Biomechanical Study of a Novel, Expandable, Non-Metallic and Radiolucent CF/PEEK Vertebral Body Replacement (VBR)
Abstract
1. Introduction
2. Materials and Methods
3. Study Protocol
- (1)
- Flexibility test, Native (native):Corpectomy and instrumentation with VBR and pedicle fixation with CF/PEEK rod.
- (2)
- Flexibility test—CF/PEEK VBR instrumented with CF/PEEK rod (CF_CF):Change of posterior rod fixation to titanium rod.
- (3)
- Flexibility test—CF/PEEK VBR Instrumented with titanium rod, (CF_Ti):Change of VBR to titanium.
- (4)
- Flexibility test—titanium VBR instrumented with titanium rod (Ti_Ti):Addition of two cross connectors to the posterior rods dissecting the ligamentum supraspinale/interspinale.
- (5)
- Flexibility test—titanium VBR instrumented with titanium rod and 2 cross connectors (Ti_Ti_cc):Change to CF/PEEK VBR.
- (6)
- Flexibility test—CF/PEEK VBR instrumented with titanium rod and 2 cross connectors (CF_Ti_cc):Change rods to CF/PEEK.
- (7)
- Flexibility test—CF/PEEK VBR Instrumented with CF/PEEK rod and 2 cross connectors (CF_CF_cc).
4. Results
4.1. Index Segments (Th12-L2)
4.2. Caudal Adjacent Segment (L2/3)
4.3. Cranial Adjacent Segment (Th11/12)
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, K.S.; Wenger, K.H.; Schlegel, J.D.; Dunn, H.K. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine 1994, 19, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Gradl, G. Combined stabilization of thoracolumbar spine fractures. Eur. J. Trauma 2006, 32, 249–252. [Google Scholar] [CrossRef]
- Schnake, K.J.; Stavridis, S.I.; Kandziora, F. Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J. Neurosurg. Spine 2014, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ulmar, B.; Erhart, S.; Unger, S.; Weise, K.; Schmoelz, W. Biomechanical analysis of a new expandable vertebral body replacement combined with a new polyaxial antero-lateral plate and/or pedicle screws and rods. Eur. Spine J. 2012, 21, 546–553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohlmann, A.; Zander, T.; Fehrmann, M.; Klockner, C.; Bergmann, G. Influence of implants for vertebral body replacement on the mechanical behavior of the lumbar spine. Orthopade 2002, 31, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Knop, C.; Lange, U.; Bastian, L.; Blauth, M. Three-dimensional motion analysis with Synex. Comparative biomechanical test series with a new vertebral body replacement for the thoracolumbar spine. Eur. Spine J. 2000, 9, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Khodadadyan-Klostermann, C.; Schaefer, J.; Schleicher, P.; Pflugmacher, R.; Eindorf, T.; Haas, N.P.; Kandziora, F. Expandable cages: biomechanical comparison of different cages for ventral spondylodesis in the thoracolumbar spine. Chirurg 2004, 75, 694–701. [Google Scholar] [PubMed]
- Pflugmacher, R.; Schleicher, P.; Schaefe, J.; Scholz, M.; Ludwig, K.; Khodadadyan-Klostermann, C.; Haas, N.P.; Kandziora, F. Biomechanical comparison of expandable cages for the vertebral body replacement in the thoracolumbar spine. Spine 2004, 29, 1413–1419. [Google Scholar] [CrossRef]
- Wilke, H.J.; Wenger, K.; Claes, L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 1998, 7, 148–154. [Google Scholar] [CrossRef]
- Schulte, M.; Schultheiss, M.; Hartwig, E.; Wilke, H.J.; Wolf, S.; Sokiranski, R.; Fleiter, T.; Kinzl, L.; Claes, L. Vertebral body replacement with a bioglass-polyurethane composite in spine metastases—Clinical, radiological and biomechanical results. Eur. Spine J. 2000, 9, 437–444. [Google Scholar] [CrossRef]
- Disch, A.C.; Luzatti, A.; Melcher, I.; Schaser, K.D.; Feraboli, F.; Schmoelz, W. Three-dimensional stiffness in a thoracolumbar en-bloc spondylectomy model: A biomechanical in vitro study. Clin. Biomech. 2007, 22, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.C.; Schaser, K.D.; Melcher, I.; Luzzati, A.; Feraboli, F.; Schmoelz, W. En bloc spondylectomy reconstructions in a biomechanics in-vitro study. Eur. Spine J. 2008, 17, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.C.; Knop, C.; Schaser, K.D.; Blauth, M.; Schmoelz, W. Angular stable anterior plating following thoracolumbar corpectomy reveals superior segmental stability compared to conventional polyaxial plate fixation. Spine 2008, 33, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.D. Orthopedic surgical management of skeletal complications of malignancy. Cancer 1997, 80, 1614–1627. [Google Scholar] [CrossRef]
- Adler, D.; Kriegsmann, M.; Sinn, P.; Schneeweiss, A.; Almansour, H.; Lehner, B.; Akbar, M. Metastatic breast cancer in the spine: Molecular predictors for choosing adequate treatment strategies. Orthopade 2018, 47, 594–603. [Google Scholar] [CrossRef]
- Adler, D.; Almansour, H.; Akbar, M. What is actually adult spinal deformity? Development, classification, and indications for surgical treatment. Orthopade 2018, 47, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; Biagini, R.; De Lure, F.; Bertoni, F.; Malaguti, M.C.; Di Fiore, M.; Zanoni, A. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine 1996, 21, 1927–1931. [Google Scholar] [CrossRef]
- Sundaresan, N.; Steinberger, A.A.; Moore, F.; Sachdev, V.P.; Krol, G.; Hough, L.; Kelliher, K. Indications and results of combined anterior-posterior approaches for spine tumor surgery. J. Neurosurg. 1996, 85, 438–446. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Baba, H.; Tsuchiya, H.; Nagata, S.; Toribatake, Y. Total en bloc spondylectomy for solitary spinal metastases. Int. Orthop. 1994, 18, 291–298. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Bouchard, J.A.; Koka, A.; Bensusan, J.S.; Stevenson, S.; Emery, S.E. Effects of irradiation on posterior spinal fusions. A rabbit model. Spine 1994, 19, 1836–1841. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Mansfield, F.L.; Borges, L.F. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine 2001, 26, 818–824. [Google Scholar] [CrossRef]
- Thongtrangan, I.; Balabhadra, R.S.; Le, H.; Park, J.; Kim, D.H. Vertebral body replacement with an expandable cage for reconstruction after spinal tumor resection. Neurosurg. Focus 2003, 15, 1–6. [Google Scholar] [CrossRef]
- Brandão, R.A.C.S.; Martins, W.C.D.S.; Arantes Jr, A.A.; Gusmão, S.N.S.; Perrin, G.; Barrey, C. Titanium versus polyetheretherketone implants for vertebral body replacement in the treatment of 77 thoracolumbar spinal fractures. Surg. Neurol. Int. 2017, 8, 191. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Krag, M.; Summers, D.; Videman, T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J. Orthop. Res. 1985, 3, 292–300. [Google Scholar] [CrossRef]
- Wilke, H.J.; Jungkunz, B.; Wenger, K.; Claes, L.E. Spinal segment range of motion as a function of in vitro test conditions: effects of exposure period, accumulated cycles, angular deformation rate, and moisture condition. Anat. Rec. 1998, 251, 15–19. [Google Scholar] [CrossRef]
- Panjabi, M.M. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine 1988, 13, 1129–1134. [Google Scholar] [CrossRef]
- Wippermann, B.W.; Schratt, H.E.; Steeg, S.; Tscherne, H. Complications of spongiosa harvesting of the ilial crest. A retrospective analysis of 1191 cases. Chirurg 1997, 68, 1286–1291. [Google Scholar] [CrossRef]
- Schmoelz, W.; Schaser, K.D.; Knop, C.; Blauth, M.; Disch, A.C. Extent of corpectomy determines primary stability following isolated anterior reconstruction in a thoracolumbar fracture model. Clin. Biomech. 2010, 25, 16–20. [Google Scholar] [CrossRef]
- Eichholz, K.M.; Hitchon, P.W.; From, A.; Rubenbauer, P.; Nakamura, S.; Lim, T.H.; Torner, J. Biomechanical testing of anterior and posterior thoracolumbar instrumentation in the cadaveric spine. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves. J. Neurosurg. Spine 2004, 1, 116–121. [Google Scholar] [CrossRef]
- Vahldiek, M.J.; Panjabi, M.M. Stability potential of spinal instrumentations in tumor vertebral body replacement surgery. Spine 1998, 23, 543–550. [Google Scholar] [CrossRef]
- Brantigen, J.W.; Steffee, A.D.; Geiger, J.M. A carbon Fiber implant to aid interbody lumbar fusion. Mechanical testing. Spine 1991, 16, 277–282. [Google Scholar] [CrossRef]
- Brantigen, J.W.; Steffee, A.D. A carbon Fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine 1993, 18, 2106–2107. [Google Scholar] [CrossRef]
- Oda, I.; Cunningham, B.W.; Abumi, K.; Kaneda, K.; McAfee, P.C. The stability of reconstruction methods after thoracolumbar total spondylectomy. An in vitro investigation. Spine 1999, 24, 1634–1638. [Google Scholar] [CrossRef]
- Shannon, F.J.; DiResta, G.R.; Ottaviano, D.; Castro, A.; Healey, J.H.; Boland, P.J. Biomechanical analysis of anterior poly-methylmethacrylate reconstruction following total spondylectomy for metastatic disease. Spine 2004, 29, 2012–2096. [Google Scholar] [CrossRef]
- Knoller, S.M.; Meyer, G.; Eckhardt, C.; Lill, C.A.; Schneider, E.; Linke, B. Range of motion in reconstruction situations following corpectomy in the lumbar spine: a question of bone mineral density? Spine 2005, 30, E229–E235. [Google Scholar] [CrossRef]
- Lange, T.; Schulte, T.L.; Gosheger, G.; Boevingloh, A.S.; Mayr, R.; Schmoelz, W. Effects of multilevel posterior ligament dissection after spinal instrumentation on adjacent segment biomechanics as a potential risk factor for proximal junctional kyphosis: a biomechanical study. BMC Musculoskelet. Disord. 2018, 19, 57. [Google Scholar] [CrossRef]
- Gedet, P.; Thistlethwaite, P.A.; Ferguson, S.J. Minimizing errors during in vitro testing of multisegmental spine specimens: considerations for component selection and kinematic measurement. J. Biomech. 2007, 40, 1881–1885. [Google Scholar] [CrossRef]



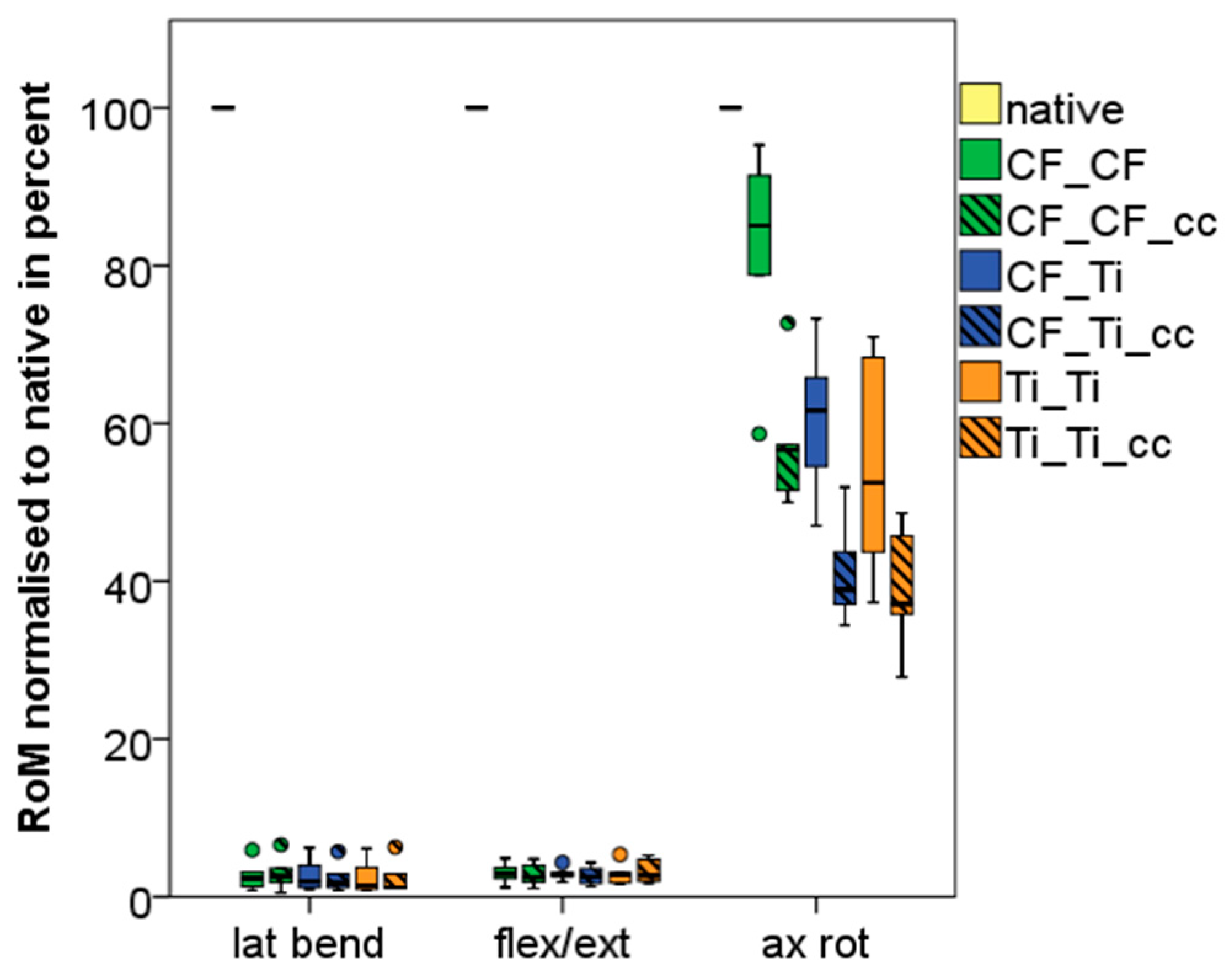
| Lateral Bending Th12-L2 | Flexion/Extension Th12-L2 | Axial Rotation Th12-L2 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Native | 11.17 | 3.26 | 10.32 | 2,40 | 4.83 | 0.95 |
| CF_CF | 0.27 | 0.18 | 0.33 | 0.18 | 3.95 | 0.90 |
| CF_CF_cc | 0.30 | 0.21 | 0.30 | 0.20 | 2.74 | 0.46 |
| CF_Ti | 0.27 | 0.20 | 0.30 | 0.12 | 2.88 | 0.40 |
| CF_Ti_cc | 0.24 | 0.18 | 0.29 | 0.17 | 1.95 | 0.33 |
| Ti_Ti | 0.25 | 0.21 | 0.31 | 0.17 | 2.55 | 0.53 |
| Ti_Ti_cc | 0.24 | 0.21 | 0.35 | 0.22 | 1.84 | 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adler, D.; Akbar, M.; Spicher, A.; Goerke, S.-A.; Schmoelz, W. Biomechanical Study of a Novel, Expandable, Non-Metallic and Radiolucent CF/PEEK Vertebral Body Replacement (VBR). Materials 2019, 12, 2732. https://doi.org/10.3390/ma12172732
Adler D, Akbar M, Spicher A, Goerke S-A, Schmoelz W. Biomechanical Study of a Novel, Expandable, Non-Metallic and Radiolucent CF/PEEK Vertebral Body Replacement (VBR). Materials. 2019; 12(17):2732. https://doi.org/10.3390/ma12172732
Chicago/Turabian StyleAdler, Daniel, Michael Akbar, Anna Spicher, Stephanie-Alice Goerke, and Werner Schmoelz. 2019. "Biomechanical Study of a Novel, Expandable, Non-Metallic and Radiolucent CF/PEEK Vertebral Body Replacement (VBR)" Materials 12, no. 17: 2732. https://doi.org/10.3390/ma12172732
APA StyleAdler, D., Akbar, M., Spicher, A., Goerke, S.-A., & Schmoelz, W. (2019). Biomechanical Study of a Novel, Expandable, Non-Metallic and Radiolucent CF/PEEK Vertebral Body Replacement (VBR). Materials, 12(17), 2732. https://doi.org/10.3390/ma12172732






