Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Groups
2.3. Preparation of Eluates
2.4. Cell Viability (MTT Assay)
2.5. Quantification of Cytokines Released from Dental Pulp Cells in Culture
2.6. Statistical Analysis
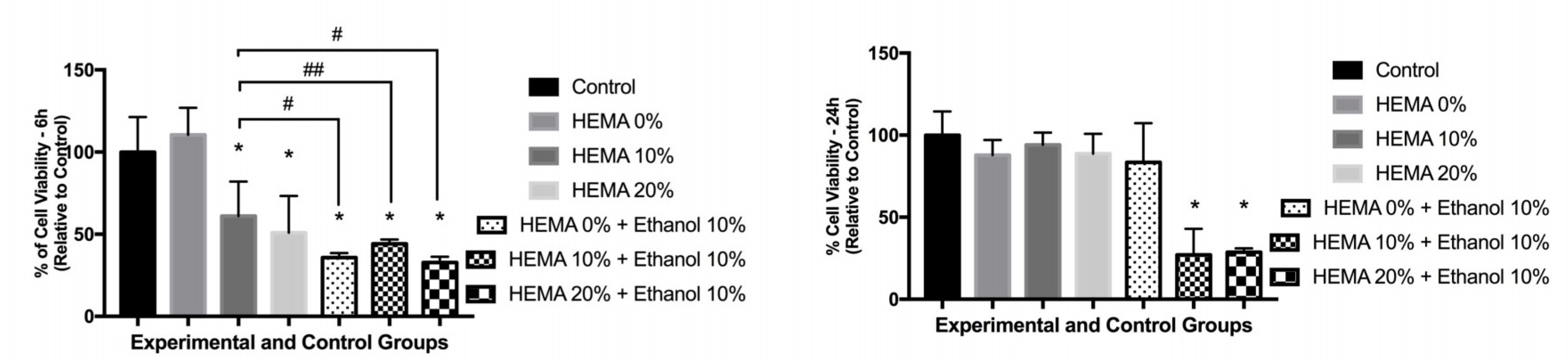
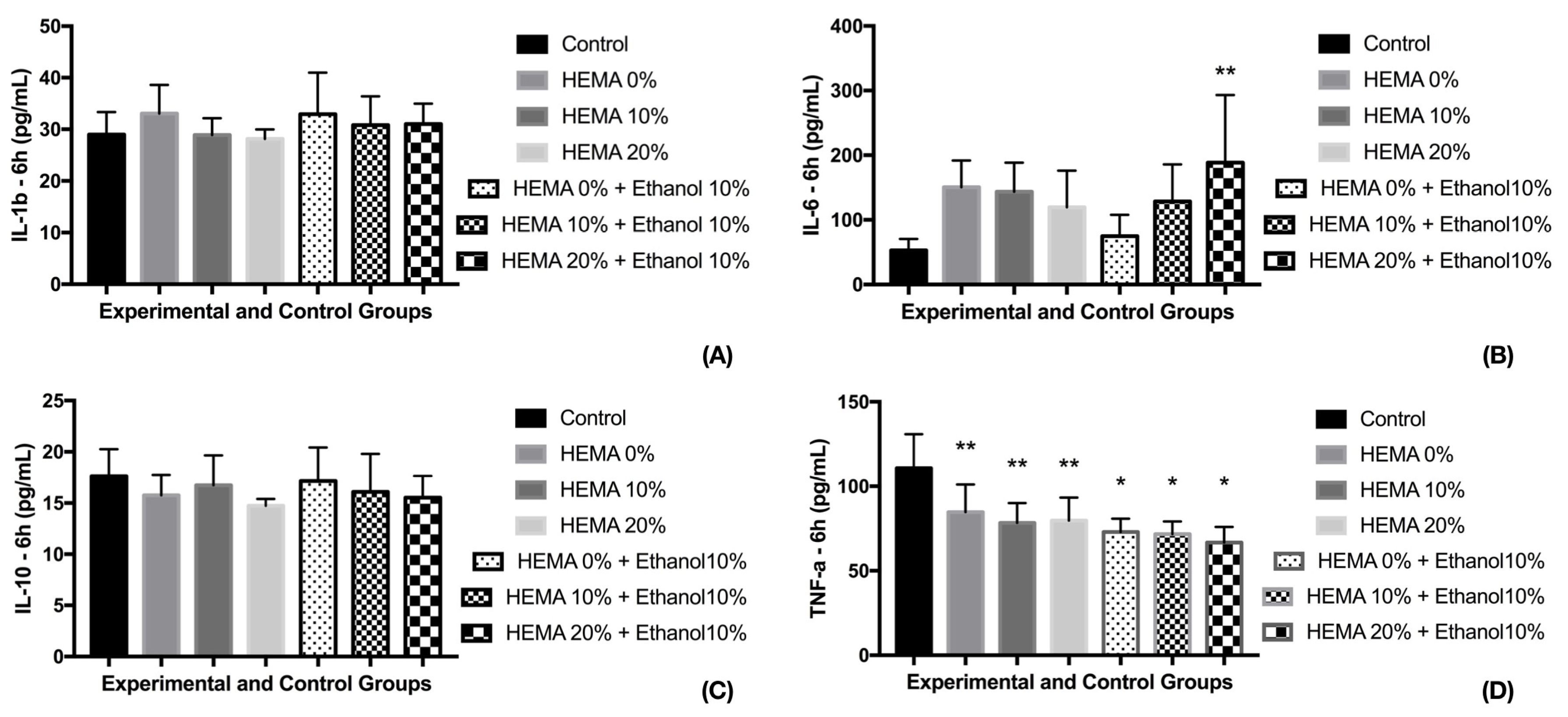
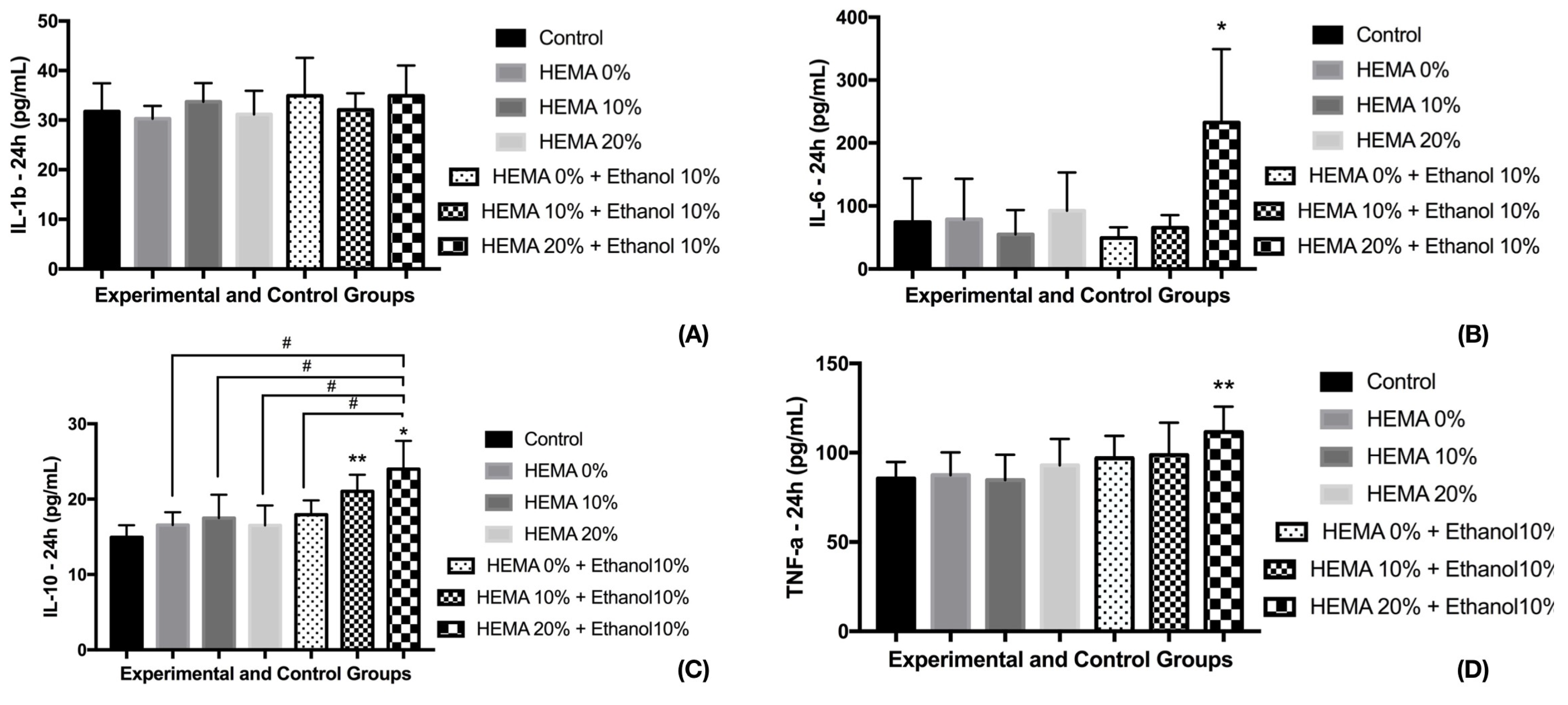
3. Results
3.1. Cell Viability and Cytokine Release (6 h)
3.2. Cell Viability and Cytokine Release (24 h)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lima, A.F.; Soares, G.P.; Vasconcellos, P.H.; Ambrosano, G.M.; Marchi, G.M.; Lovadino, J.R.; Aguiar, F.H. Effect of surface sealants on microleakage of Class II restorations after thermocycling and long-term water storage. J. Adhes. Dent. 2011, 13, 249–254. [Google Scholar] [PubMed]
- Leite, T.V.; Cavalcanti, A.N.; Lima, A.F.; Goncalves, L.S.; Watts, D.C.; Baron, G.M.M.; Martins, L.R.M. Light curing resin cements containing iodonium salts promote suitable apical bonding of posts to radicular dentin. Braz. Oral. Res. 2018, 32, e116. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Ferreira, S.F.; Catelan, A.; Palialol, A.R.; Goncalves, L.S.; Aguiar, F.H.; Marchi, G.M. The effect of surface treatment and bonding procedures on the bond strength of silorane composite repairs. Acta Odontol. Scand. 2014, 72, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.N.; de Lima, A.F.; Peris, A.R.; Mitsui, F.H.; Marchi, G.M. Effect of surface treatments and bonding agents on the bond strength of repaired composites. J. Esthet. Restor. Dent. 2007, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Fabiao, M.D.; Stape, T.H.S.; Yanikian, C.R.F.; de Lima, A.F.; Pizi, E.C.G.; Baron, G.M.M.; Martins, L.R.M. Influence of different adhesive protocols on ceramic bond strength and degree of conversion of resin cements. Int. J. Adhes. Adhes. 2015, 62, 7–13. [Google Scholar] [CrossRef]
- Papakonstantinou, A.E.; Eliades, T.; Cellesi, F.; Watts, D.C.; Silikas, N. Evaluation of UDMA’s potential as a substitute for Bis-GMA in orthodontic adhesives. Dent. Mater. 2013, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.E.; Lima, A.F.; Soares, G.P.; Ambrosano, G.M.; Marchi, G.M.; Lovadino, J.R.; Aguiar, F.H. Effect of preheating resin composite and light-curing units on the microleakage of Class II restorations submitted to thermocycling. Oper. Dent. 2011, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Lombardo, G.; Balloni, S.; Bodo, M.; Cianetti, S.; Barbati, A.; Montaseri, A.; Marinucci, L. Cytotoxicity of universal dental adhesive systems: Assessment in vitro assays on human gingival fibroblasts. Toxicol. Vitr. 2019, 60, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Ribeiro, A.P.; Basso, F.G.; Bagnato, V.S.; Hebling, J.; Marchi, G.M.; de Souza Costa, C.A. Effect of low-level laser therapy on odontoblast-like cells exposed to bleaching agent. Lasers Med. Sci. 2014, 29, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Ribeiro, A.P.; Soares, D.G.; Sacono, N.T.; Hebling, J.; de Souza Costa, C.A. Toxic effects of daily applications of 10% carbamide peroxide on odontoblast-like MDPC-23 cells. Acta Odontol. Scand. 2013, 71, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Lessa, F.C.; Mancini, M.N.; Hebling, J.; Costa, C.A.; Marchi, G.M. Transdentinal protective role of sodium ascorbate against the cytopathic effects of H2O2 released from bleaching agents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e70–e76. [Google Scholar] [CrossRef]
- de Souza Costa, C.A.; Teixeira, H.M.; do Nascimento, A.B.L.; Hebling, J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 175–184. [Google Scholar] [CrossRef] [PubMed]
- de Souza Costa, C.A.; Hebling, J.; Randall, R.C. Human pulp response to resin cements used to bond inlay restorations. Dent. Mater. 2006, 22, 954–962. [Google Scholar] [CrossRef]
- Costa, C.A.; Giro, E.M.; do Nascimento, A.B.; Teixeira, H.M.; Hebling, J. Short-term evaluation of the pulpo-dentin complex response to a resin-modified glass-ionomer cement and a bonding agent applied in deep cavities. Dent. Mater. 2003, 19, 739–746. [Google Scholar] [CrossRef]
- de Souza Costa, C.A.; do Nascimento, A.B.; Teixeira, H.M. Response of human pulps following acid conditioning and application of a bonding agent in deep cavities. Dent. Mater. 2002, 18, 543–551. [Google Scholar] [CrossRef]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation-regeneration interplay in the dentine-pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar]
- Ratanasathien, S.; Wataha, J.C.; Hanks, C.T.; Dennison, J.B. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J. Dent. Res. 1995, 74, 1602–1606. [Google Scholar] [CrossRef]
- Mahdhaoui, K.; Fournier, B.; Derbanne, M.A. Unbound monomers do diffuse through the dentin barrier. Dent. Mater. 2017, 33, 743–751. [Google Scholar] [CrossRef]
- Putzeys, E.; Duca, R.C.; Coppens, L.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. In-vitro transdentinal diffusion of monomers from adhesives. J. Dent. 2018, 75, 91–97. [Google Scholar] [CrossRef]
- Schweikl, H.; Gallorini, M.; Poschl, G.; Urmann, V.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W. Functions of transcription factors NF-kappaB and Nrf2 in the inhibition of LPS-stimulated cytokine release by the resin monomer HEMA. Dent. Mater. 2018, 34, 1661–1678. [Google Scholar] [CrossRef]
- Argolo, S.; Mathias, P.; Aguiar, T.; Lima, A.; Santos, S.; Foxton, R.; Cavalcanti, A. Effect of agitation and storage temperature on water sorption and solubility of adhesive systems. Dent. Mater. J. 2015, 34, 1–6. [Google Scholar] [CrossRef]
- Malacarne-Zanon, J.; Pashley, D.H.; Agee, K.A.; Foulger, S.; Alves, M.C.; Breschi, L.; Cadenaro, M.; Garcia, F.P.; Carrilho, M.R. Effects of ethanol addition on the water sorption/solubility and percent conversion of comonomers in model dental adhesives. Dent. Mater. 2009, 25, 1275–1284. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, L.I.; Chan, C.P.; Lee, J.J.; Wang, T.M.; Yang, T.T.; Lin, P.S.; Lin, H.J.; Chang, H.H.; Jeng, J.H. The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials 2010, 31, 8164–8171. [Google Scholar] [CrossRef]
- Eckhardt, A.; Gerstmayr, N.; Hiller, K.A.; Bolay, C.; Waha, C.; Spagnuolo, G.; Camargo, C.; Schmalz, G.; Schweikl, H. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials 2009, 30, 2006–2014. [Google Scholar] [CrossRef]
- de Lima, A.F.; Lessa, F.C.; Gasparoto Mancini, M.N.; Hebling, J.; de Souza Costa, C.A.; Marchi, G.M. Cytotoxic effects of different concentrations of a carbamide peroxide bleaching gel on odontoblast-like cells MDPC-23. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 907–912. [Google Scholar] [CrossRef]
- Krifka, S.; Petzel, C.; Hiller, K.A.; Frank, E.M.; Bosl, C.; Spagnuolo, G.; Reichl, F.X.; Schmalz, G.; Schweikl, H. Resin monomer-induced differential activation of MAP kinases and apoptosis in mouse macrophages and human pulp cells. Biomaterials 2010, 31, 2964–2975. [Google Scholar] [CrossRef]
- de Lima, F.M.; Villaverde, A.B.; Salgado, M.A.; Castro-Faria-Neto, H.C.; Munin, E.; Albertini, R.; Aimbire, F. Low intensity laser therapy (LILT) in vivo acts on the neutrophils recruitment and chemokines/cytokines levels in a model of acute pulmonary inflammation induced by aerosol of lipopolysaccharide from Escherichia coli in rat. J. Photochem. Photobiol. B 2010, 101, 271–278. [Google Scholar] [CrossRef]
- Collares, F.M.; Ogliari, F.A.; Zanchi, C.H.; Petzhold, C.L.; Piva, E.; Samuel, S.M. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J. Adhes. Dent. 2011, 13, 125–129. [Google Scholar]
- Van Landuyt, K.L.; Snauwaert, J.; Peumans, M.; De Munck, J.; Lambrechts, P.; Van Meerbeek, B. The role of HEMA in one-step self-etch adhesives. Dent. Mater. 2008, 24, 1412–1419. [Google Scholar] [CrossRef]
- Ginzkey, C.; Zinnitsch, S.; Steussloff, G.; Koehler, C.; Hackenberg, S.; Hagen, R.; Kleinsasser, N.H.; Froelich, K. Assessment of HEMA and TEGDMA induced DNA damage by multiple genotoxicological endpoints in human lymphocytes. Dent. Mater. 2015, 31, 865–876. [Google Scholar] [CrossRef]
- Cadenaro, M.; Breschi, L.; Rueggeberg, F.A.; Suchko, M.; Grodin, E.; Agee, K.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent. Mater. 2009, 25, 621–628. [Google Scholar] [CrossRef]
- Faria-e-Silva, A.L.; Lima, A.F.; Moraes, R.R.; Piva, E.; Martins, L.R. Degree of conversion of etch-and-rinse and self-etch adhesives light-cured using QTH or LED. Oper. Dent. 2010, 35, 649–654. [Google Scholar] [CrossRef]
- Gaglianone, L.A.; Lima, A.F.; Goncalves, L.S.; Cavalcanti, A.N.; Aguiar, F.H.; Marchi, G.M. Mechanical properties and degree of conversion of etch-and-rinse and self-etch adhesive systems cured by a quartz tungsten halogen lamp and a light-emitting diode. J. Mech. Behav. Biomed. Mater. 2012, 12, 139–143. [Google Scholar] [CrossRef]
- Cadenaro, M.; Breschi, L.; Antoniolli, F.; Navarra, C.O.; Mazzoni, A.; Tay, F.R.; Di Lenarda, R.; Pashley, D.H. Degree of conversion of resin blends in relation to ethanol content and hydrophilicity. Dent. Mater. 2008, 24, 1194–1200. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Chang, M.C.; Lin, L.D.; Chan, C.P.; Chang, H.H.; Chen, L.I.; Lin, H.J.; Yeh, H.W.; Tseng, W.Y.; Lin, P.S.; Lin, C.C.; et al. The effect of BisGMA on cyclooxygenase-2 expression, PGE2 production and cytotoxicity via reactive oxygen species- and MEK/ERK-dependent and -independent pathways. Biomaterials 2009, 30, 4070–4077. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef]
- Wellner, P.; Mayer, W.; Hickel, R.; Reichl, F.X.; Durner, J. Cytokine release from human leukocytes exposed to silorane- and methacrylate-based dental materials. Dent. Mater. 2012, 28, 743–748. [Google Scholar] [CrossRef]
- Hack, C.E.; Aarden, L.A.; Thijs, L.G. Role of cytokines in sepsis. Adv. Immunol. 1997, 66, 101–195. [Google Scholar]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J. Intensive Care Med. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Chernoff, A.E.; Granowitz, E.V.; Shapiro, L.; Vannier, E.; Lonnemann, G.; Angel, J.B.; Kennedy, J.S.; Rabson, A.R.; Wolff, S.M.; Dinarello, C.A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J. Immunol. 1995, 154, 5492–5499. [Google Scholar]
- Polydorou, O.; Konig, A.; Hellwig, E.; Kummerer, K. Long-term release of monomers from modern dental-composite materials. Eur. J. Oral Sci. 2009, 117, 68–75. [Google Scholar] [CrossRef]
- Yang, Y.; Reichl, F.X.; Ilie, N.; Shi, J.; Dhein, J.; Hickel, R.; Hogg, C. Antioxidants as a novel dental resin-composite component: Effect on elution and degree of conversion. Dent. Mater. 2019, 35, 650–661. [Google Scholar] [CrossRef]
- Cetinguc, A.; Olmez, S.; Vural, N. HEMA diffusion from dentin bonding agents in young and old primary molars in vitro. Dent. Mater. 2007, 23, 302–307. [Google Scholar] [CrossRef]
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Goncalves, L.S.; Hadis, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78. [Google Scholar] [CrossRef]
- Andrade, K.M.; Palialol, A.R.; Lancellotti, A.C.; Aguiar, F.H.; Watts, D.C.; Goncalves, L.S.; Lima, A.F.; Marchi, G.M. Effect of diphenyliodonium hexafluorphosphate on resin cements containing different concentrations of ethyl 4-(dimethylamino)benzoate and 2-(dimethylamino)ethyl methacrylate as co-initiators. Dent. Mater. 2016, 32, 749–755. [Google Scholar] [CrossRef]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1,2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of nanohydroxyapatite, antibiotic, and mucosal defensive agent on the mechanical and thermal properties of glass ionomer cements for special needs patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
| Group | Composition (%) | |||
|---|---|---|---|---|
| Bis-GMA | TEGDMA | Ethanol | HEMA | |
| G1 | 50 | 50 | − | − |
| G2 | 45 | 45 | − | 10 |
| G3 | 40 | 40 | − | 20 |
| G4 | 45 | 45 | 10 | − |
| G5 | 40 | 40 | 10 | 10 |
| G6 | 35 | 35 | 10 | 20 |
| G0—Control (without treatment) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, H.; Zambelli, L.F.A.; Britto, A.A.d.; Vieira, R.P.; Ligeiro-de-Oliveira, A.P.; Andia, D.C.; Oliveira, M.T.; Lima, A.F. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials 2019, 12, 2750. https://doi.org/10.3390/ma12172750
Massaro H, Zambelli LFA, Britto AAd, Vieira RP, Ligeiro-de-Oliveira AP, Andia DC, Oliveira MT, Lima AF. Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials. 2019; 12(17):2750. https://doi.org/10.3390/ma12172750
Chicago/Turabian StyleMassaro, Helder, Lígia F. A. Zambelli, Auriléia A. de Britto, Rodolfo P. Vieira, Ana P. Ligeiro-de-Oliveira, Denise C. Andia, Marcelo T. Oliveira, and Adriano F. Lima. 2019. "Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells" Materials 12, no. 17: 2750. https://doi.org/10.3390/ma12172750
APA StyleMassaro, H., Zambelli, L. F. A., Britto, A. A. d., Vieira, R. P., Ligeiro-de-Oliveira, A. P., Andia, D. C., Oliveira, M. T., & Lima, A. F. (2019). Solvent and HEMA Increase Adhesive Toxicity and Cytokine Release from Dental Pulp Cells. Materials, 12(17), 2750. https://doi.org/10.3390/ma12172750






