Effects of Substrate-Coating Materials on the Wound-Healing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Cell Viability Assay
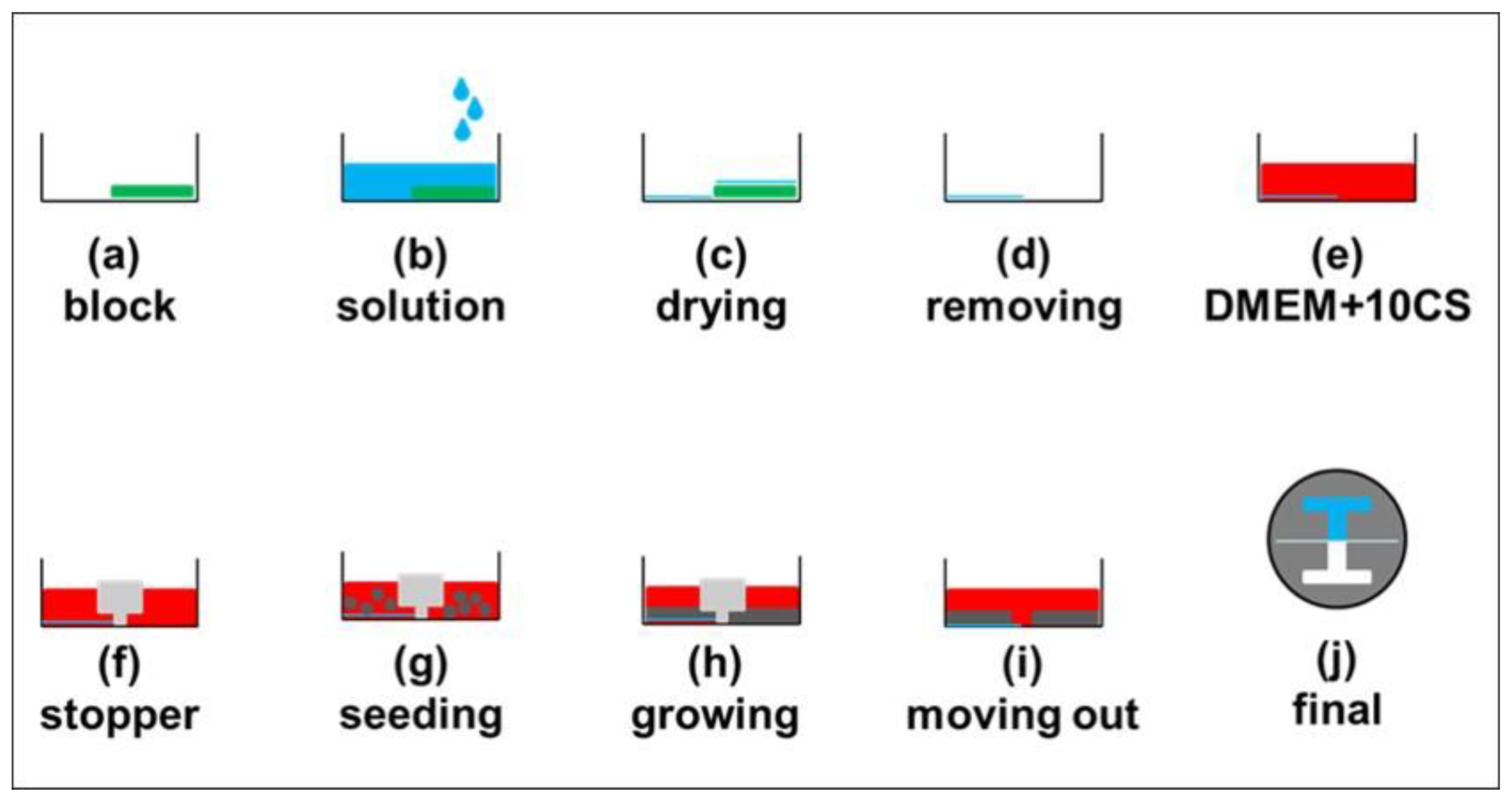
2.3. Fabrication of Barrier
2.4. Experimental Procedure
2.5. Data Analysis
3. Results and Discussion
3.1. Verifying the Presence of Coating Materials
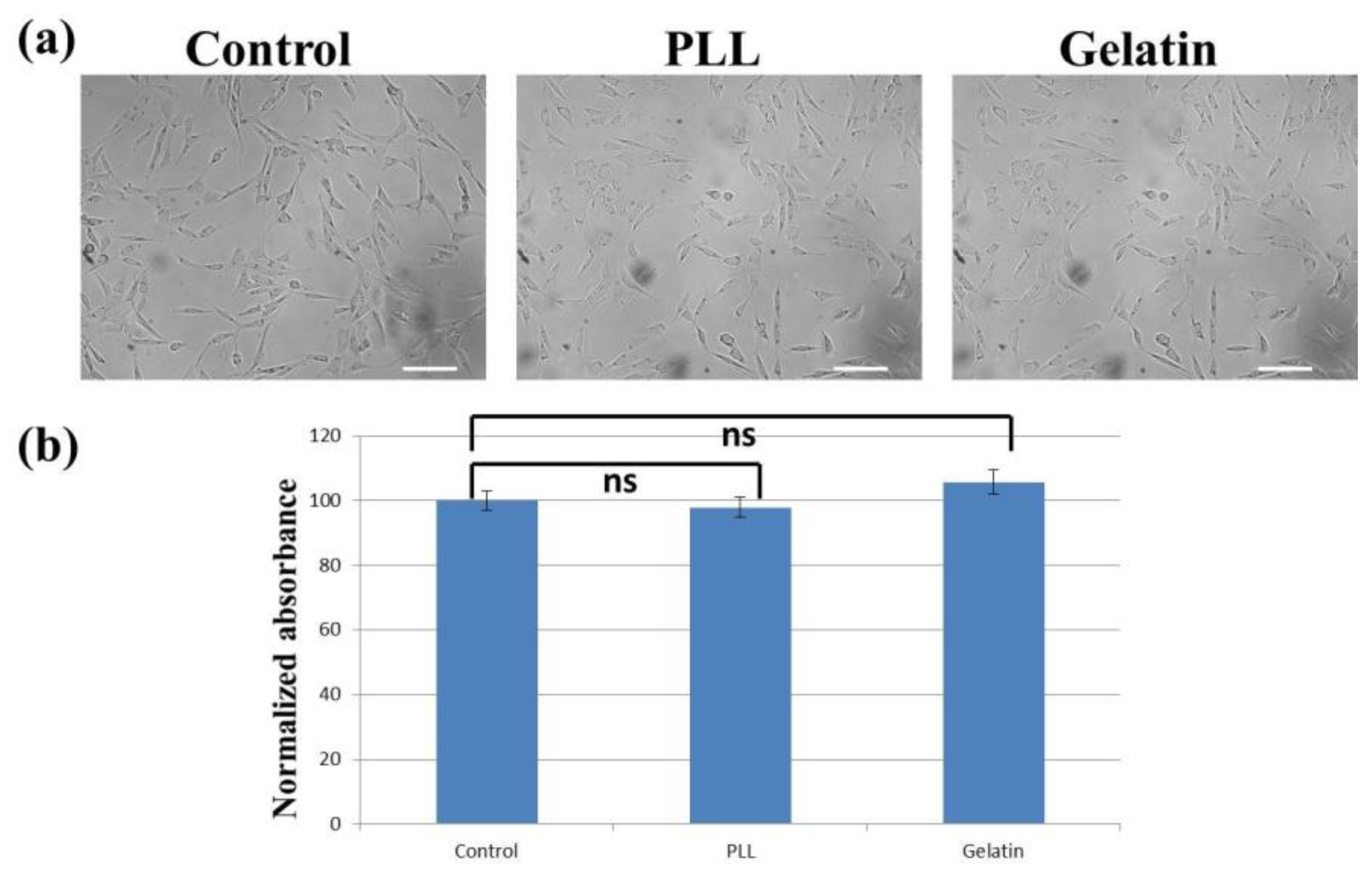
3.2. Cell Morphology and Proliferation/Viability
3.3. Dependence of Substrate-Coating Materials on the Wound-Healing Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roussos, E.T.; Condeelis, J.S.; Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer 2011, 11, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.R.; Sikes, R.A.; Nicholson, B.E.; Sun, Y.X.; Pienta, K.J.; Taichman, R.S. Cancer cells homing to bone: The significance of chemotaxis and cell adhesion. Cancer Treat. Res. 2004, 118, 291–309. [Google Scholar] [PubMed]
- Dormann, D.; Weijer, C.J. Chemotactic cell movement during Dictyostelium development and gastrulation. Curr. Opin. Genet. Dev. 2006, 16, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dormann, D.; Weijer, C.J. Chemotactic cell movement during development. Curr. Opin. Genet. Dev. 2003, 13, 358–364. [Google Scholar] [CrossRef]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 34117. [Google Scholar] [CrossRef]
- Tai, G.; Reid, B.; Cao, L.; Zhao, M. Electrotaxis and wound healing: Experimental methods to study electric fields as a directional signal for cell migration. Methods Mol. Biol. 2009, 571, 77–97. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Lan, C.C.; Lu, E.Y.; Pan, H.J.; Lee, C.H. Directional migration of cancer cells induced by a blue light intensity gradient. Biomed. Opt. Express 2015, 6, 2624–2632. [Google Scholar] [CrossRef]
- Mazzag, B.C.; Zhulin, I.B.; Mogilner, A. Model of bacterial band formation in aerotaxis. Biophys. J. 2003, 85, 3558–3574. [Google Scholar] [CrossRef]
- Bahat, A.; Caplan, S.R.; Eisenbach, M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 2012, 7, e41915. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Pink, D.; Chronopoulos, A.; Karim, S.A.; Morton, J.P.; Del Rio Hernandez, A.E. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci. Rep. 2017, 7, 2506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, X.; Li, N.; Wu, L.F.; Luo, C.; Ouyang, Q.; Tu, Y.; Chen, G. Angle sensing in magnetotaxis of Magnetospirillum magneticum AMB-1. Integr. Biol. Quant. Biosci. Nano Macro 2014, 6, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Yang, Y.; Zhang, D.D.; Wong, P.K. Advances in wound-healing assays for probing collective cell migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Shnyder, S.D.; Bibby, M.; Double, J.A.; Bicknel, R.; Jayson, G.C. Quantitative angiogenesis assays in vivo—A review. Angiogenesis 2004, 7, 1–16. [Google Scholar] [CrossRef]
- Varankar, S.S.; Bapat, S.A. Migratory Metrics of Wound Healing: A Quantification Approach for in vitro Scratch Assays. Front. Oncol. 2018, 8, 633. [Google Scholar] [CrossRef]
- Goetsch, K.P.; Niesler, C.U. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal. Biochem. 2011, 411, 158–160. [Google Scholar] [CrossRef]
- Yarrow, J.C.; Perlman, Z.E.; Westwood, N.J.; Mitchison, T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004, 4, 21. [Google Scholar] [CrossRef]
- Harma, V.; Schukov, H.P.; Happonen, A.; Ahonen, I.; Virtanen, J.; Siitari, H.; Akerfelt, M.; Lotjonen, J.; Nees, M. Quantification of dynamic morphological drug responses in 3D organotypic cell cultures by automated image analysis. PLoS ONE 2014, 9, e96426. [Google Scholar] [CrossRef]
- Van Horssen, R.; Ten Hagen, T.L. Crossing barriers: The new dimension of 2D cell migration assays. J. Cell. Physiol. 2011, 226, 288–290. [Google Scholar] [CrossRef]
- Zordan, M.D.; Mill, C.P.; Riese, D.J.; Leary, J.F. A high throughput, interactive imaging, bright-field wound healing assay. Cytom. Part A J. Int. Soc. Anal. Cytol. 2011, 79, 227–232. [Google Scholar] [CrossRef]
- Wu, S.Y.; Sun, Y.S.; Cheng, K.C.; Lo, K.Y. A Wound-Healing Assay Based on Ultraviolet Light Ablation. SLAS Technol. 2017, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Murrell, M.; Kamm, R.; Matsudaira, P. Tension, free space, and cell damage in a microfluidic wound healing assay. PLoS ONE 2011, 6, e24283. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, A.D.; Vermeul, K.; Poot, A.A.; Feijen, J.; Vermes, I. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H719–H725. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Wu, C.H.; Wang, M.H.; Chen, B.S.; Chiou, L.L.; Lee, H.S. Cooperative regulation of substrate stiffness and extracellular matrix proteins in skin wound healing of axolotls. BioMed Res. Int. 2015, 2015, 712546. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Prantl, L.; Vykoukal, J.; Loibl, M.; Felthaus, O. Differential Effects of Coating Materials on Viability and Migration of Schwann Cells. Materials 2016, 9, 150. [Google Scholar] [CrossRef]
- Liberio, M.S.; Sadowski, M.C.; Soekmadji, C.; Davis, R.A.; Nelson, C.C. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior. PLoS ONE 2014, 9, e112122. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef]
- Sloan, P. Current concepts of the role of fibroblasts and extracellular matrix in wound healing and their relevance to oral implantology. J. Dent. 1991, 19, 107–109. [Google Scholar] [CrossRef]
- Lacroix, B.; Didier, E.; Grenier, J.F. Role of pantothenic and ascorbic acid in wound healing processes: In vitro study on fibroblasts. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam. Ernahrungsforschung. J. Int. Vitaminol. Nutr. 1988, 58, 407–413. [Google Scholar]
- Pitingolo, G.; Riaud, A.; Nastruzzi, C.; Taly, V. Gelatin-Coated Microfluidic Channels for 3D Microtissue Formation: On-Chip Production and Characterization. Micromachines 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Tovar, G.E.M.; Hoch, E.; Southan, A. Gelatin methacrylamide as coating material in cell culture. Biointerphases 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ammann, K.R.; DeCook, K.J.; Tran, P.L.; Merkle, V.M.; Wong, P.K.; Slepian, M.J. Collective cell migration of smooth muscle and endothelial cells: Impact of injury versus non-injury stimuli. J. Biol. Eng. 2015, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Mazia, D.; Schatten, G.; Sale, W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 1975, 66, 198–200. [Google Scholar] [CrossRef] [PubMed]
- McKeehan, W.L.; Ham, R.G. Stimulation of clonal growth of normal fibroblasts with substrata coated with basic polymers. J. Cell Biol. 1976, 71, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-Y.; Lo, K.-Y.; Sun, Y.-S. Effects of Substrate-Coating Materials on the Wound-Healing Process. Materials 2019, 12, 2775. https://doi.org/10.3390/ma12172775
Lin J-Y, Lo K-Y, Sun Y-S. Effects of Substrate-Coating Materials on the Wound-Healing Process. Materials. 2019; 12(17):2775. https://doi.org/10.3390/ma12172775
Chicago/Turabian StyleLin, Jin-Young, Kai-Yin Lo, and Yung-Shin Sun. 2019. "Effects of Substrate-Coating Materials on the Wound-Healing Process" Materials 12, no. 17: 2775. https://doi.org/10.3390/ma12172775
APA StyleLin, J.-Y., Lo, K.-Y., & Sun, Y.-S. (2019). Effects of Substrate-Coating Materials on the Wound-Healing Process. Materials, 12(17), 2775. https://doi.org/10.3390/ma12172775





