Effect of Carbonation on Abrasion Resistance of Alkali-Activated Slag with Various Activators
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Mix Proportions
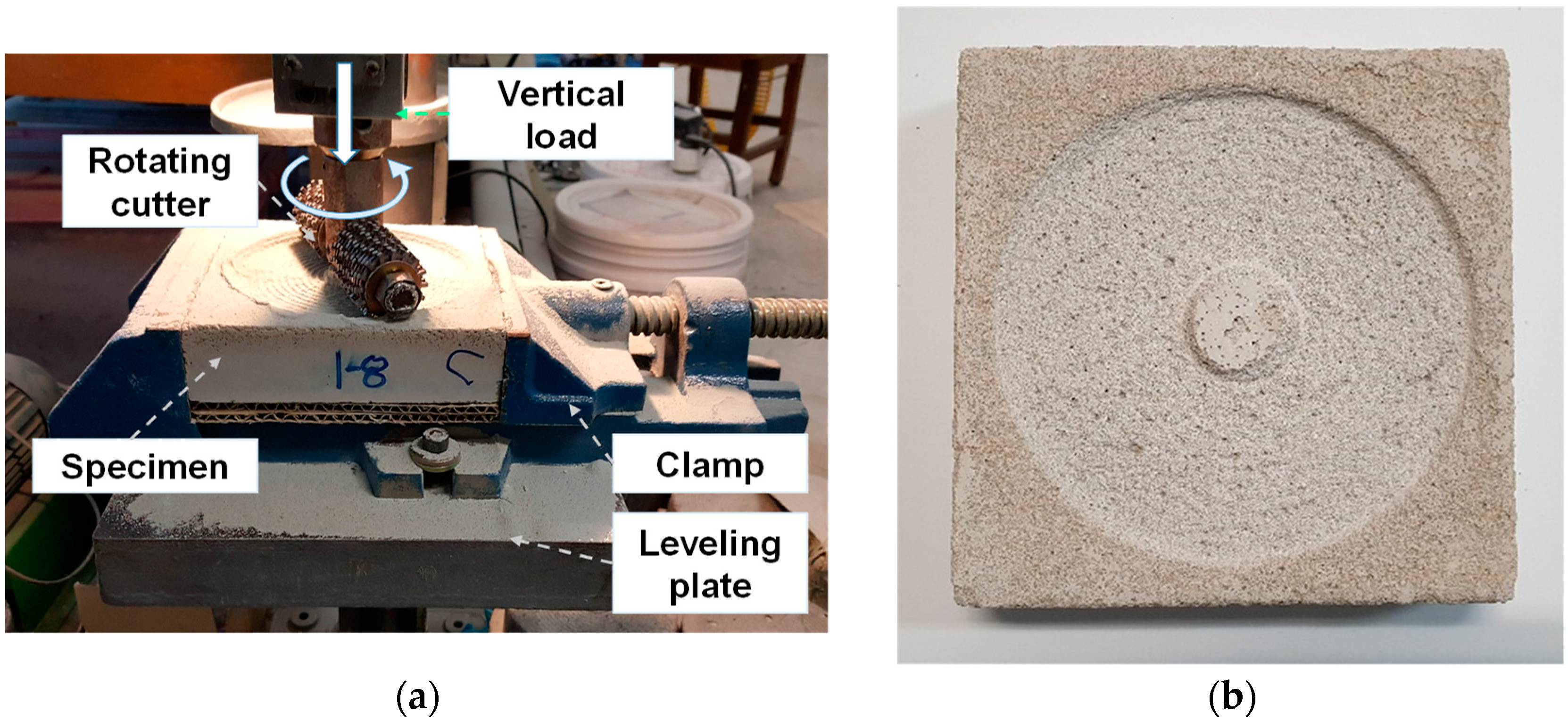
2.2. Experimental Details
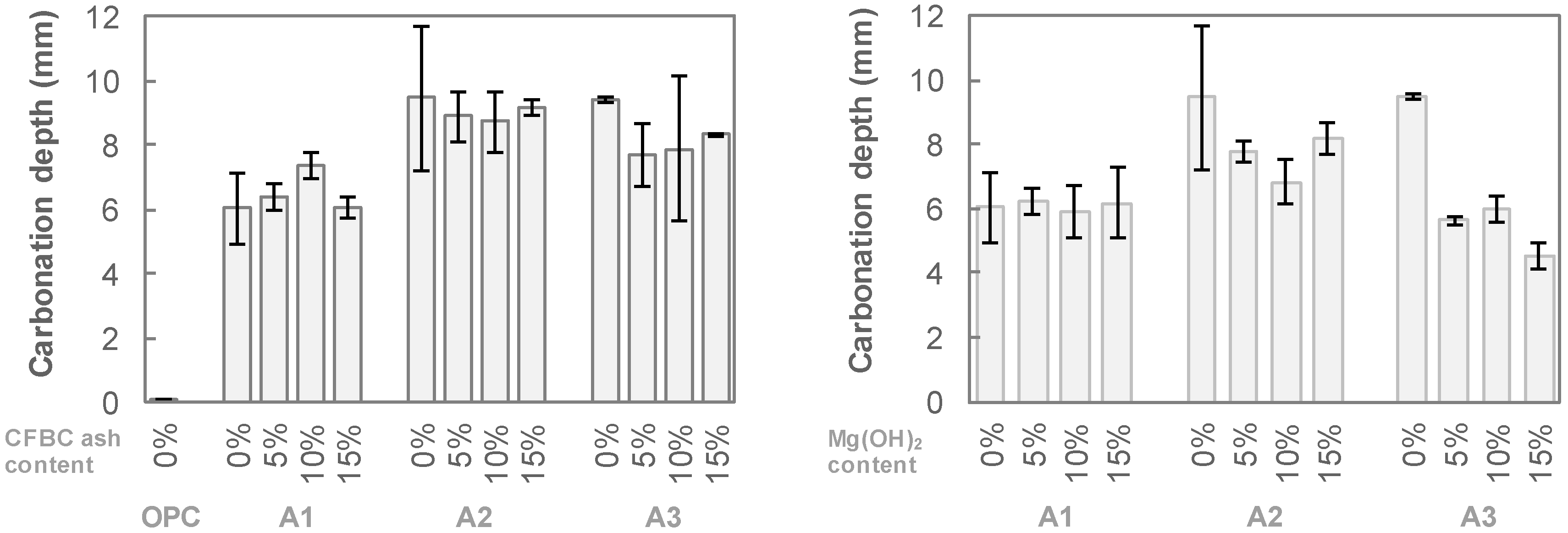
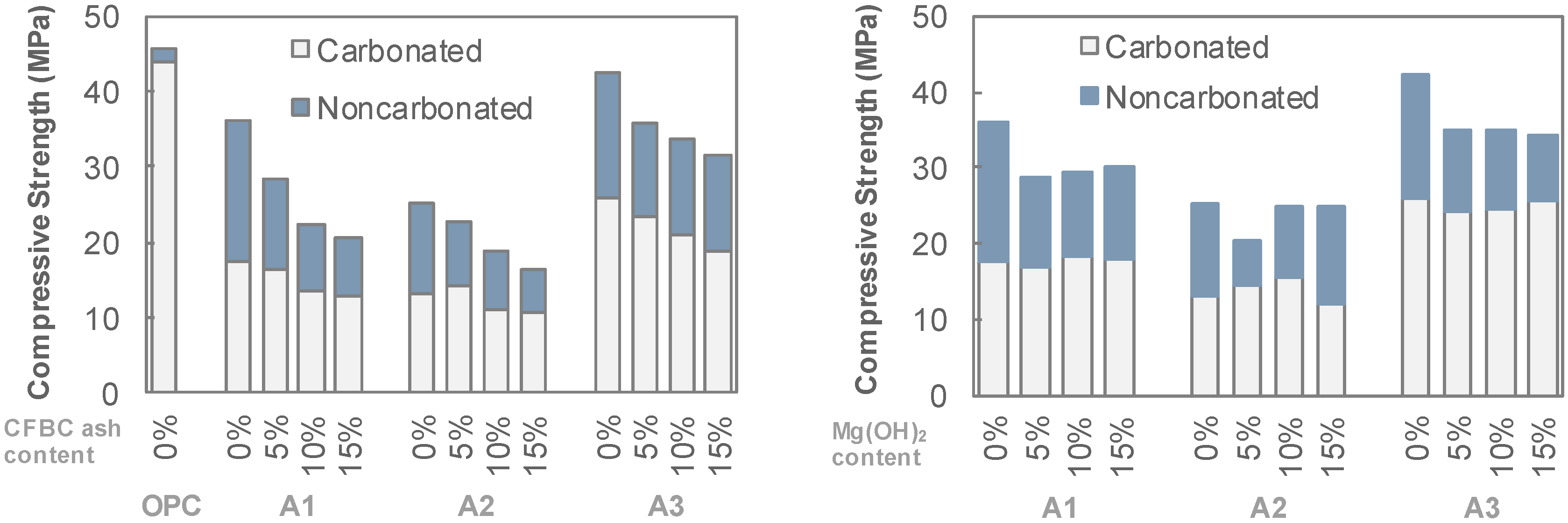
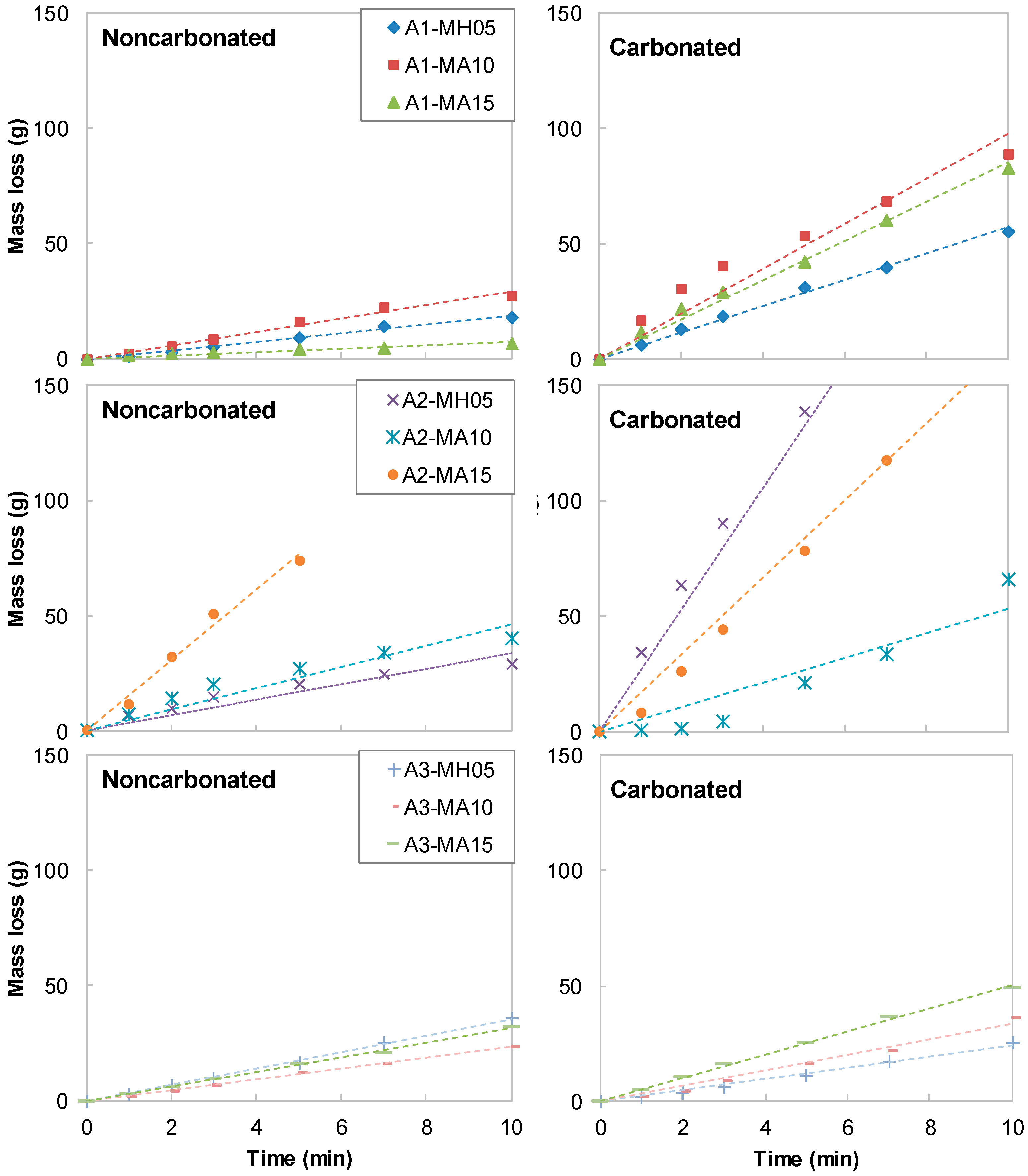
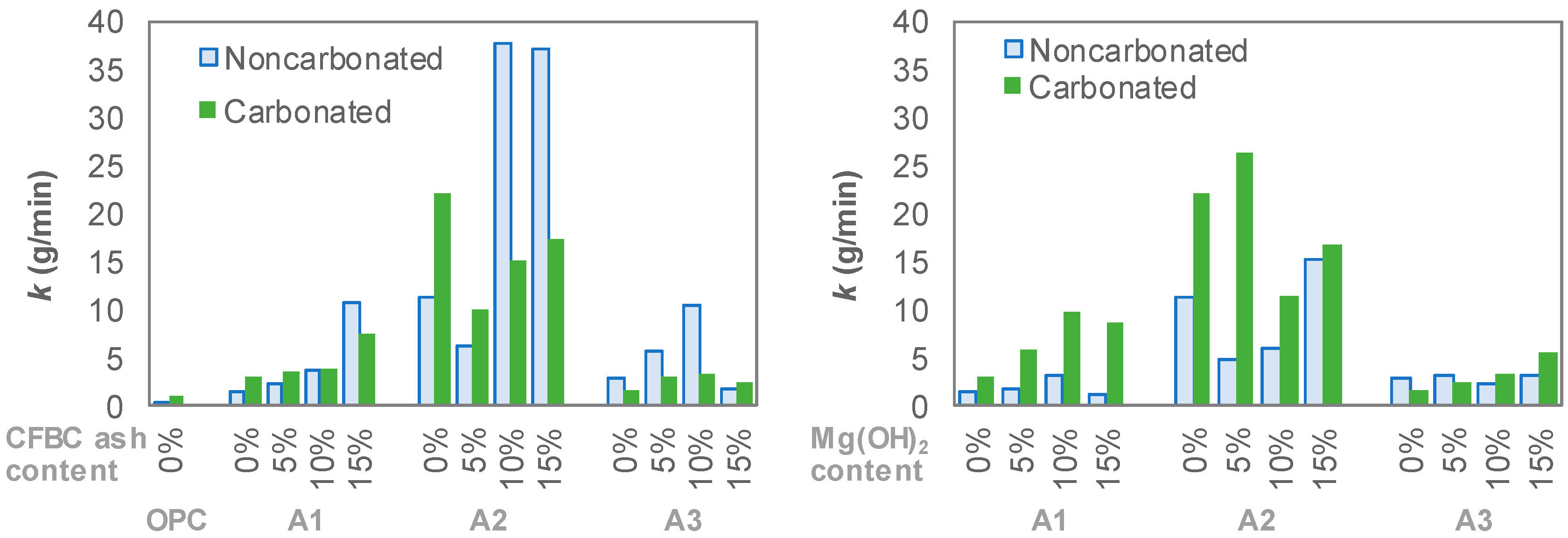
3. Experimental Results
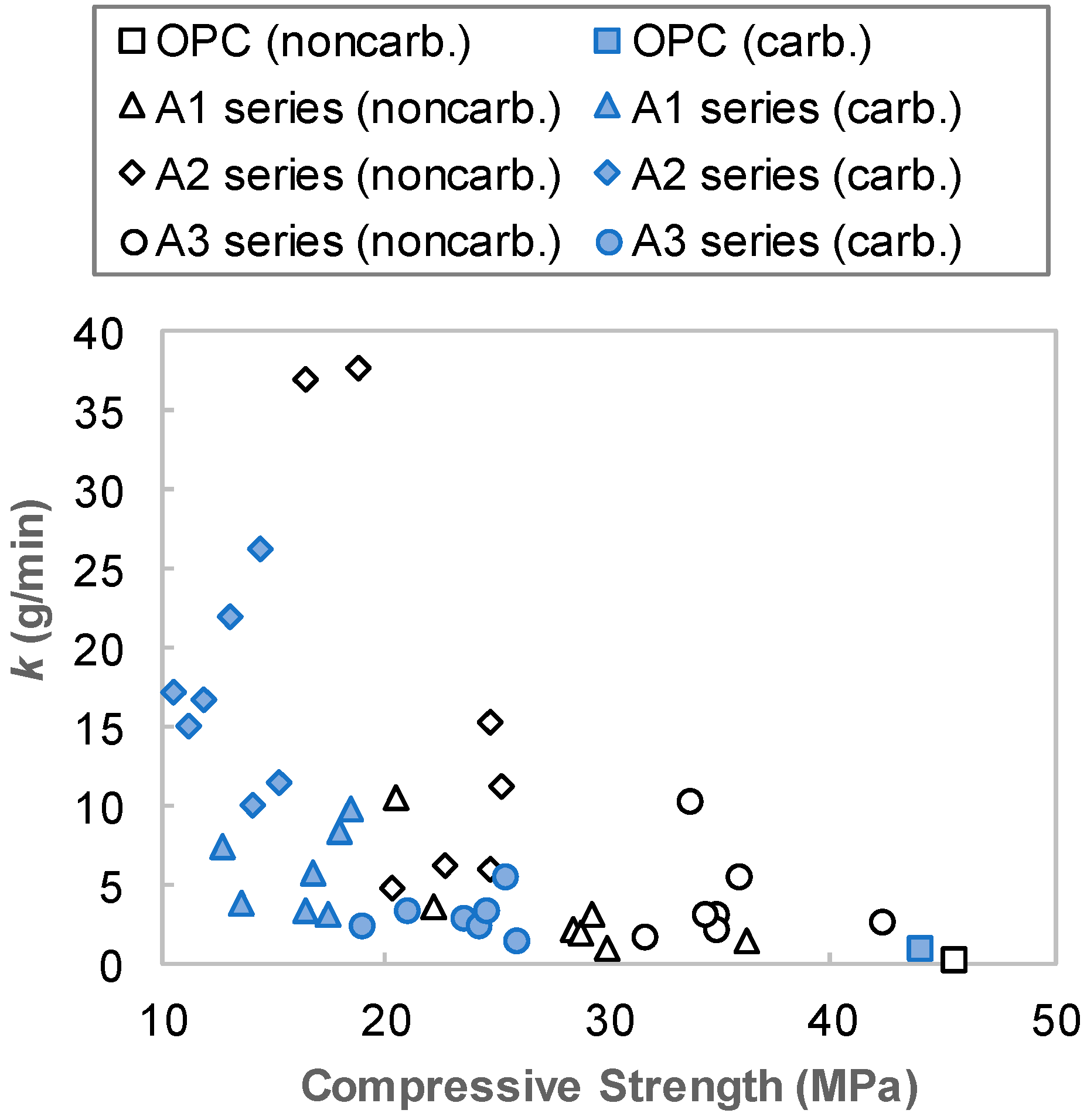
4. Discussion
5. Conclusions
- (1)
- The resistance of AAS to carbonation and abrasion were varied sensitively with regard to the activator type. Employing a combination of Na- and Ca-based activators showed a relatively higher abrasion resistance than that of a sole Na-based activator. For the mixtures with a combination of Ca(OH)2, Na2SO4 and Na2SiF6 (A3 series), although the compressive strength decreased after carbonation, the abrasion resistance rather increased. Abrasion mass loss of the A3 series specimen was reduced by about 40% while the compressive strength decreased in the range of 30%.
- (2)
- Mg(OH)2 and CFBC ash, i.e., additional binders tested in the present work, gave negative effects on both compressive strength and abrasion resistance of AAS mixtures. Use of these additional binders by up to 15 wt% of total binder led to a decrease in the compressive strength by approximately 30% and an increase in abrasion mass loss up to 30%.
- (3)
- Nevertheless, the highest abrasion resistance of AAS mixtures in the present study was approximately half of that of OPC with the same w/b. The other mixtures had a very-low abrasion resistance of approximately 1/5–1/100, compared with OPC. Therefore, it can be concluded that additional caution is required when using AAS where abrasion may have occurred.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External sulfate attack on alkali-activated slag. Construct. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- De Belie, N.; Monteny, J.; Beeldens, A.; Vincke, E.; Van Gemert, D.; Verstraete, W. Experimental research and prediction of the effect of chemical and biogenic sulfuric acid on different types of commercially produced concrete sewer pipes. Cem. Concr. Res. 2004, 34, 2223–2236. [Google Scholar] [CrossRef]
- Karahan, O.; Yakupoğlu, A. Resistance of alkali-activated slag mortar to abrasion and fire. Adv. Cem. Res. 2011, 23, 289–297. [Google Scholar] [CrossRef]
- Mohebi, R.; Behfarnia, K.; Shojaei, M. Abrasion resistance of alkali-activated slag concrete designed by Taguchi method. Construct. Build. Mater. 2015, 98, 792–798. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Song, K.-I. Characteristics of Self-Compacting and Precast Concrete Mixtures Using Powder-Type Alkali-Activated Slag (AAS). Ph.D. Thesis, Chonnam National University, Gwangju, Korea, February 2017. [Google Scholar]
- Lu, D.Y.; Hughes, R.W.; Anthony, E.J. Ca-based sorbent looping combustion for CO2 capture in pilot-scale dual fluidized beds. Fuel Process. Technol. 2008, 89, 1386–1395. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, H.; Sagawa, T.; Hama, Y. Compressive strength development and durability of an environmental load-reduction material manufactured using circulating fluidized bed ash and blast-furnace slag. Construct. Build. Mater. 2017, 146, 102–113. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. The role of brucite, ground granulated blastfurnace slag, and magnesium silicates in the carbonation and performance of MgO cements. Construct. Build. Mater. 2015, 94, 629–643. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.-M.; Chung, S.; Ahn, J.-W.; Kim, H.-K. Utilization of circulating fluidized bed combustion ash in producing controlled low-strength materials with cement or sodium carbonate as activator. Construct. Build. Mater. 2018, 159, 642–651. [Google Scholar] [CrossRef]
- Lee, N.K.; Abate, S.Y.; Kim, H.-K. Use of recycled aggregates as internal curing agent for alkali-activated slag system. Construct. Build. Mater. 2018, 159, 286–296. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Pyo, S.; Abate, S.Y.; Kim, H.-K. Abrasion resistance of ultra high performance concrete incorporating coarser aggregate. Construct. Build. Mater. 2018, 165, 11–16. [Google Scholar] [CrossRef]
- Song, K.I.; Song, J.K.; Lee, B.Y.; Yang, K.H. Carbonation characteristics of alkali-activated blast-furnace slag mortar. Adv. Mater. Sci. Eng. 2014, 2014, 326458. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]


| wt% | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | Others | LOI a |
|---|---|---|---|---|---|---|---|---|---|
| Slag | 45.7 | 29.1 | 11.7 | 0.5 | 3.1 | 2.0 | 0.4 | 5.7 | 1.8 |
| CFBC ash | 56.5 | 6.2 | 4.6 | 8.5 | 1.8 | 16.9 | 0.1 | 1.8 | 3.6 |
| OPC | 60.6 | 23.0 | 3.4 | 3.1 | 3.7 | 3.0 | - | - | 2.2 |
| Series | Slag | Basic Activator Set | Additional Binders | ||||
|---|---|---|---|---|---|---|---|
| NaOH | Na2CO3 | CFBC | Mg(OH)2 | ||||
| A1 | 93 | 4 | 3 | - | - | ||
| A1-CA05 | 88.4 | 3.8 | 2.85 | 5 | - | ||
| A1-CA10 | 83.7 | 3.6 | 2.7 | 10 | - | ||
| A1-CA15 | 79.1 | 3.4 | 2.55 | 15 | - | ||
| A1-MH05 | 88.4 | 3.8 | 2.85 | - | 5 | ||
| A1-MH10 | 83.7 | 3.6 | 2.7 | - | 10 | ||
| A1-MH15 | 79.1 | 3.4 | 2.55 | - | 15 | ||
| Series | Slag | Na2SiO3 | CFBC | Mg(OH)2 | |||
| A2 | 90 | 10 | - | - | |||
| A2-CA05 | 85.5 | 9.5 | 5 | - | |||
| A2-CA10 | 81 | 9 | 10 | - | |||
| A2-CA15 | 76.5 | 8.5 | 15 | - | |||
| A2-MH05 | 85.5 | 9.5 | - | 5 | |||
| A2-MH10 | 81 | 9 | - | 10 | |||
| A2-MH15 | 76.5 | 8.5 | - | 15 | |||
| Series | Slag | Ca(OH)2 | Na2SO4 | Na2SiF6 | CFBC | Mg(OH)2 | |
| A3 | 88 | 7 | 3 | 2 | - | - | |
| A3-CA05 | 83.6 | 6.65 | 2.85 | 1.9 | 5 | - | |
| A3-CA10 | 79.2 | 6.3 | 2.7 | 1.8 | 10 | - | |
| A3-CA15 | 74.8 | 5.95 | 2.55 | 1.7 | 15 | - | |
| A3-MH05 | 83.6 | 6.65 | 2.85 | 1.9 | - | 5 | |
| A3-MH10 | 79.2 | 6.3 | 2.7 | 1.8 | - | 10 | |
| A3-MH15 | 74.8 | 5.95 | 2.55 | 1.7 | - | 15 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Song, K.-I.; Song, J.-K.; Jang, J.G. Effect of Carbonation on Abrasion Resistance of Alkali-Activated Slag with Various Activators. Materials 2019, 12, 2812. https://doi.org/10.3390/ma12172812
Kim H-K, Song K-I, Song J-K, Jang JG. Effect of Carbonation on Abrasion Resistance of Alkali-Activated Slag with Various Activators. Materials. 2019; 12(17):2812. https://doi.org/10.3390/ma12172812
Chicago/Turabian StyleKim, Hyeong-Ki, Keum-Il Song, Jin-Kyu Song, and Jeong Gook Jang. 2019. "Effect of Carbonation on Abrasion Resistance of Alkali-Activated Slag with Various Activators" Materials 12, no. 17: 2812. https://doi.org/10.3390/ma12172812
APA StyleKim, H.-K., Song, K.-I., Song, J.-K., & Jang, J. G. (2019). Effect of Carbonation on Abrasion Resistance of Alkali-Activated Slag with Various Activators. Materials, 12(17), 2812. https://doi.org/10.3390/ma12172812






