A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Doxorubicin-Containing Liposomes
2.2. Release Profile of Doxorubicin
2.3. Formation of Cell Spheroids with Human Gingiva-Derived Stem Cells
2.4. Evaluation of Spheroid Morphology and Determination of Quantitative Cell Viability
2.5. Uptake of Doxorubicin by Cell Spheroids with Human Gingiva-Derived Stem Cells
2.6. Osteogenic Differentiation Using Alkaline Phosphatase Activity Assays and Alizarin Red S Staining
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
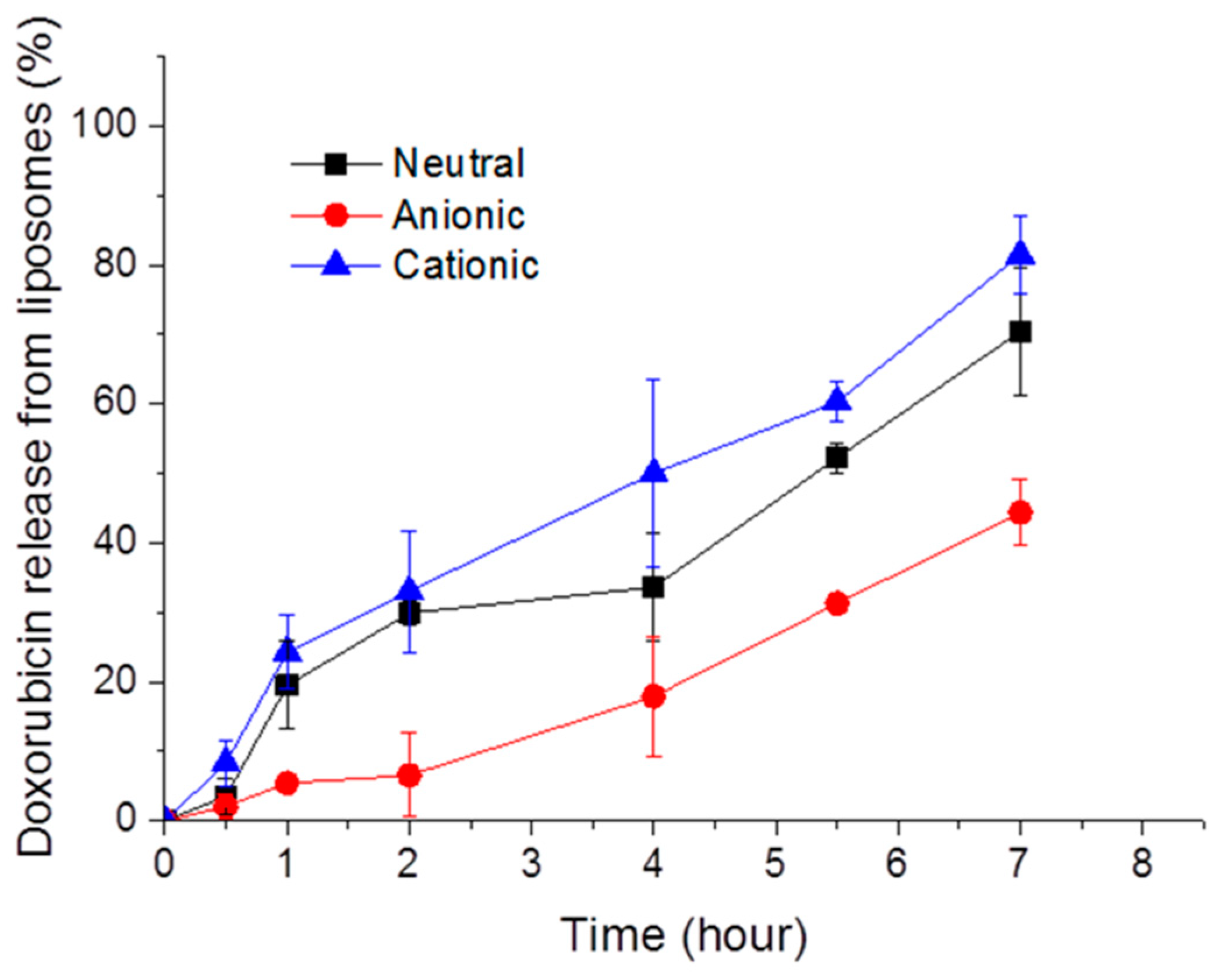
3.1. Release Profile of Doxorubicin
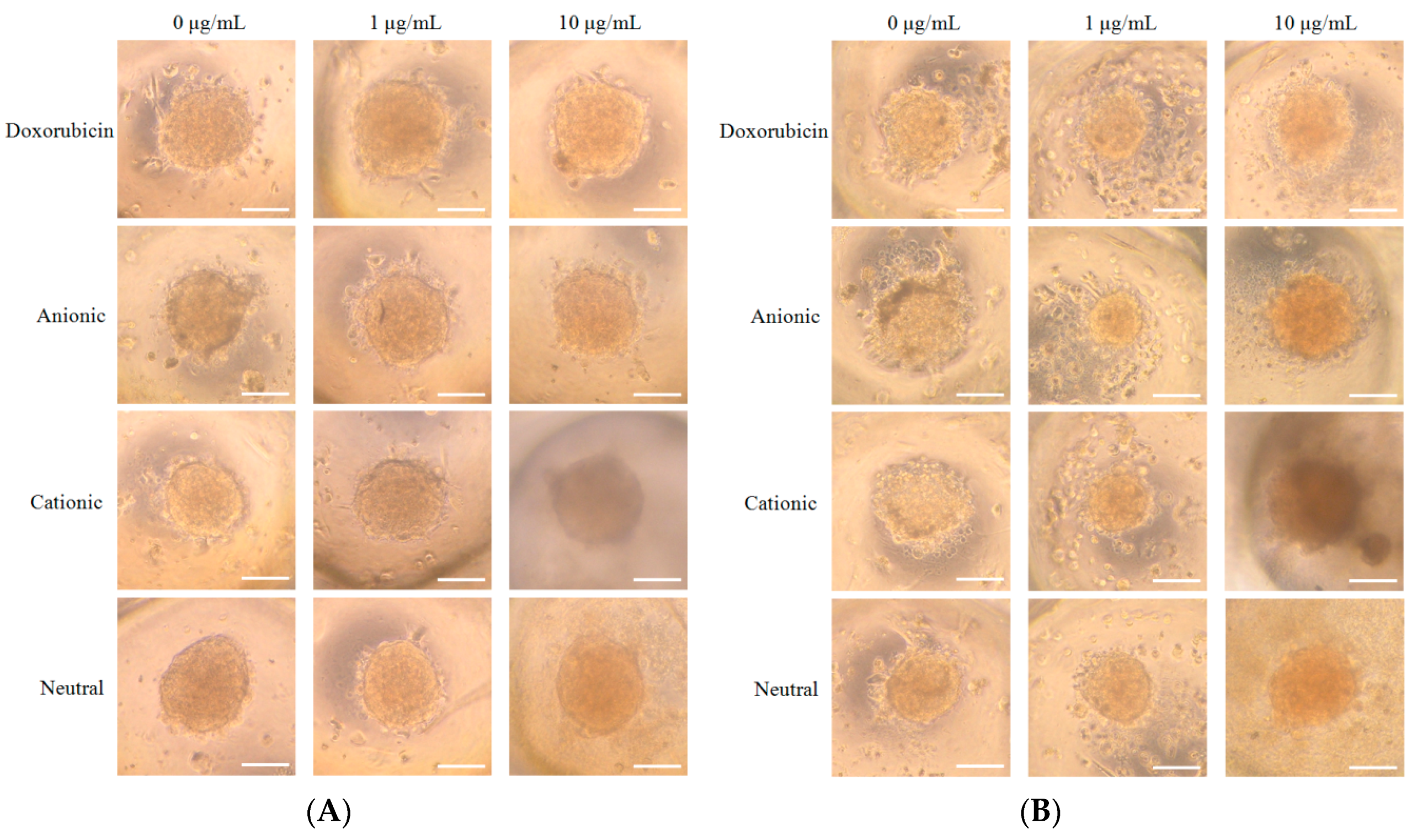
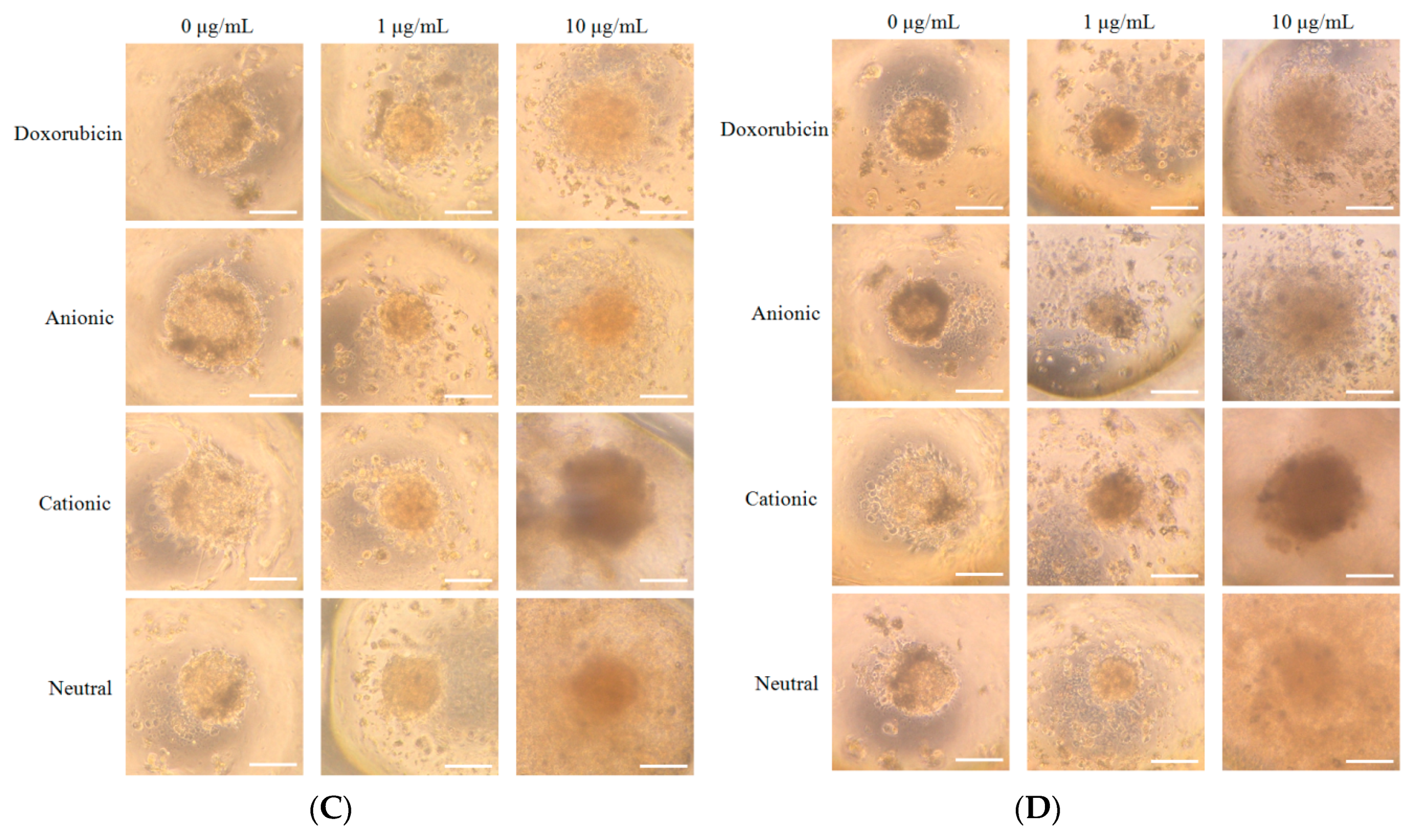
3.2. Cellular Morphology and Cellular Viability
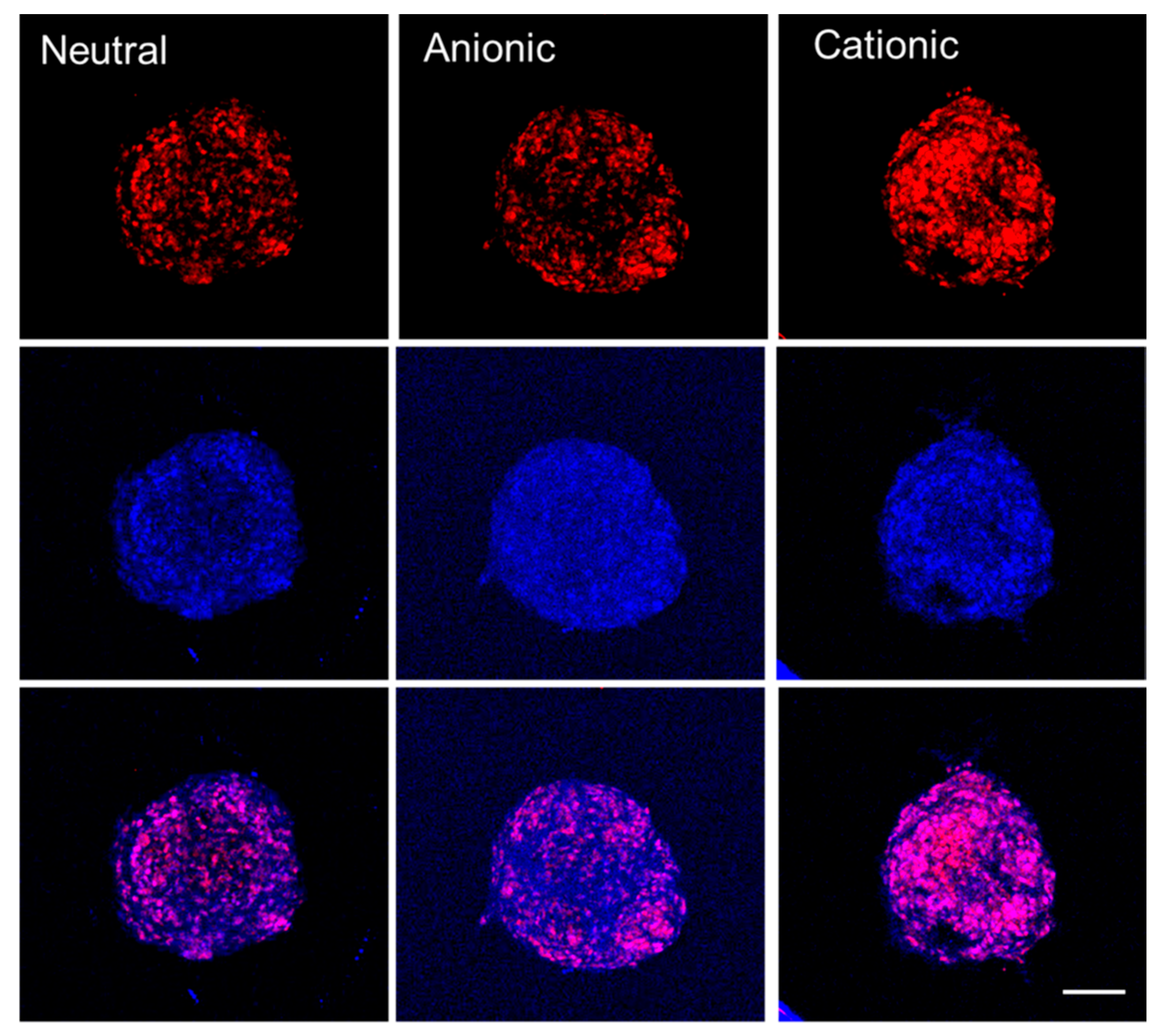
3.3. Uptake of Doxorubicin by Cell Spheroids with Human Gingiva-Derived Stem Cells
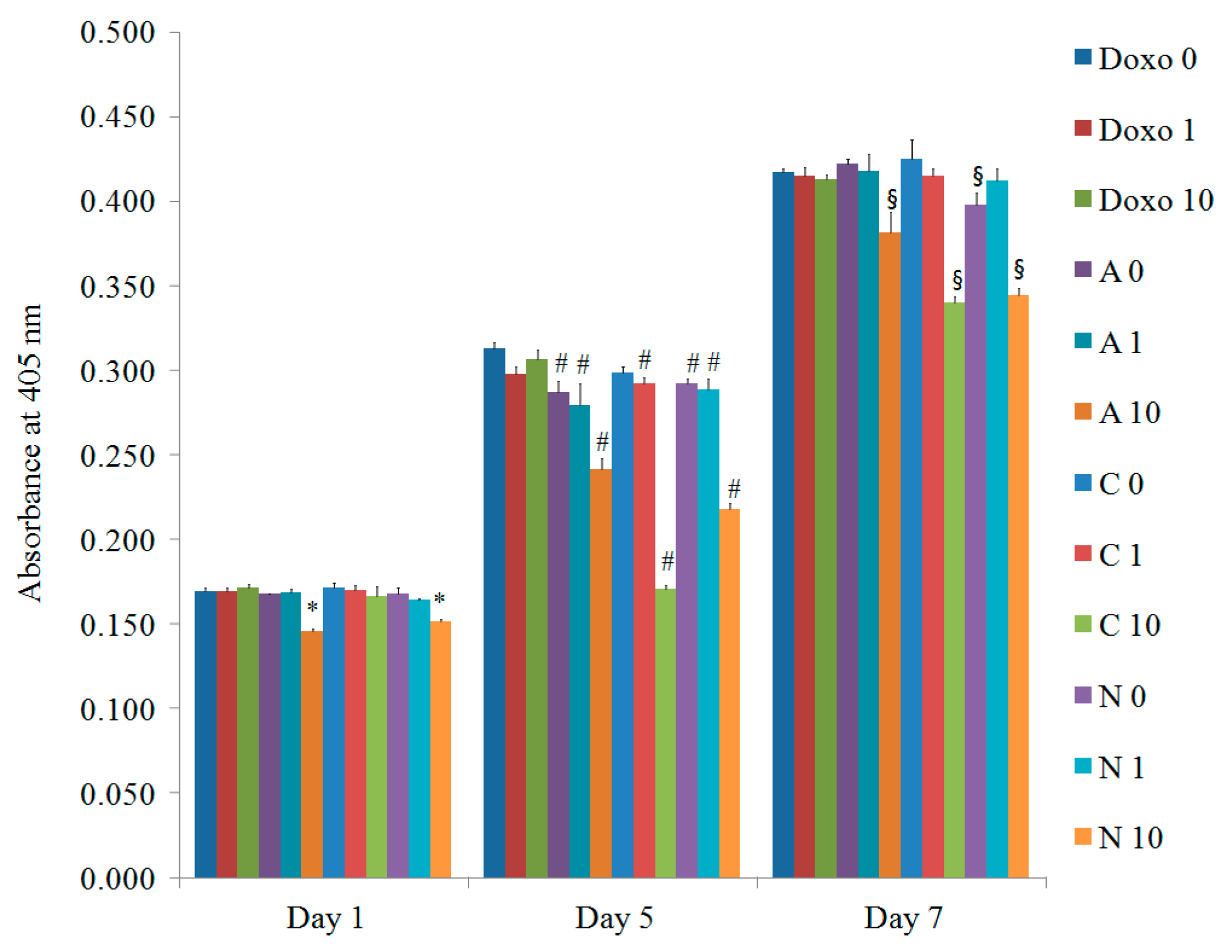
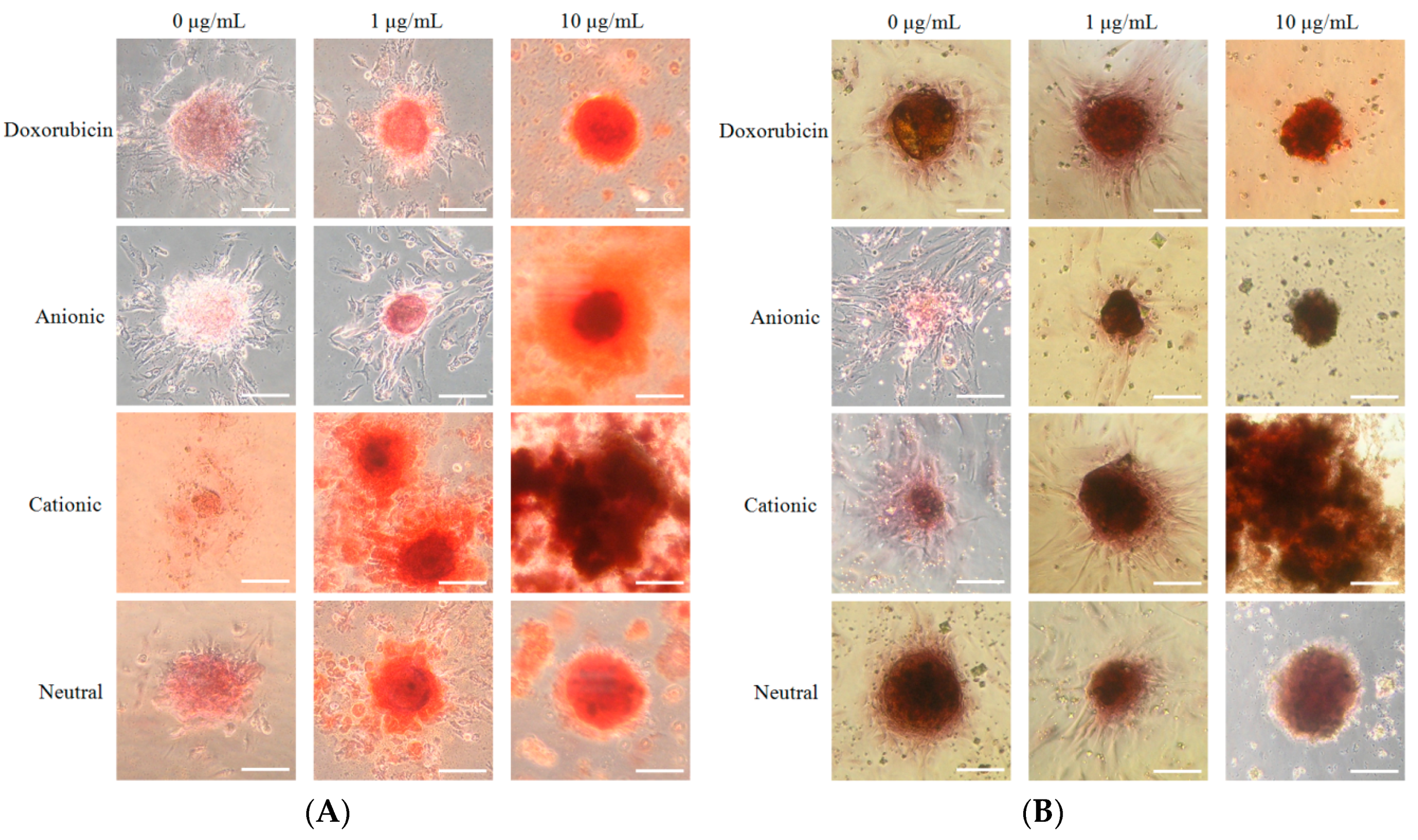
3.4. Evaluation of Osteogenic Differentiation
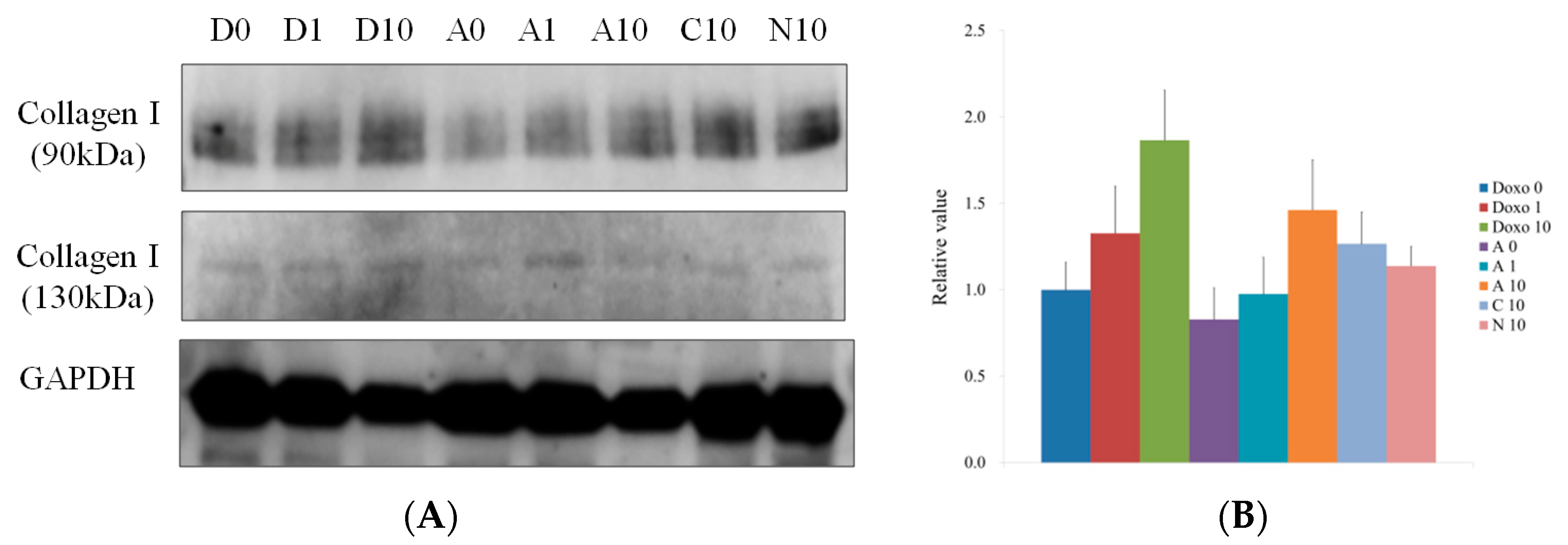
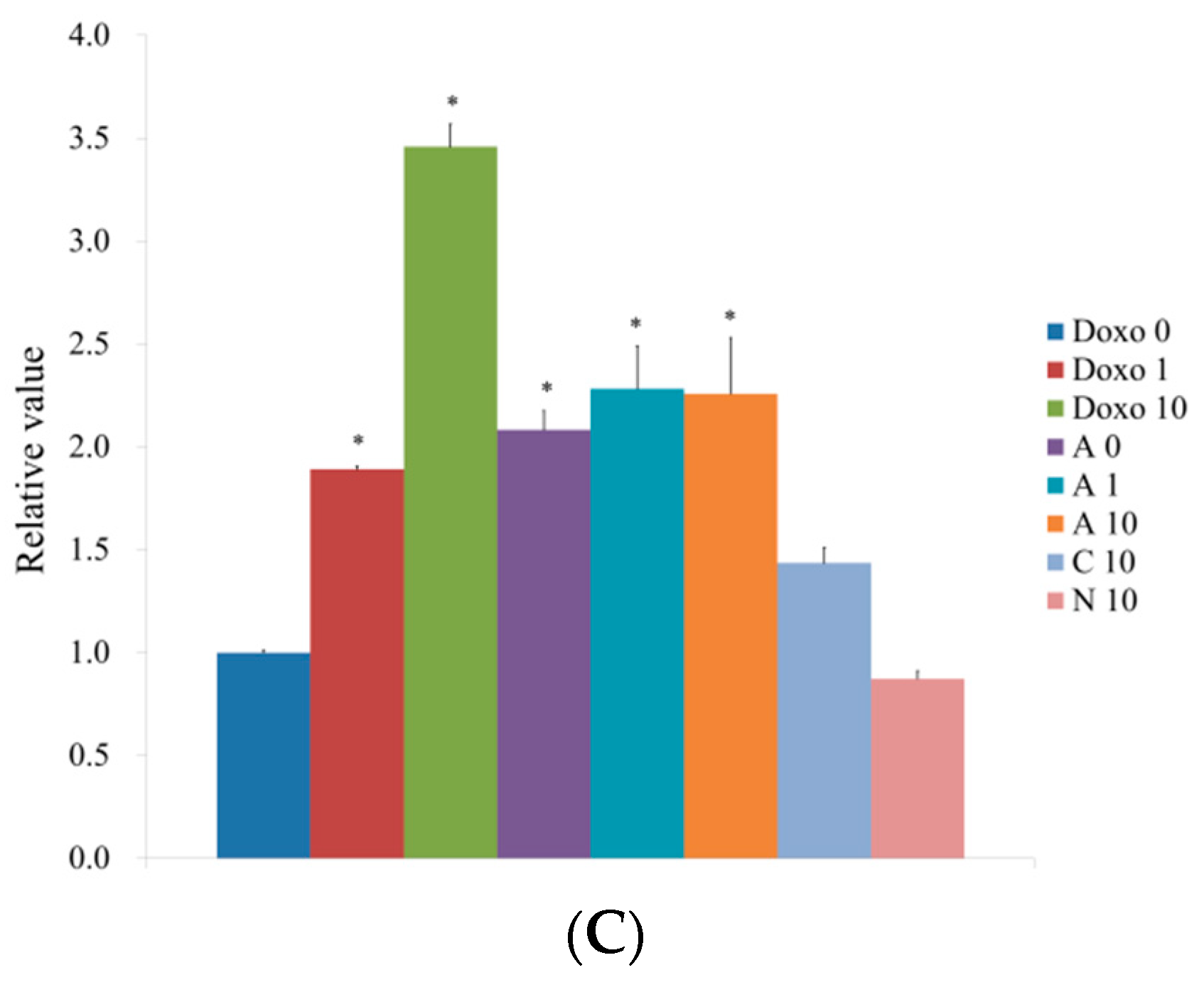
3.5. Western Blot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lomovskaya, N.; Otten, S.L.; Doi-Katayama, Y.; Fonstein, L.; Liu, X.-C.; Takatsu, T.; Inventi-Solari, A.; Filippini, S.; Torti, F.; Colombo, A.L.; et al. Doxorubicin Overproduction in Streptomyces peucetius: Cloning and Characterization of the dnrU Ketoreductase and dnrV Genes and the doxA Cytochrome P-450 Hydroxylase Gene. J. Bacteriol. 1999, 181, 305–318. [Google Scholar] [PubMed]
- Yoo, H.S.; Lee, K.H.; Oh, J.E.; Park, T.G. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin–PLGA conjugates. J. Control. Release 2000, 68, 419–431. [Google Scholar] [CrossRef]
- Pearce, A.K.; Simpson, J.D.; Fletcher, N.L.; Houston, Z.H.; Fuchs, A.V.; Russell, P.J.; Whittaker, A.K.; Thurecht, K.J. Localised delivery of doxorubicin to prostate cancer cells through a PSMA-targeted hyperbranched polymer theranostic. Biomaterials 2017, 141, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, R.M.; Eaton, K. Toxicity of chemotherapy. Hematol. Oncol. Clin. N. Am. 1996, 10, 967–990. [Google Scholar] [CrossRef]
- Burridge, P.W.; Li, Y.F.; Matsa, E.; Wu, H.; Ong, S.-G.; Sharma, A.; Holmström, A.; Chang, A.C.; Coronado, M.J.; Ebert, A.D.; et al. Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes Recapitulate the Predilection of Breast Cancer Patients to Doxorubicin–Induced Cardiotoxicity. Nat. Med. 2016, 22, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, S.H.; Hussein, S.; Hasan, M.M.; Deraz, R.H.A.; Hussein, W.F.; Sabik, L.M.E. Functional and Structural Assessment of the Effect of Human Umbilical Cord Blood Mesenchymal Stem Cells in Doxorubicin-Induced Cardiotoxicity. J. Cell. Biochem. 2017, 118, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dal-Pra, S.; Mirotsou, M.; Jayawardena, T.M.; Hodgkinson, C.P.; Bursac, N.; Dzau, V.J. Tissue-engineered 3-dimensional (3D) microenvironment enhances the direct reprogramming of fibroblasts into cardiomyocytes by microRNAs. Sci. Rep. 2016, 6, 38815. [Google Scholar] [CrossRef]
- Lo, Y.-P.; Liu, Y.-S.; Rimando, M.G.; Ho, J.H.-C.; Lin, K.-H.; Lee, O.K. Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells. Sci. Rep. 2016, 6, 21253. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J. Bone Jt. Surg. Am. Vol. 1984, 66, 602–607. [Google Scholar] [CrossRef]
- Singh, K.; Smucker, J.D.; Ugbo, J.L.; Tortolani, P.J.; Tsai, L.; Fei, Q.; Kuh, S.; Rumi, M.; Heller, J.G.; Boden, S.D.; et al. rhBMP-2 enhancement of posterolateral spinal fusion in a rabbit model in the presence of concurrently administered doxorubicin. Spine J. 2007, 7, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.A.; Waterhouse, D.N.; Mayer, L.D.; Cullis, P.R.; Madden, T.D.; Bally, M.B. The Liposomal Formulation of Doxorubicin. Methods Enzymol. 2005, 391, 71–97. [Google Scholar] [PubMed]
- Lee, S.; Ko, Y.; Park, J. Evaluation of the osteogenic differentiation of gingiva-derived stem cells grown on culture plates or in stem cell spheroids: Comparison of two- and three-dimensional cultures. Exp. Ther. Med. 2017, 14, 2434–2438. [Google Scholar] [CrossRef] [PubMed]
- Tae, J.; Lee, H.; Lee, H.; Ko, Y.; Park, J. Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells. Exp. Ther. Med. 2018, 16, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Na, C.-B.; Park, J.-B. Effects of Simvastatin on the Viability and Secretion of Vascular Endothelial Growth Factor of Cell Spheroids Cultured in Growth Media. Implant Dent. 2018, 27, 480–487. [Google Scholar] [CrossRef]
- Jin, S.H.; Lee, J.E.; Yun, J.H.; Kim, I.; Ko, Y.; Park, J.B. Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J. Periodontal Res. 2015, 50, 461–467. [Google Scholar] [CrossRef]
- Lee, H.; Son, J.; Yi, G.; Koo, H.; Park, J. Cellular viability and osteogenic differentiation potential of human gingiva-derived stem cells in 2D culture following treatment with anionic, cationic, and neutral liposomes containing doxorubicin. Exp. Ther. Med. 2018, 16, 4457–4462. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-Y.; Park, S.; Ryu, J.H.; Na, J.H.; Koo, H.; Lee, K.E.; Jeon, H.; Kwon, I.C.; Kim, K.; et al. In vivo NIRF Imaging of Tumor Targetability of Nanosized Liposomes in Tumor-Bearing Mice. Macromol. Biosci. 2012, 12, 849–856. [Google Scholar] [CrossRef]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFbeta. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Shah, S.; Chandra, A.; Kaur, A.; Sabnis, N.; Lacko, A.; Gryczynski, Z.; Fudala, R.; Gryczynski, I. Fluorescence properties of doxorubicin in PBS buffer and PVA films. J. Photochem. Photobiol. B Boil. 2017, 170, 65–69. [Google Scholar] [CrossRef]
- Anh, D.J.; Dimai, H.P.; Hall, S.L.; Farley, J.R. Skeletal Alkaline Phosphatase Activity Is Primarily Released from Human Osteoblasts in an Insoluble Form, and the Net Release Is Inhibited by Calcium and Skeletal Growth Factors. Calcif. Tissue Int. 1998, 62, 332–340. [Google Scholar] [CrossRef]
- Jin, S.-H.; Kweon, H.; Park, J.-B.; Kim, C.-H. The effects of tetracycline-loaded silk fibroin membrane on proliferation and osteogenic potential of mesenchymal stem cells. J. Surg. Res. 2014, 192, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Ko, S.-Y.; Lee, J.H.; Kim, D.-H.; Yun, J.-H. Evaluation of the periodontal regenerative properties of patterned human periodontal ligament stem cell sheets. J. Periodontal Implant Sci. 2017, 47, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Cui, H. Intermittent parathyroid hormone (1–34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp. Ther. Med. 2016, 11, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Zhang, H.; Lin, C.Y.; Chung, C.P.; Byun, Y.; Park, Y.S.; Yang, V.C. Simvastatin maintains osteoblastic viability while promoting differentiation by partially regulating the expressions of estrogen receptors alpha. J. Surg. Res. 2012, 174, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-H.; Pathak, S.; Yong, C.S.; Kim, J.O.; Jeong, J.-H.; Park, J.-B. Potential differentiation ability of gingiva originated human mesenchymal stem cell in the presence of tacrolimus. Sci. Rep. 2016, 6, 34910. [Google Scholar] [CrossRef] [PubMed]
| Day | Groups (n = 3) | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Doxo0 | Doxo1 | Doxo10 | A0 | A1 | A10 | C0 | C1 | C10 | N0 | N1 | N10 | ||
| 1 | 1.976 ± 0.060 | 1.902 ± 0.032 | 1.887 ± 0.039 | 2.084 ± 0.021 | 1.994 ± 0.037 | 1.949 ± 0.022 | 2.181 ± 0.022 | 2.194 ± 0.022 | 2.018 ± 0.041 | 2.060 ± 0.075 | 2.077 ± 0.024 | 2.137 ± 0.048 | 0.001 |
| 3 | 0.335 ± 0.005 | 0.317 ± 0.004 | 0.311 ± 0.003 | 0.322 ± 0.001 | 0.308 ± 0.001 | 0.287 ± 0.001 | 0.344 ± 0.001 | 0.311 ± 0.001 | 0.363 ± 0.001 | 0.318 ± 0.004 | 0.314 ± 0.002 | 0.308 ± 0.002 | 0.000 |
| 5 | 0.372 ± 0.002 | 0.265 ± 0.005 | 0.233 ± 0.030 | 0.273 ± 0.011 | 0.264 ± 0.006 | 0.261 ± 0.002 | 0.267 ± 0.003 | 0.257 ± 0.008 | 0.251 ± 0.002 | 0.262 ± 0.003 | 0.244 ± 0.004 | 0.243 ± 0.007 | 0.007 |
| 7 | 0.236 ± 0.003 | 0.229 ± 0.003 | 0.230 ± 0.005 | 0.237 ± 0.003 | 0.236 ± 0.001 | 0.218 ± 0.002 | 0.238 ± 0.002 | 0.222 ± 0.003 | 0.225 ± 0.003 | 0.230 ± 0.003 | 0.221 ± 0.002 | 0.227 ± 0.001 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Son, J.; Min, S.K.; Na, C.-B.; Yi, G.; Koo, H.; Park, J.-B. A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva. Materials 2019, 12, 2693. https://doi.org/10.3390/ma12172693
Lee H, Son J, Min SK, Na C-B, Yi G, Koo H, Park J-B. A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva. Materials. 2019; 12(17):2693. https://doi.org/10.3390/ma12172693
Chicago/Turabian StyleLee, Hyunjin, Jihwan Son, Sae Kyung Min, Chae-Bin Na, Gawon Yi, Heebeom Koo, and Jun-Beom Park. 2019. "A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva" Materials 12, no. 17: 2693. https://doi.org/10.3390/ma12172693
APA StyleLee, H., Son, J., Min, S. K., Na, C.-B., Yi, G., Koo, H., & Park, J.-B. (2019). A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva. Materials, 12(17), 2693. https://doi.org/10.3390/ma12172693







