teichert, S.; Demyanov, S.; Sivakov, V. Silver nanostructures evolution in porous SiO2/p-Si matrices for wide wavelength surface-enhanced Raman scattering applications. MRS Commun. 2018, 8, 95–99. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Surface-enhanced Raman scattering (SERS)-active substrates from silver plated-porous silicon for detection of crystal violet. Appl. Surf. Sci. 2015, 331, 241–247. [Google Scholar] [CrossRef]
- Kalimuthu, V.; Rath, S. One-step synthesis of Au-coated porous silicon as a surface enhanced Raman scattering substrate for biomolecule detection. Mater. Lett. 2017, 204, 115–119. [Google Scholar] [CrossRef]
- Mikac, L.; Ivanda, M.; Derek, V.; Gotić, M. Influence of mesoporous silicon preparation condition on silver clustering and SERS enhancement. J. Raman Spectrosc. 2016, 47, 1036–1041. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Rezaloo, F.; Rezaei, B. Electrochemical sensor based on porous silicon/silver nanocomposite for the determination of hydrogen peroxide. Sens. Actuators B Chem. 2016, 231, 239–244. [Google Scholar] [CrossRef]
- Wang, Y.W.; Kao, K.C.; Wang, J.K.; Mou, C.Y. Large-Scale Uniform Two-Dimensional Hexagonal Arrays of Gold Nanoparticles Templated from Mesoporous Silica Film for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2016, 120, 24382–24388. [Google Scholar] [CrossRef]
- Tsao, C.W.; Yang, Z.J. High Sensitivity and High Detection Specificity of Gold-Nanoparticle-Grafted Nanostructured Silicon Mass Spectrometry for Glucose Analysis. ACS Appl. Mater. Interfaces 2015, 7, 22630–22637. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chang, H.; Zhu, W.; Xu, C.; Feng, X. Rhodium Nanoparticle-mesoporous Silicon Nanowire Nanohybrids for Hydrogen Peroxide Detection with High Selectivity. Sci. Rep. 2015, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. A Novel Disposable Biosensor Based on SiNWs/AuNPs Modified-Screen Printed Electrode for Dengue Virus DNA Oligomer Detection. IEEE Sens. J. 2015, 15, 4420–4421. [Google Scholar] [CrossRef]
- Kumar, A.; Karadan, P.; Barshilia, H.C. Synthesis of silver nanowires towards the development the ultrasensitive AgNWs/SiNPLs hybrid photodetector and flexible transparent conductor. Mater. Sci. Semicond. Process. 2018, 75, 239–246. [Google Scholar] [CrossRef]
- Lee, J.; Hong, M.H.; Han, S.; Na, J.; Kim, I.; Kwon, Y.J.; Lim, Y.B.; Choi, H.J. Sensitive and Selective Detection of HIV-1 RRE RNA Using Vertical Silicon Nanowire Electrode Array. Nanoscale Res. Lett. 2016, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silina, Y.E.; Koch, M.; Herbeck-Engel, P.; Iatsunskyi, I. Exploring the potential of high resolution inductively coupled plasma mass spectrometry towards non-destructive control and validation of electroless gold nanoparticles onto silicon nanowires hybrids. Anal. Methods 2019, 11, 3987–3995. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, Y.; Chen, X.; Wu, J. Two-Dimensional Electrochemiluminescence on Porous Silicon Platform for Explosive Detection and Discrimination. ACS Sens. 2018, 3, 1439–1444. [Google Scholar] [CrossRef]
- Arzumanyan, G.; Doroshkevich, N.; Mamatkulov, K.; Shashkov, S.; Girel, K.; Bandarenka, H.; Borisenko, V. Phospholipid detection by surface-enhanced Raman scattering using silvered porous silicon substrates. Phys. Status Solidi 2017, 214, 1600915. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Ahmadi, N.; Rezaei, B.; Abarghoui, M.M. A new electrochemical sensor for the simultaneous determination of acetaminophen and codeine based on porous silicon/palladium nanostructure. Talanta 2015, 134, 745–753. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z. Metal Nanoparticles/Porous Silicon Microcavity Enhanced Surface Plasmon Resonance Fluorescence for the Detection of DNA. Sensors 2018, 18, 661. [Google Scholar] [CrossRef] [PubMed]
- Kosovic, M.; Balarin, M.; Ivanda, M.; Derek, V.; Marcius, M.; Ristic, M.; Gamulin, O. Porous silicon covered with silver nanoparticles as Surface-Enhanced Raman Scattering (SERS) substrate for ultra-low concentration detection. Appl. Spectrosc. 2015, 69, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Abarghoui, M.M.; Rezaei, B. Simultaneous determination of morphine and codeine using Pt nanoparticles supported on porous silicon flour modified ionic liquid carbon paste electrode. Sens. Actuators B Chem. 2015, 219, 1–9. [Google Scholar] [CrossRef]
- Harraz, F.A.; Faisal, M.; Al-Salami, A.E.; El-Toni, A.M.; Almadiy, A.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Silver nanoparticles decorated stain-etched mesoporous silicon for sensitive, selective detection of ascorbic acid. Mater. Lett. 2019, 234, 96–100. [Google Scholar] [CrossRef]
- Colombelli, A.; Manera, M.G.; Taurino, A.; Catalano, M.; Convertino, A.; Rella, R. Au nanoparticles decoration of silica nanowires for improved optical bio-sensing. Sens. Actuators B Chem. 2016, 226, 589–597. [Google Scholar] [CrossRef]
- Sainato, M.; Strambini, L.M.; Rella, S.; Mazzotta, E.; Barillaro, G. Sub-Parts Per Million NO2 Chemi-Transistor Sensors Based on Composite Porous Silicon/Gold Nanostructures Prepared by Metal-Assisted Etching. ACS Appl. Mater. Interfaces 2015, 7, 7136–7145. [Google Scholar] [CrossRef]
- Convertino, A.; Mussi, V.; Maiolo, L. Disordered array of Au covered Silicon nanowires for SERS biosensing combined with electrochemical detection. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Yun, J.; Moon, D.-I.; Choi, Y.-K.; Park, I. Self-heated silicon nanowires for high performance hydrogen gas detection. Nanotechnology 2015, 26, 095501. [Google Scholar] [CrossRef]
- Convertino, A.; Mussi, V.; Maiolo, L.; Ledda, M.; Lolli, M.G.; Bovino, F.A.; Fortunato, G.; Rocchia, M.; Lisi, A. Array of disordered silicon nanowires coated by a gold film for combined NIR photothermal treatment of cancer cells and Raman monitoring of the process evolution. Nanotechnology 2018, 29, 415102. [Google Scholar] [CrossRef]
- Rindzevicius, T.; Barten, J.; Vorobiev, M.; Schmidt, M.S.; Castillo, J.J.; Boisen, A. Detection of surface-linked polychlorinated biphenyls using surface-enhanced Raman scattering spectroscopy. Vib. Spectrosc. 2017, 90, 1–6. [Google Scholar] [CrossRef]
- Xu, D.; Teng, F.; Wang, Z.; Lu, N. Droplet-Confined Electroless Deposition of Silver Nanoparticles on Ordered Superhydrophobic Structures for High Uniform SERS Measurements. ACS Appl. Mater. Interfaces 2017, 9, 21548–21553. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, R.K.; Sommer, L.M.; Johansen, H.K.; Rindzevicius, T.; Molin, S.; Jelsbak, L.; Engelsen, S.B.; Boisen, A. SERS detection of the biomarker hydrogen cyanide from Pseudomonas aeruginosa cultures isolated from cystic fibrosis patients. Sci. Rep. 2017, 7, 45264. [Google Scholar] [CrossRef]
- Maiolo, L.; Polese, D.; Pecora, A.; Fortunato, G.; Shacham-Diamand, Y.; Convertino, A. Highly Disordered Array of Silicon Nanowires: An Effective and Scalable Approach for Performing and Flexible Electrochemical Biosensors. Adv. Healthc. Mater. 2016, 5, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Nayef, U.M.; Khudhair, I.M.; Kayahan, E. Organic vapor sensor using photoluminescence of laser ablated gold nanoparticles on porous silicon. Optik (Stuttg) 2017, 144, 546–552. [Google Scholar] [CrossRef]
- Hassan, K.; Uddin, A.S.M.I.; Chung, G.S. Hydrogen sensing properties of Pt/Pd bimetal decorated on highly hydrophobic Si nanowires. Int. J. Hydrogen Energy 2016, 41, 10991–11001. [Google Scholar] [CrossRef]
- Durucan, O.; Wu, K.; Viehrig, M.; Rindzevicius, T.; Boisen, A. Nanopillar-Assisted SERS Chromatography. ACS Sens. 2018, 3, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Wu, K.; Hansen, M.F.; Schmidt, M.S.; Boisen, A.; Sun, Y. Quantitative detection of trace level cloxacillin in food samples using magnetic molecularly imprinted polymer extraction and surface-Enhanced raman spectroscopy nanopillars. Anal. Chem. 2017, 89, 11484–11490. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, S.; Deng, S.; Chen, H.; Wang, C. Flexible, Transparent, and Free-Standing Silicon Nanowire SERS Platform for in Situ Food Inspection. ACS Sens. 2017, 2, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Shan, M.; Paltrinieri, L.; Evers, W.H.; Chu, L.; Poltorak, L.; Klootwijk, J.H.; Seoane, B.; Gascon, J.; Sudhölter, E.J.R.; et al. Enhanced vapour sensing using silicon nanowire devices coated with Pt nanoparticle functionalized porous organic frameworks. Nanoscale 2018, 10, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rashid, J.I.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. Surface modifications to boost sensitivities of electrochemical biosensors using gold nanoparticles/silicon nanowires and response surface methodology approach. J. Mater. Sci. 2016, 51, 1083–1097. [Google Scholar] [CrossRef]
- Li, Y.; Dykes, J.; Gilliam, T.; Chopra, N. A new heterostructured SERS substrate: Free-standing silicon nanowires decorated with graphene-encapsulated gold nanoparticles. Nanoscale 2017, 9, 5263–5272. [Google Scholar] [CrossRef] [PubMed]
- Shiraz, H.G.; Astaraei, F.R.; Fardindoost, S.; Hosseini, Z.S. Decorated CNT based on porous silicon for hydrogen gas sensing at room temperature. RSC Adv. 2016, 6, 44410–44414. [Google Scholar] [CrossRef]
- Das, N.; Basu, J.; RoyChaudhuri, C. Graphene coated nanoporous silicon immunosensor for food toxin detection. Int. J. Adv. Eng. Sci. Appl. Math. 2015, 7, 204–209. [Google Scholar] [CrossRef]
- Moretta, R.; Terracciano, M.; Dardano, P.; Casalino, M.; De Stefano, L.; Schiattarella, C.; Rea, I. Toward Multi-Parametric Porous Silicon Transducers Based on Covalent Grafting of Graphene Oxide for Biosensing Applications. Front. Chem. 2018, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shiraz, H.G. Efficient room temperature hydrogen gas sensing based on graphene oxide and decorated porous silicon. Int. J. Hydrogen Energy 2017, 42, 15966–15972. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.Y.; Kim, S.; Lee, D.H.; Kim, J.H.; Kim, J.M.; Kang, H.; Han, J.S.; Park, J.W.; Lee, H.; et al. Precise and selective sensing of DNA-DNA hybridization by graphene/Si-nanowires diode-type biosensors. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.C.; Zhang, C.; Liu, X.Y.; Gao, S.S.; Hu, L.T.; Guo, J.; Ma, Y.; Jiang, S.Z.; Si, H.P. High-performance SERS substrate based on hybrid structure of graphene oxide/AgNPs/Cu film@pyramid Si. Sci. Rep. 2016, 6, 2–11. [Google Scholar] [CrossRef]
- Eom, N.; Cho, H.-B.; Song, Y.; Lee, W.; Sekino, T.; Choa, Y.-H. Room-Temperature H2 Gas Sensing Characterization of Graphene-Doped Porous Silicon via a Facile Solution Dropping Method. Sensors 2017, 17, 2750. [Google Scholar] [CrossRef]
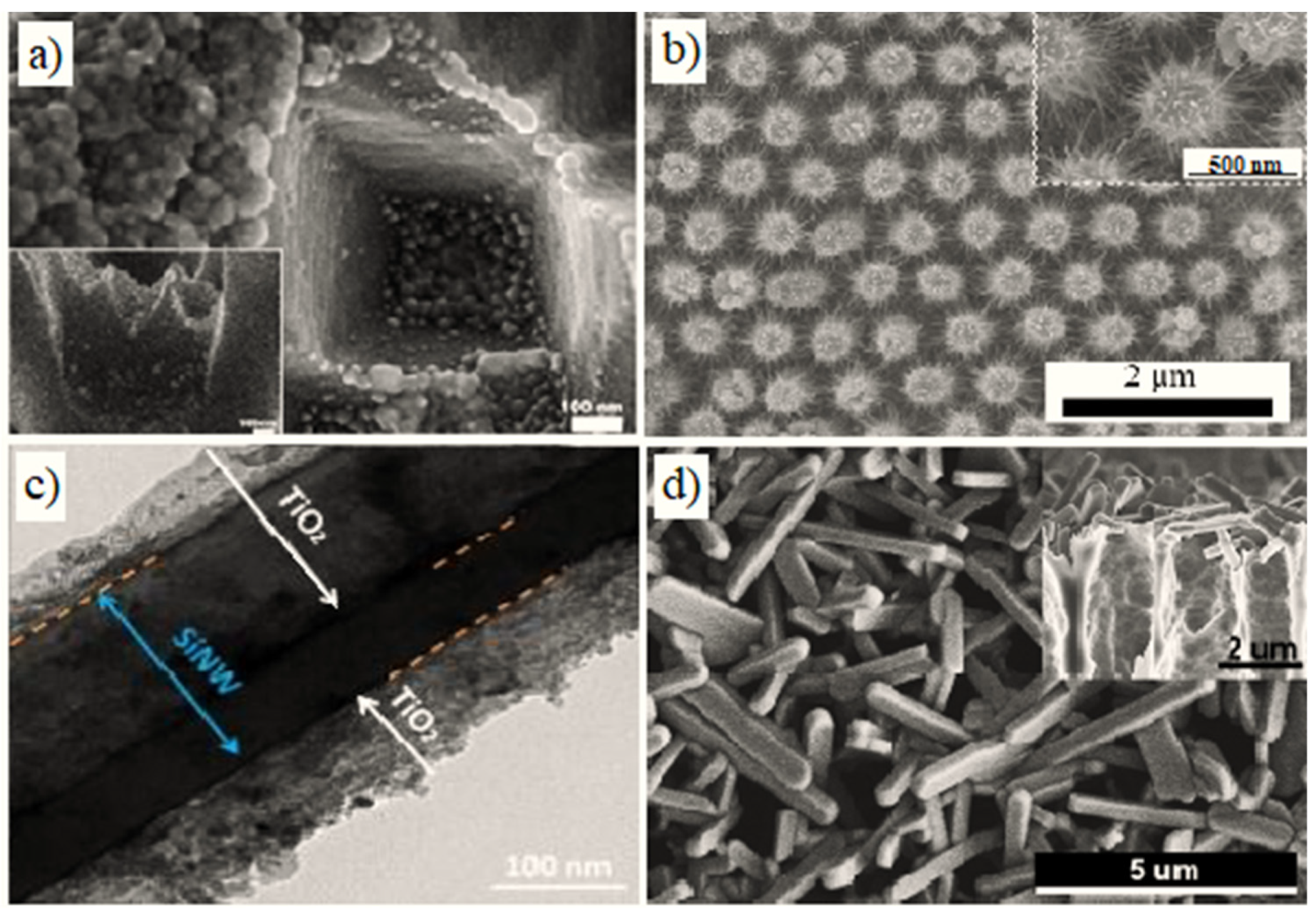
| (Bio)sensors Based on PSi, SiNWs, SiNPs and Their Composites with Polymers | ||||
| Type of transducer | Detection approach | Material for detection | LOD a/Sensitivity b range/Sensitivity c | Reference |
| PSi | Photoluminescence | Glucose, urea | b 0–3.0 mM | [18] |
| Cu2+, Pb2+, Cd2+ | c 10 nM | |||
| Colorimetric sensing | Autoimmune antibodies | a 10 fg/mL | [73] | |
| Fluorescence | J774 macrophage cells | a few and/or single cells | [75] | |
| Visual colorimetric sensing | Non-pathogenic E. coli | a 1.5 ± 0.4 × 105 CFU/mL | [100] | |
| SiNWs | Resistance | H2O | b 10–50 ppm | [117] |
| Capacitance | Pressure | a 0.1 Pa | [116] | |
| Luminescence | Streptavidin | a 1.6 fM | [72] | |
| I–V curves | Near-infrared (NIR) light | c 14.86–844.33 mA/W | [111] | |
| SiNPLs | I–V curves | Relative humidity (RH) | a 10% | [49] |
| Refractive index | Isopropyl alcohol | a 579.5 nm/RIU | [71] | |
| I–V curves | Ethanol, acetone gas | a 0.25% | [108] | |
| I–V curves | Light | c 1.3 mA/W | [109] | |
| UV light | c 0.82 mA/W | |||
| (Bio)sensors based on nano-Si and MOx nanocomposites | ||||
| PSi/WO3 | Resistance | NO2 | a 100 ppb b 100 ppb–3 ppm | [129] |
| PSi/ZnO | Electrochemical impedance analysis | Ethanol solution | b 0.05–0.6 M | [134] |
| PSi/TiO2 | Fluorescence | Aflatoxins B1 | a 15.4 pg/mL | [26] |
| Ochratoxin A | a 1.48 pg/mL | |||
| Fumonisin B1 | a 0.21 pg/mL | |||
| PSi/ZnO | Photocurrent | UV Light (325 nm) | c 1.98 A/W | [123] |
| PSi/TiO2 | I–V curve | UV illumination | c 0.045 A/W | [132] |
| PSi/SnO2:Sn | Capacitance | Relative Humidity | b 11–95% | [25] |
| SiNWs/TeO2/Pd | Resistance | C6H6, CO, C7H8, N2O | b 10–50 ppm | [121] |
| SiNWs/ZnO | I–V curves | Glucose | a 12 μM c 129 μA mM−1 | [130] |
| SiNWs/WO3 | Resistance | N2O | b 0.25–5 ppm | [126] |
| SiNWs/ZnO | Resistance | N2O | b 5–50 ppm | [131] |
| SiNPLs/Fe2O3/Ag | SERS | Malachite green (MG) | a 10−8 M | [38] |
| SiNPLs/TiO2 | I–V curves | CH4 | a 20 ppm | [136] |
| (Bio)sensors based on nano-Si and metals nanoparticles | ||||
| PSi/Ag | SERS | Rhodamine 6G | a 10−15 M | [168] |
| Crystal violet | a 100 pM | [153] | ||
| Porphyrin CuTMPyP4 | a 10−11 M | [151] | ||
| PSi/Au | Photoluminescence | Aflatoxin B1 | a 2.5 ± 0.5 pg/mL b 0.01–10 ng/ml | [13] |
| PSi/Ag | Amperometric response | Ascorbic acid | a 0.83 μM c 1.279 mA mM−1 cm−2 b 20–600 μM | [170] |
| SiNWs/Au | Differential pulse voltammetry | DNA | a 1.63 × 10−12 M | [160] |
| SiNWs/Ag | Resistance | NO2 | a10 ppb | [27] |
| SiNWs/Au | I–V measurements | Glucose | a 11 μM b 55.1 μM–16.53 mM | [148] |
| SiNWs/Au | Impedance measurements | Avidin | a 10 × 10−12 M | [179] |
| SiNWs/Pd/Pt | Resistance | H2 | b 1–40,000 ppm | [181] |
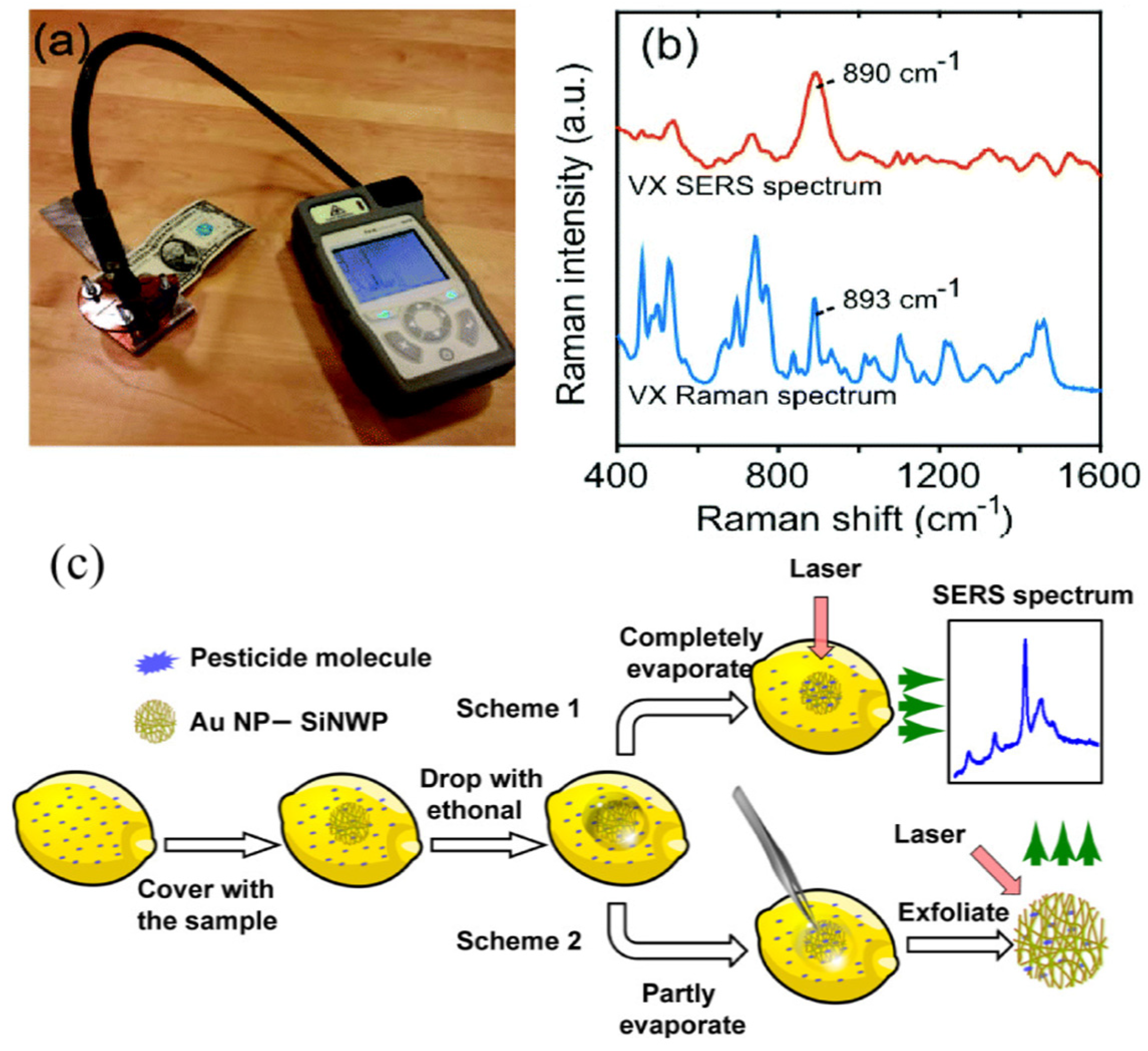
| SiNPs/Au | SERS | Nerve gases VX | a 13 fM | [12] |
| Tabun | a 630 fM | |||
| SiNPs/Ag | SERS | Rhodamine 6G | a 10−11 M | [177] |
| a 10−13 M b 10−7–10−13 M | [149] | |||
| SiNPs/Au | SERS | Rhodamine 6G | b 10−10–10−6 M | [51] |
| Cloxacillin | b 15.6–500 pM | [183] | ||
| (Bio)sensors based on nano-Si and carbon-based nanomaterials | ||||
| PSi/GO substrate | Impedance | Aflatoxin B1 | b 1 fg/mL–1 pg/mL | [189] |
| PSi/GO AgNPs/PCu | SERS | Rhodamine 6 G | a 10−15 M | [193] |
| PSi/Pd/GO | Resistance | H2 | a 200 ppm at 15 °C | [191] |
| PSi/Graphene | I–V curves | H2 | b 100–1000 ppm | [194] |
| SiNWs/Graphene | SERS | R6G | a 10−6 M | [187] |
| SiNWs/Graphene | I–V curves characterization, PL measurements | DNA | b 0.1–500 nM | [192] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myndrul, V.; Iatsunskyi, I. Nanosilicon-Based Composites for (Bio)sensing Applications: Current Status, Advantages, and Perspectives. Materials 2019, 12, 2880. https://doi.org/10.3390/ma12182880
Myndrul V, Iatsunskyi I. Nanosilicon-Based Composites for (Bio)sensing Applications: Current Status, Advantages, and Perspectives. Materials. 2019; 12(18):2880. https://doi.org/10.3390/ma12182880
Chicago/Turabian StyleMyndrul, Valerii, and Igor Iatsunskyi. 2019. "Nanosilicon-Based Composites for (Bio)sensing Applications: Current Status, Advantages, and Perspectives" Materials 12, no. 18: 2880. https://doi.org/10.3390/ma12182880
APA StyleMyndrul, V., & Iatsunskyi, I. (2019). Nanosilicon-Based Composites for (Bio)sensing Applications: Current Status, Advantages, and Perspectives. Materials, 12(18), 2880. https://doi.org/10.3390/ma12182880