Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prachumrak, N.; Pojanasopa, S.; Namuangruk, S.; Kaewin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Novel bis[5-(fluoren-2-yl)thiophen-2-yl]benzothiadiazole end-capped with carbazole dendrons as highly efficient solution-processed nondoped red emitters for organic light-emitting diodes. ACS Appl. Mater. Interfaces 2013, 5, 8694–8703. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Komiyama, H.; Yasuda, T. Pyrimidine-based twisted donor–acceptor delayed fluorescence molecules: A new universal platform for highly efficient blue electroluminescence. Chem. Sci. 2017, 8, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Adachi, C.; Yasuda, T. High-efficiency blue organic light-emitting diodes based on thermally activated delayed fluorescence from phenoxaphosphine and phenoxathiin derivatives. Adv. Mater. 2016, 28, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-R.; Lee, C.W.; Lee, J.Y.; Gong, M.-S. Design of ortho-linkage carbazole-triazine structure for high-efficiency blue thermally activated delayed fluorescent emitters. Dyes Pigments 2016, 134, 562–568. [Google Scholar] [CrossRef]
- Sun, K.; Sun, Y.; Jiang, W.; Huang, S.; Tian, W.; Sun, Y. Highly efficient and color tunable thermally activated delayed fluorescent emitters and their applications for the solution-processed oleds. Dyes Pigments 2017, 139, 326–333. [Google Scholar] [CrossRef]
- Kim, K.-S.; Jeong, S.; Kim, C.; Kwon, Y.; Choi, B.-D.; Han, Y.S. Synthesis and electro-optical properties of carbazole derivatives with high band gap energy. Thin Solid Films 2009, 518, 284–289. [Google Scholar] [CrossRef]
- Takahashi, T.; Shizu, K.; Yasuda, T.; Togashi, K.; Adachi, C. Donor–acceptor-structured 1,4-diazatriphenylene derivatives exhibiting thermally activated delayed fluorescence: Design and synthesis, photophysical properties and oled characteristics. Sci. Technol. Adv. Mater. 2014, 15, 034202. [Google Scholar] [CrossRef]
- Liu, M.; Seino, Y.; Chen, D.; Inomata, S.; Su, S.-J.; Sasabe, H.; Kido, J. Blue thermally activated delayed fluorescence materials based on bis(phenylsulfonyl)benzene derivatives. Chem. Commun. 2015, 51, 16353–16356. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, I.-J.; Wang, Y.; Kim, S.H.; Kim, M.; Chai, K.Y. Acridine-triphenylamine based hole-transporting and hole-injecting material for highly efficient phosphorescent-based organic light emitting diodes. Appl. Sci. 2018, 8, 1168. [Google Scholar] [CrossRef]
- Usluer, O.; Demic, S.; Egbe, D.A.M.; Birckner, E.; Tozlu, C.; Pivrikas, A.; Ramil, A.M.; Sariciftci, N.S. Fluorene-carbazole dendrimers: Synthesis, thermal, photophysical and electroluminescent device properties. Adv. Funct. Mater. 2010, 20, 4152–4161. [Google Scholar] [CrossRef]
- Huh, D.H.; Kim, G.W.; Kim, G.H.; Kulshreshtha, C.; Kwon, J.H. High hole mobility hole transport material for organic light-emitting devices. Synth. Met. 2013, 180, 79–84. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, H.W.; Ko, I.J.; Qiong, W.; Nguyen, Q.P.B.; Jayashantha, P.G.S.; Kwon, J.H.; Chai, K.Y. Thermally stable efficient hole transporting materials based on carbazole and triphenylamine core for red phosphorescent oleds. Org. Electron. 2017, 51, 463–470. [Google Scholar] [CrossRef]
- Griniene, R.; Grazulevicius, J.V.; Tseng, K.Y.; Wang, W.B.; Jou, J.H.; Grigalevicius, S. Aryl substituted 9-(2,2-diphenylvinyl)carbazoles as efficient materials for hole transporting layers of oleds. Synth. Met. 2011, 161, 2466–2470. [Google Scholar] [CrossRef]
- Zheng, Z.; Dong, Q.; Gou, L.; Su, J.-H.; Huang, J. Novel hole transport materials based on n,n′-disubstituted-dihydrophenazine derivatives for electroluminescent diodes. J. Mater. Chem. C 2014, 2, 9858–9865. [Google Scholar] [CrossRef]
- Shih, P.-I.; Chien, C.-H.; Chuang, C.-Y.; Shu, C.-F.; Yang, C.-H.; Chen, J.-H.; Chi, Y. Novel host material for highly efficient blue phosphorescent oleds. J. Mater. Chem. 2007, 17, 1692–1698. [Google Scholar] [CrossRef]
- Lyu, Y.-Y.; Kwak, J.; Jeon, W.S.; Byun, Y.; Lee, H.S.; Kim, D.; Lee, C.; Char, K. Highly efficient red phosphorescent oleds based on non-conjugated silicon-cored spirobifluorene derivative doped with ir-complexes. Adv. Funct. Mater. 2009, 19, 420–427. [Google Scholar] [CrossRef]
- Tokito, S.; Iijima, T.; Suzuri, Y.; Kita, H.; Tsuzuki, T.; Sato, F. Confinement of triplet energy on phosphorescent molecules for highly-efficient organic blue-light-emitting devices. Appl. Phys. Lett. 2003, 83, 569–571. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Holmes, R.J.; Djurovich, P.I.; Forrest, S.R.; Thompson, M.E. Ultrahigh energy gap hosts in deep blue organic electrophosphorescent devices. Chem. Mater. 2004, 16, 4743–4747. [Google Scholar] [CrossRef]
- Yeh, S.-J.; Wu, M.-F.; Chen, C.-T.; Song, Y.-H.; Chi, Y.; Ho, M.-H.; Hsu, S.-F.; Chen, C.H. New dopant and host materials for blue-light-emitting phosphorescent organic electroluminescent devices. Adv. Mater. 2005, 17, 285–289. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yasuda, T.; Yang, Y.S.; Zhang, Q.; Adachi, C. Luminous butterflies: Efficient exciton harvesting by benzophenone derivatives for full-color delayed fluorescence oleds. Angew. Chem. Int. Ed. 2014, 53, 6402–6406. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Xiao, L.; Su, S.-J.; Agata, Y.; Lan, H.; Kido, J. Nearly 100% internal quantum efficiency in an organic blue-light electrophosphorescent device using a weak electron transporting material with a wide energy gap. Adv. Mater. 2009, 21, 1271–1274. [Google Scholar] [CrossRef]
- Taneda, M.; Shizu, K.; Tanaka, H.; Adachi, C. High efficiency thermally activated delayed fluorescence based on 1,3,5-tris(4-(diphenylamino)phenyl)-2,4,6-tricyanobenzene. Chem. Commun. 2015, 51, 5028–5031. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Lee, J.; Yasuda, T. High-performance blue organic light-emitting diodes with 20% external electroluminescence quantum efficiency based on pyrimidine-containing thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 7911–7916. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Lee, G.H.; Kim, Y.S. Theoretical study of imidazole derivatives for blue thermally activated delayed fluorescence emitter. J. Nanosci. Nanotechnol. 2016, 16, 11014–11017. [Google Scholar] [CrossRef]
- Yu, H.; Dai, X.; Yao, F.; Wei, X.; Cao, J.; Jhun, C. Efficient white phosphorescent organic light-emitting diodes using ultrathin emissive layers (. Sci. Rep. 2018, 8, 6068. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhou, L.; Teng, M.-Y.; Xu, Q.-L.; Lin, C.; Zheng, Y.-X.; Zuo, J.-L.; Zhang, H.-J.; You, X.-Z. Highly efficient green phosphorescent oleds based on a novel iridium complex. J. Mater. Chem. C 2013, 1, 560–565. [Google Scholar] [CrossRef]
- Thomas, S.T. Iridium(iii) complexes for oled application. In Iridium(iii) in Optoelectronic and Photonics Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 205–274. [Google Scholar]
- Yang, C.-H.; Mauro, M.; Polo, F.; Watanabe, S.; Muenster, I.; Fröhlich, R.; De Cola, L. Deep-blue-emitting heteroleptic iridium(iii) complexes suited for highly efficient phosphorescent oleds. Chem. Mater. 2012, 24, 3684–3695. [Google Scholar] [CrossRef]
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational design of phosphorescent iridium(iii) complexes for emission color tunability and their applications in oleds. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Cebrián, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Li, K.; Ming Tong, G.S.; Wan, Q.; Cheng, G.; Tong, W.-Y.; Ang, W.-H.; Kwong, W.-L.; Che, C.-M. Highly phosphorescent platinum(ii) emitters: Photophysics, materials and biological applications. Chem. Sci. 2016, 7, 1653–1673. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Li, G.; Ye, K.; Wang, C.; Zhao, S.; Liu, Y.; Hou, Z.; Wang, Y. Highly efficient phosphorescent oleds with host-independent and concentration-insensitive properties based on a bipolar iridium complex. J. Mater. Chem. C 2013, 1, 2920–2926. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, R.; Shi, H.; Li, C.; Zhu, H. High efficiency green phosphorescent oleds using double-host materials. Dyes Pigments 2017, 143, 196–202. [Google Scholar] [CrossRef]
- May, F.; Al-Helwi, M.; Baumeier, B.; Kowalsky, W.; Fuchs, E.; Lennartz, C.; Andrienko, D. Design rules for charge-transport efficient host materials for phosphorescent organic light-emitting diodes. J. Am. Chem. Soc. 2012, 134, 13818–13822. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Park, T.J.; Jeon, W.S.; Park, J.J. Bipolar Host Materials for Red and Green Phosphorescent Oled. In Proceedings of the Volume 6828, Light-Emitting Diode Materials and Devices II, Beijing, China, 11–15 November 2007. [Google Scholar] [CrossRef]
- Zhan, G.; Liu, Z.; Bian, Z.; Huang, C. Recent advances in organic light-emitting diodes based on pure organic room temperature phosphorescence materials. Front. Chem. 2019, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-T.; Chi, Y. Phosphorescent dyes for organic light-emitting diodes. Chem. Eur. J. 2007, 13, 380–395. [Google Scholar] [CrossRef]
- Tung, Y.-L.; Lee, S.-W.; Chi, Y.; Chen, L.-S.; Shu, C.-F.; Wu, F.-I.; Carty, A.J.; Chou, P.-T.; Peng, S.-M.; Lee, G.-H. Organic light-emitting diodes based on charge-neutral ruii phosphorescent emitters. Adv. Mater. 2005, 17, 1059–1064. [Google Scholar] [CrossRef]
- Wu, S.; Aonuma, M.; Zhang, Q.; Huang, S.; Nakagawa, T.; Kuwabara, K.; Adachi, C. High-efficiency deep-blue organic light-emitting diodes based on a thermally activated delayed fluorescence emitter. J. Mater. Chem. C 2014, 2, 421–424. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Sigmund, E.; Meyer, A.; Röck, L.; Klemm, P.; Lautenschlager, S.; Schmid, A.; Yost, S.R.; Van Voorhis, T.; Bange, S.; et al. Metal-free oled triplet emitters by side-stepping kasha’s rule. Angew. Chem. Int. Ed. 2013, 52, 13449–13452. [Google Scholar] [CrossRef]
- Chou, P.-T.; Chi, Y. Osmium- and ruthenium-based phosphorescent materials: Design, photophysics, and utilization in oled fabrication. Eur. J. Inorg. Chem. 2006, 2006, 3319–3332. [Google Scholar] [CrossRef]
- Lin, C.-C.; Huang, M.-J.; Chiu, M.-J.; Huang, M.-P.; Chang, C.-C.; Liao, C.-Y.; Chiang, K.-M.; Shiau, Y.-J.; Chou, T.-Y.; Chu, L.-K.; et al. Molecular design of highly efficient thermally activated delayed fluorescence hosts for blue phosphorescent and fluorescent organic light-emitting diodes. Chem. Mater. 2017, 29, 1527–1537. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, S.K.; Hwang, S.-H.; Lee, J.Y. Stable blue thermally activated delayed fluorescent organic light-emitting diodes with three times longer lifetime than phosphorescent organic light-emitting diodes. Adv. Mater. 2015, 27, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Rajamalli, P.; Senthilkumar, N.; Gandeepan, P.; Ren-Wu, C.-Z.; Lin, H.-W.; Cheng, C.-H. A thermally activated delayed blue fluorescent emitter with reversible externally tunable emission. J. Mater. Chem. C 2016, 4, 900–904. [Google Scholar] [CrossRef]
- Komatsu, R.; Sasabe, H.; Nakao, K.; Hayasaka, Y.; Ohsawa, T.; Kido, J. Unlocking the potential of pyrimidine conjugate emitters to realize high-performance organic light-emitting devices. Adv. Opt. Mater. 2017, 5, 1600675. [Google Scholar] [CrossRef]
- Ihn, S.-G.; Lee, N.; Jeon, S.O.; Sim, M.; Kang, H.; Jung, Y.; Huh, D.H.; Son, Y.M.; Lee, S.Y.; Numata, M.; et al. An alternative host material for long-lifespan blue organic light-emitting diodes using thermally activated delayed fluorescence. Adv. Sci. 2017, 4, 1600502. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Reddy, S.S.; Cui, L.-S.; Nomura, H.; Hwang, S.; Kim, D.H.; Nakanotani, H.; Jin, S.H.; Adachi, C. Thermally activated delayed fluorescence of bis(9,9-dimethyl-9,10-dihydroacridine) dibenzo[b,d]thiophene 5,5-dioxide derivatives for organic light-emitting diodes. J. Lumin. 2017, 190, 485–491. [Google Scholar] [CrossRef]
- Gómez-Bombarelli, R.; Aguilera-Iparraguirre, J.; Hirzel, T.D.; Duvenaud, D.; Maclaurin, D.; Blood-Forsythe, M.A.; Chae, H.S.; Einzinger, M.; Ha, D.-G.; Wu, T.; et al. Design of efficient molecular organic light-emitting diodes by a high-throughput virtual screening and experimental approach. Nat. Mater. 2016, 15, 1120–1127. [Google Scholar] [CrossRef]
- Higuchi, T.; Nakanotani, H.; Adachi, C. High-efficiency white organic light-emitting diodes based on a blue thermally activated delayed fluorescent emitter combined with green and red fluorescent emitters. Adv. Mater. 2015, 27, 2019–2023. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, W.; Duan, L. Recent progress in solution processable tadf materials for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Liang, X.; Tu, Z.-L.; Zheng, Y.-X. Thermally activated delayed fluorescence materials: Towards realization of high efficiency through strategic small molecular design. Chem. Eur. J. 2019, 25, 5623–5642. [Google Scholar] [CrossRef]
- Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (tadf) emitters for organic light-emitting diodes (oleds). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.B.; Penfold, T.J.; Monkman, A.P. Photophysics of thermally activated delayed fluorescence molecules. Methods Appl. Fluoresc. 2017, 5, 012001. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, Y.; Namikawa, T.; Suzuki, T.; Miyata, Y.; Kita, H.; Sato, T.; Oi, S. Design and synthesis of efficient blue thermally activated delayed fluorescence molecules bearing triarylborane and 10,10-dimethyl-5,10-dihydrophenazasiline moieties. Tetrahedron Lett. 2016, 57, 4914–4917. [Google Scholar] [CrossRef]
- Chen, X.-L.; Yu, R.; Zhang, Q.-K.; Zhou, L.-J.; Wu, X.-Y.; Zhang, Q.; Lu, C.-Z. Rational design of strongly blue-emitting cuprous complexes with thermally activated delayed fluorescence and application in solution-processed oleds. Chem. Mater. 2013, 25, 3910–3920. [Google Scholar] [CrossRef]
- Gao, Y.; Su, T.; Zhao, L.; Geng, Y.; Wu, Y.; Zhang, M.; Su, Z.-M. How does a little difference in structure determine whether molecules have thermally activated delayed fluorescence characteristic or not? Org. Electron. 2017, 50, 70–76. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Peng, Q.; Meng, L.; Wang, Y.; Yi, Y.; Wang, P. Novel thermally activated delayed fluorescence materials–thioxanthone derivatives and their applications for highly efficient oleds. Adv. Mater. 2014, 26, 5198–5204. [Google Scholar] [CrossRef]
- Tanaka, H.; Shizu, K.; Nakanotani, H.; Adachi, C. Dual intramolecular charge-transfer fluorescence derived from a phenothiazine-triphenyltriazine derivative. J. Phys. Chem. C 2014, 118, 15985–15994. [Google Scholar] [CrossRef]
- Seo, J.-A.; Gong, M.-S.; Song, W.; Lee, J.Y. Molecular orbital controlling donor moiety for high-efficiency thermally activated delayed fluorescent emitters. Chem. Asian J. 2016, 11, 868–873. [Google Scholar] [CrossRef]
- Pan, K.-C.; Li, S.-W.; Ho, Y.-Y.; Shiu, Y.-J.; Tsai, W.-L.; Jiao, M.; Lee, W.-K.; Wu, C.-C.; Chung, C.-L.; Chatterjee, T.; et al. Efficient and tunable thermally activated delayed fluorescence emitters having orientation-adjustable cn-substituted pyridine and pyrimidine acceptor units. Adv. Funct. Mater. 2016, 26, 7560–7571. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Namikawa, T.; Suzuki, T.; Miyata, Y.; Kita, H.; Sato, T.; Oi, S. Dimesitylarylborane-based luminescent emitters exhibiting highly-efficient thermally activated delayed fluorescence for organic light-emitting diodes. Org. Electron. 2016, 34, 208–217. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, D.; Zhang, S.; Tao, Y.; Huang, W. Cn-containing donor–acceptor-type small-molecule materials for thermally activated delayed fluorescence oleds. J. Mater. Chem. C 2017, 5, 7699–7714. [Google Scholar] [CrossRef]
- Im, Y.; Kim, M.; Cho, Y.J.; Seo, J.-A.; Yook, K.S.; Lee, J.Y. Molecular design strategy of organic thermally activated delayed fluorescence emitters. Chem. Mater. 2017, 29, 1946–1963. [Google Scholar] [CrossRef]
- He, Z.; Cai, X.; Wang, Z.; Li, Y.; Xu, Z.; Liu, K.; Chen, D.; Su, S.-J. Sky-blue thermally activated delayed fluorescence material employing a diphenylethyne acceptor for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 36–42. [Google Scholar] [CrossRef]
- Chatterjee, T.; Wong, K.-T. Perspective on host materials for thermally activated delayed fluorescence organic light emitting diodes. Adv. Opt. Mater. 2019, 7, 1800565. [Google Scholar] [CrossRef]
- Im, Y.; Lee, J.Y. Recent progress of green thermally activated delayed fluorescent emitters. J. Inf. Disp. 2017, 18, 101–117. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.-K.; Huang, C.-C.; Kumar, S.; Li, S.-H.; Dong, Z.-L.; Fung, M.-K.; Jiang, Z.-Q.; Liao, L.-S. Thermally activated delayed fluorescence sensitizer for d–a–a type emitters with orange-red light emission. J. Mater. Chem. C 2018, 6, 10030–10035. [Google Scholar] [CrossRef]
- Cho, Y.J.; Jeon, S.K.; Chin, B.D.; Yu, E.; Lee, J.Y. The design of dual emitting cores for green thermally activated delayed fluorescent materials. Angew. Chem. Int. Ed. 2015, 54, 5201–5204. [Google Scholar] [CrossRef]
- Furukawa, T.; Nakanotani, H.; Inoue, M.; Adachi, C. Dual enhancement of electroluminescence efficiency and operational stability by rapid upconversion of triplet excitons in oleds. Sci. Rep. 2015, 5, 8429. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Inomata, S.; Sasabe, H.; Pu, Y.-J.; Kido, J. High-performance green oleds using thermally activated delayed fluorescence with a power efficiency of over 100 lm w−1. Adv. Mater. 2016, 28, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Gaj, M.P.; Fuentes-Hernandez, C.; Zhang, Y.; Marder, S.R.; Kippelen, B. Highly efficient organic light-emitting diodes from thermally activated delayed fluorescence using a sulfone–carbazole host material. Org. Electron. 2015, 16, 109–112. [Google Scholar] [CrossRef]
- Kim, O.Y.; Kim, B.S.; Lee, J.Y. High efficiency thermally activated delayed fluorescent devices using a mixed host of carbazole and phosphine oxide derived host materials. Synth. Met. 2015, 201, 49–53. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yook, K.S.; Lee, J.Y. A universal host material for high external quantum efficiency close to 25% and long lifetime in green fluorescent and phosphorescent oleds. Adv. Mater. 2014, 26, 4050–4055. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kim, B.S.; Lee, C.W.; Im, Y.; Yook, K.S.; Hwang, S.-H.; Lee, J.Y. Above 30% external quantum efficiency in green delayed fluorescent organic light-emitting diodes. ACS Appl. Mater. Interfaces 2015, 7, 9625–9629. [Google Scholar] [CrossRef]
- Komatsu, R.; Sasabe, H.; Seino, Y.; Nakao, K.; Kido, J. Light-blue thermally activated delayed fluorescent emitters realizing a high external quantum efficiency of 25% and unprecedented low drive voltages in oleds. J. Mater. Chem. C 2016, 4, 2274–2278. [Google Scholar] [CrossRef]
- Volz, D. Review of organic light-emitting diodes with thermally activated delayed fluorescence emitters for energy-efficient sustainable light sources and displays. J. Photonics Energy 2016, 6, 020901. [Google Scholar] [CrossRef]
- Tsai, W.-L.; Huang, M.-H.; Lee, W.-K.; Hsu, Y.-J.; Pan, K.-C.; Huang, Y.-H.; Ting, H.-C.; Sarma, M.; Ho, Y.-Y.; Hu, H.-C.; et al. A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices. Chem. Commun. 2015, 51, 13662–13665. [Google Scholar] [CrossRef]
- Braveenth, R.; Lee, H.; Kim, S.; Raagulan, K.; Kim, S.; Kwon, J.H.; Chai, K.Y. High efficiency green TADF emitters of acridine donor and triazine acceptor D–A–D structures. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef]
- Suzuki, K.; Adachi, C.; Kaji, H. Solution-processable thermally activated delayed fluorescence emitters for application in organic light emitting diodes. J. Soc. Inf. Disp. 2017, 25, 480–485. [Google Scholar] [CrossRef]
- Hirata, S.; Sakai, Y.; Masui, K.; Tanaka, H.; Lee, S.Y.; Nomura, H.; Nakamura, N.; Yasumatsu, M.; Nakanotani, H.; Zhang, Q.; et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat. Mater. 2014, 14, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shizu, K.; Yoshimura, K.; Kawada, A.; Miyazaki, H.; Adachi, C. Organic luminescent molecule with energetically equivalent singlet and triplet excited states for organic light-emitting diodes. Phys. Rev. Lett. 2013, 110, 247401. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Lee, S.Y.; Schmidt, T.D.; Yasuda, T.; Adachi, C.; Brütting, W. Efficiency enhancement of organic light-emitting diodes incorporating a highly oriented thermally activated delayed fluorescence emitter. Adv. Funct. Mater. 2014, 24, 5232–5239. [Google Scholar] [CrossRef]
- Serevičius, T.; Nakagawa, T.; Kuo, M.-C.; Cheng, S.-H.; Wong, K.-T.; Chang, C.-H.; Kwong, R.C.; Xia, S.; Adachi, C. Enhanced electroluminescence based on thermally activated delayed fluorescence from a carbazole–triazine derivative. Phys. Chem. Chem. Phys. 2013, 15, 15850–15855. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kim, M.; Jeon, S.K.; Hwang, S.-H.; Lee, C.W.; Lee, J.Y. Design strategy for 25% external quantum efficiency in green and blue thermally activated delayed fluorescent devices. Adv. Mater. 2015, 27, 5861–5867. [Google Scholar] [CrossRef]
- Yu, J.G.; Han, S.H.; Lee, H.L.; Hong, W.P.; Lee, J.Y. A novel molecular design employing a backbone freezing linker for improved efficiency, sharpened emission and long lifetime in thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2019, 7, 2919–2926. [Google Scholar] [CrossRef]
- Jung, M.; Lee, K.H.; Hong, W.P.; Lee, J.Y. The effect of frontier orbital distribution of the core structure on the photophysics and device performances of thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yun, J.H.; Han, S.H.; Lee, J.Y. Benzofuroacridine and benzothienoacridine as new donor moieties for emission color management of thermally activated delayed fluorescent emitters. J. Mater. Chem. C 2019, 7, 4573–4580. [Google Scholar] [CrossRef]
- Tanaka, H.; Shizu, K.; Miyazaki, H.; Adachi, C. Efficient green thermally activated delayed fluorescence (tadf) from a phenoxazine–triphenyltriazine (pxz–trz) derivative. Chem. Commun. 2012, 48, 11392–11394. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shizu, K.; Nakanotani, H.; Adachi, C. Twisted intramolecular charge transfer state for long-wavelength thermally activated delayed fluorescence. Chem. Mater. 2013, 25, 3766–3771. [Google Scholar] [CrossRef]
- Shizu, K.; Uejima, M.; Nomura, H.; Sato, T.; Tanaka, K.; Kaji, H.; Adachi, C. Enhanced electroluminescence from a thermally activated delayed-fluorescence emitter by suppressing nonradiative decay. Phys. Rev. Appl. 2015, 3, 014001. [Google Scholar] [CrossRef]
- Wada, Y.; Shizu, K.; Kubo, S.; Suzuki, K.; Tanaka, H.; Adachi, C.; Kaji, H. Highly efficient electroluminescence from a solution-processable thermally activated delayed fluorescence emitter. Appl. Phys. Lett. 2015, 107, 183303. [Google Scholar] [CrossRef]
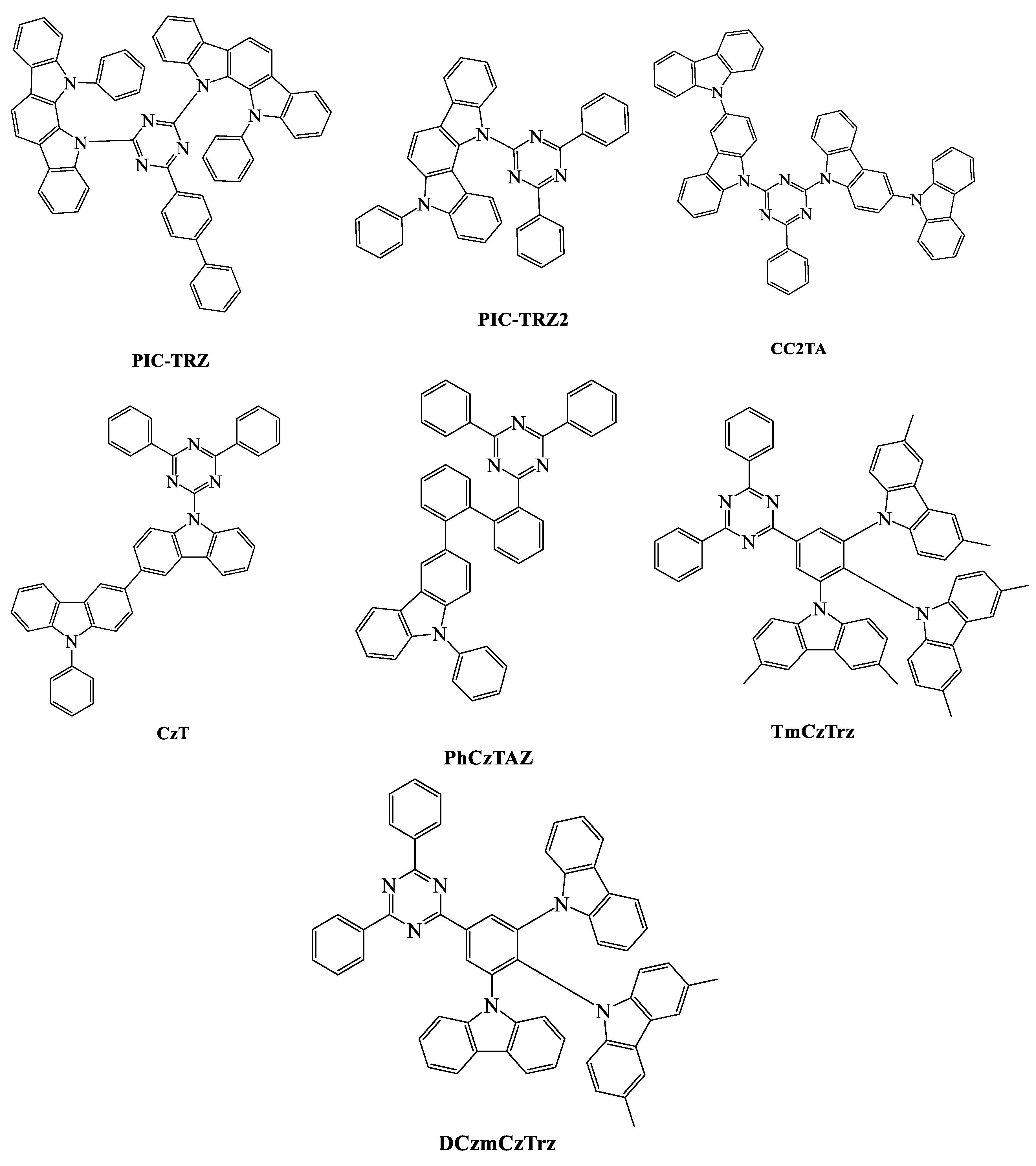
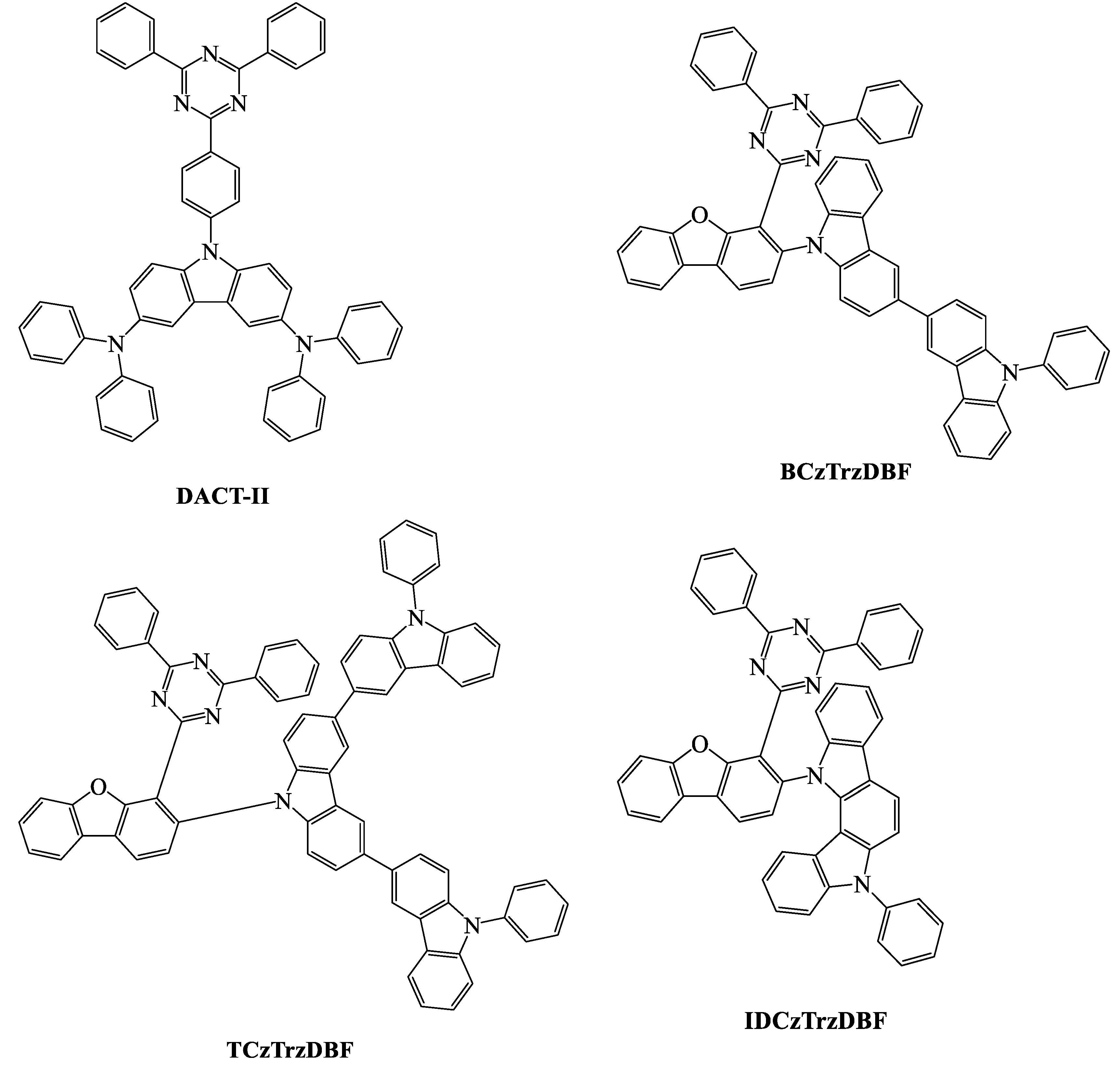
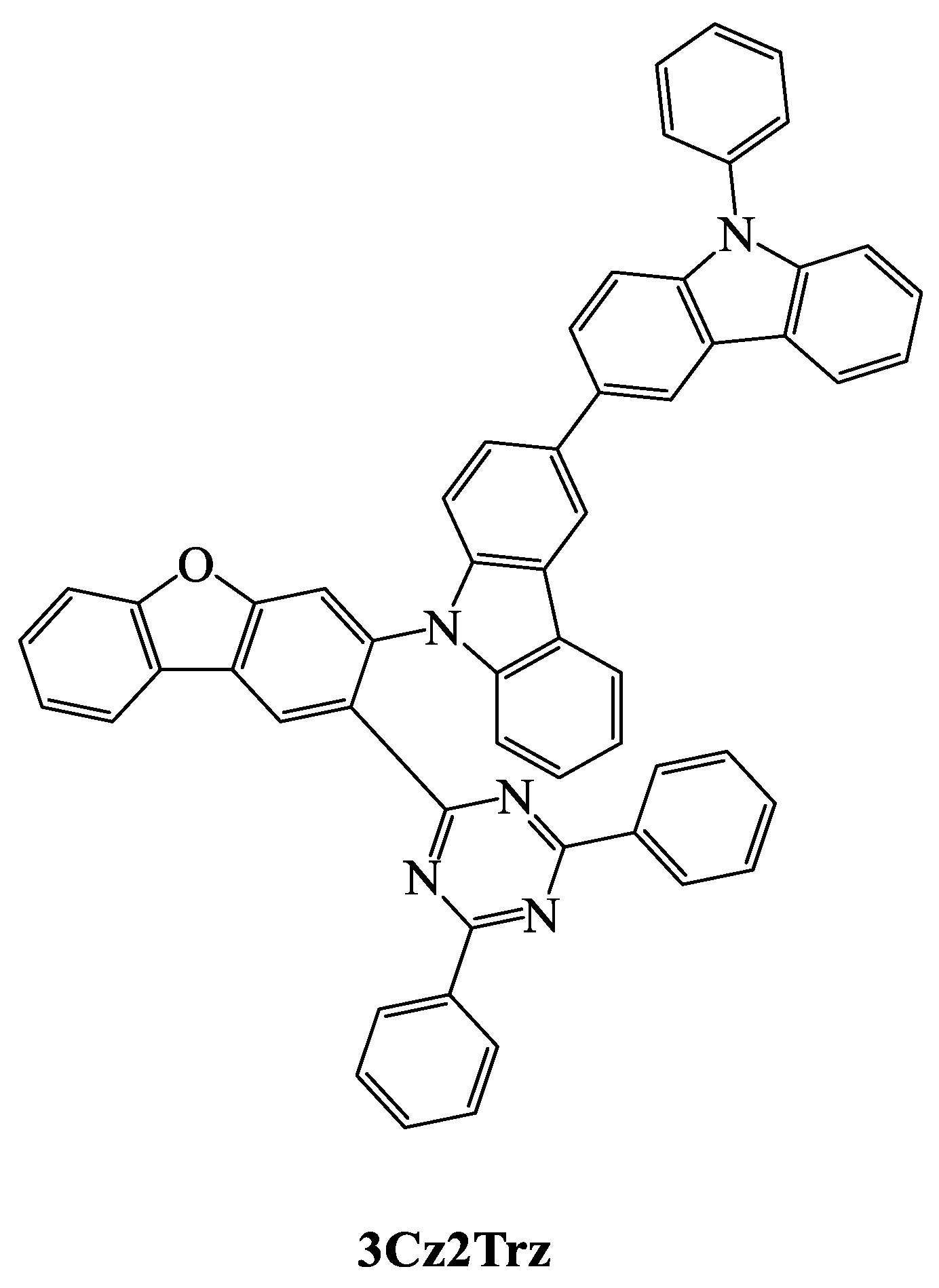
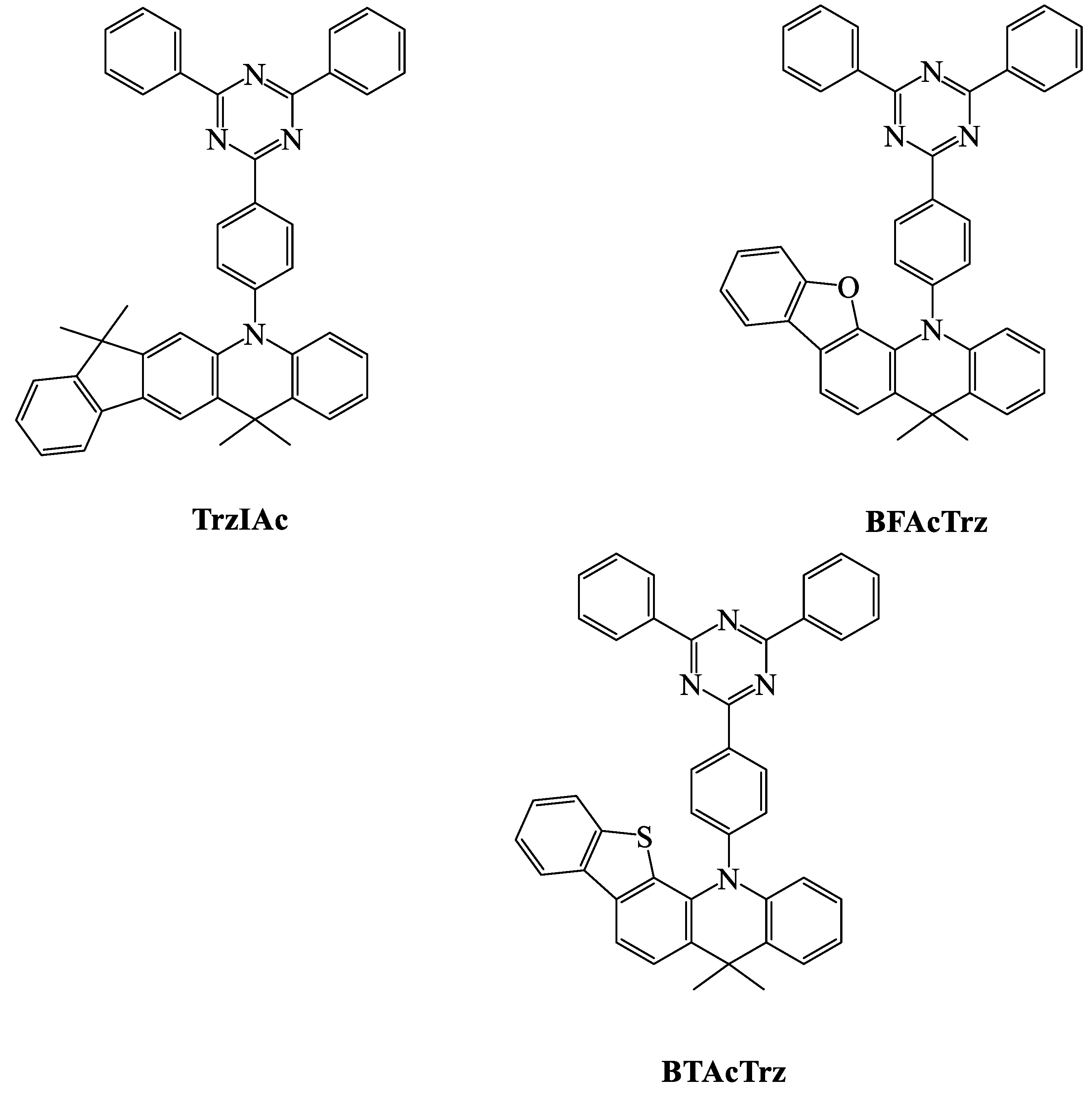
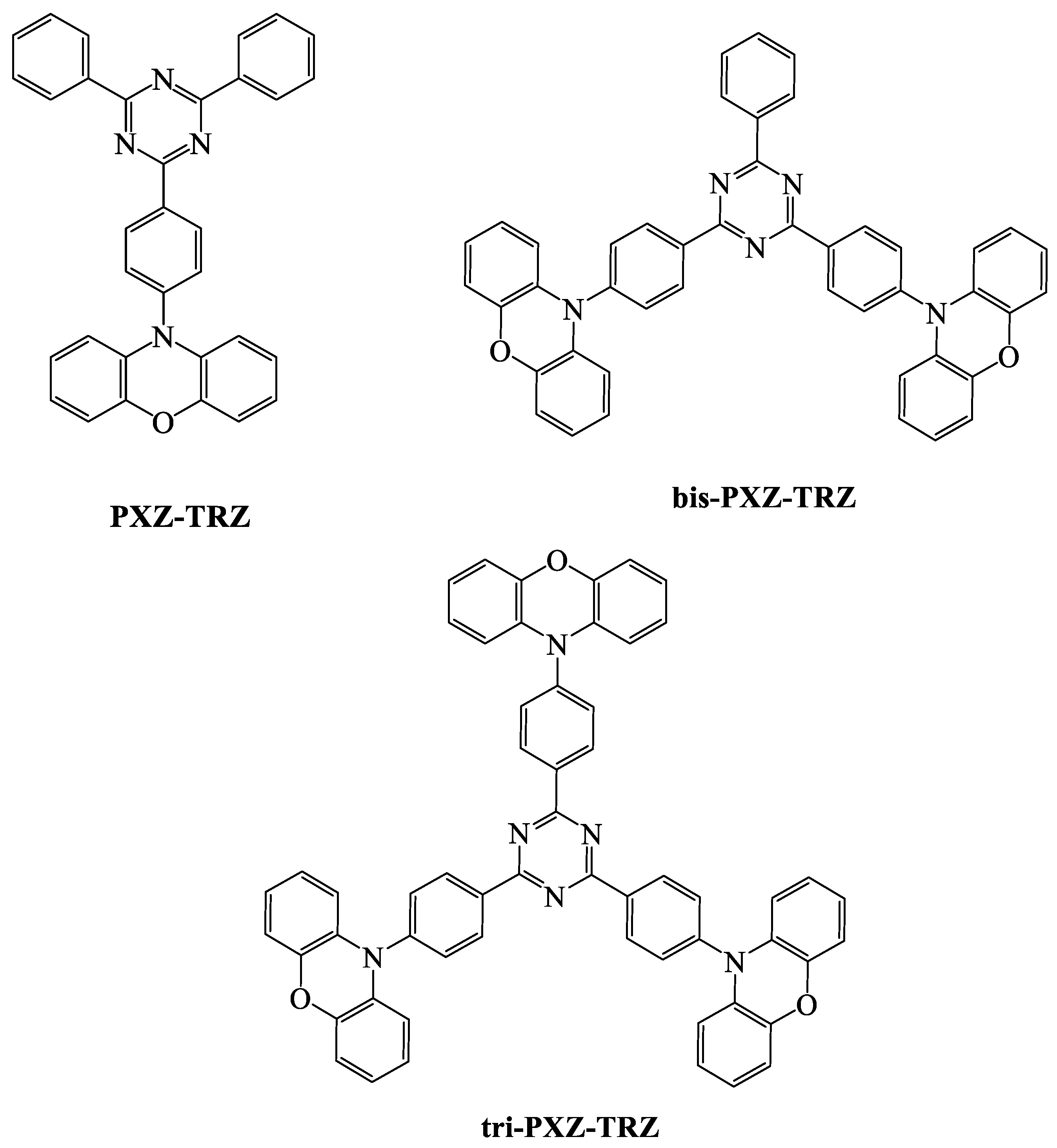
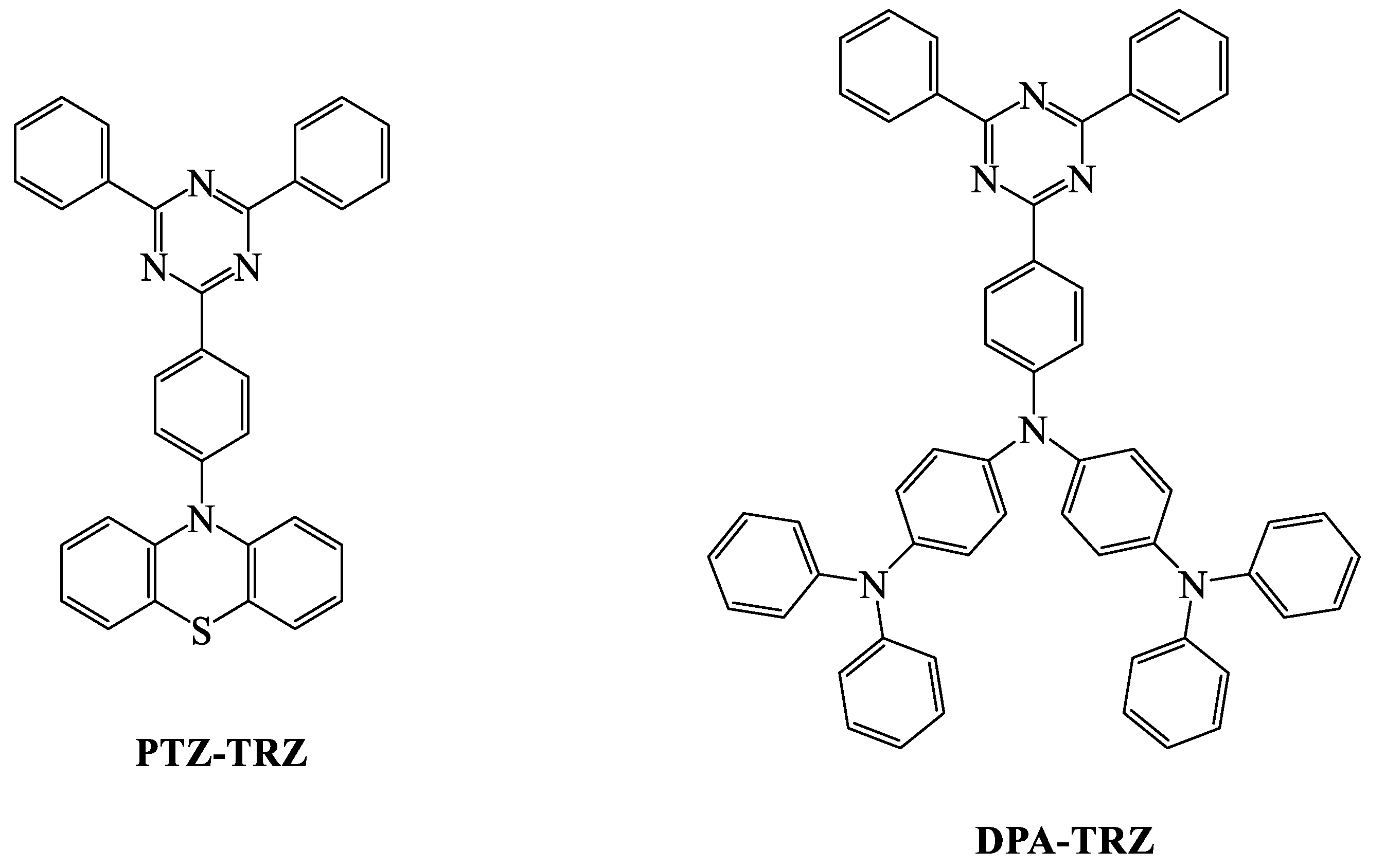
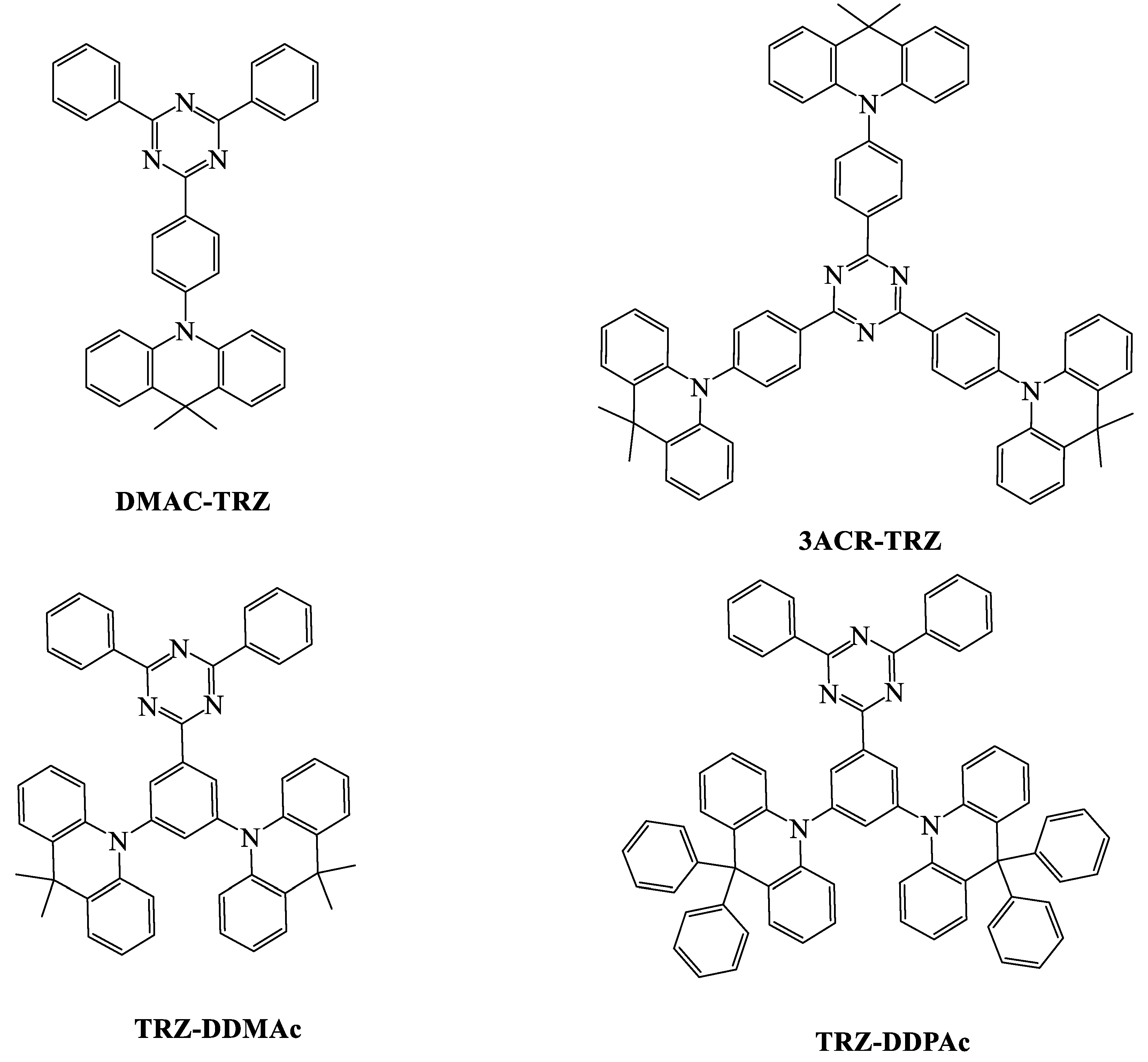
| TADF Emitter | HOMO (eV) | LUMO (eV) | PL (nm) | ∆EST (eV) | ΦPL (%) | τd (μs) | Reference |
|---|---|---|---|---|---|---|---|
| PIC-TRZ | - | - | 500 | 0.11 | 39 | 230 | [86] |
| PXZ-TRZ | 5.5 | 3.1 | 540 | 0.07 | 65.7 | 0.68 | [89] |
| CC2TA | 5.9 | 2.6 | 513a | 0.05 | 62 | 22 | [87] |
| PIC-TRZ2 | - | - | - | 0.02 | 45 | 2.7 | [86] |
| Bis-PXZ-TRZ | 5.7 | 3.4 | 560 a | 0.054 b | 64 | 1.33 a | [90] |
| Tri-PXZ-TRZ | 5.7 | 3.4 | 568 a | 0.065 b | 58 | 1.10 a | [90] |
| CzT | - | - | 502 | 0.07 | 39.7 | 42.6 a | [88] |
| PTZ-TRZ | 5.5 | 3.0 | 420,520 | 0.18 b | 65.8 | 0.52 a | [89] |
| DMAC-TRZ | 5.3 | 2.78 | 510 | 0.05 | 83 | 3.6 | [82] |
| DACT-II | 5.5 | 3.2 | 520 | 0.009 | 100 | - | [84] |
| DPA-TRZ | - | - | 540 | 0.11 | 100 | 160 | [91] |
| TrzIAc | 5.75 | 3.34 | 519 | 0.06 | 97 a | 1.6 | [61] |
| 3ACR-TRZ | - | - | 504 | 0.015 | 98 a | 6.7 | [93] |
| BCzTrzDBF | 5.85 | 3.34 | - | 0.06 | 82.4 | 5.4 | [95] |
| TCzTrzDBF | 5.87 | 3.43 | - | 0.01 | 86.3 | 4.4 | [95] |
| IDCzTrzDBF | 5.88 | 3.34 | - | 0.05 | 85.4 | 2.8 | [95] |
| BFAcTrz | 5.84 | 3.23 | - | 0.11 | 92.3 | 14.2 | [94] |
| BTAcTrz | 5.8 | 3.24 | - | 0.02 | 100 | 9.3 | [94] |
| TmCzTrz | 5.19 b | 2.11 b | - | 0.07 | 100 | 13.3 | [92] |
| DCzmCzTrz | 5.26 b | 2.15 b | - | 0.20 | 98 | 9.7 | [92] |
| TRZ-DDMAc | 5.70 | 2.89 | 529 | 0.03 | 52.7 | 10.32 | [83] |
| TRZ-DDPAc | 5.72 | 2.87 | 511 | 0.05 | 79.7 | 10.37 | [83] |
| 2Cz3Trz | 5.78 | 3.32 | - | 0.06 | 74.2 | 4.80 | [96] |
| 3Cz2Trz | 5.77 | 3.20 | - | 0.05 | 69.7 | 2.84 | [96] |
| TADF Emitter | Device Structure | EML (nm) | ELmax (nm) | CIE Color | CE (cd/A) | PE (lm/W) | EQE (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| PIC-TRZ | ITO/NPD/α-mCP/6 wt % PIC-TRZ: mCP/BP4mPy/LiF/Al | 15 | 500 | - | - | - | 5.3 | [86] |
| PXZ-TRZ | ITO/α-NPD/6 wt % PXZ-TRZ: CBP/TPBi/LiF/Al | 15 | 529 | - | - | - | 12.5 | [89] |
| CC2TA | ITO/α-NPD/6 wt % CC2TA: mCP/6 wt % CC2TA: DPEPO/DPEPO/TPBi/LiF/Al | 30 | 490 | - | - | - | 11.0 | [87] |
| PIC-TRZ2 | ITO/TAPC/6 wt % PIC-TRZ2: PYD2/DPEPO/TmPyPBi/LiF/Al | 20 | 505 | - | - | - | 14.0 | [86] |
| Bis-PXZ-TRZ | ITO/α-NPD/6 wt % Bis-PXZ-TRZ: mCBP/TPBi/LiF/Al | 15 | 552 | - | - | - | 9.1 | [90] |
| Tri-PXZ-TRZ | ITO/α-NPD/6 wt % Tri-PXZ-TRZ: mCBP/TPBi/LiF/Al | 15 | 553 | - | - | - | 13.3 | [90] |
| CzT | ITO/α-NPD/TCTA/CzSi/3 wt % CzT: DPEPO/DPEPO/TPBi/LiF/Al | 20 | 520 | 0.23, 0.40 | - | 9.7 | 6.0 | [88] |
| PTZ-TRZ | ITO/α-NPD/2 wt % PTZ-TRZ: mCBP/TPBi/LiF/Al | 15 | 532 | - | - | - | 10.8 | [89] |
| DMAC-TRZ | ITO/PEDOT: PSS/TAPC/mCP/8 wt % DMAC-TRZ: mCPCN/DPSS/3TPYMB/LiF/Al (nondoped device): ITO/PEDOT: PSS/TAPC/mCp/DMAC-TRZ/3TPYMB/LiF/Al | 20 | - | - | 66.8 61.1 | 65.6 45.7 | 26.5 20.0 | [82] |
| DACT-II | ITO/TAPC/9 wt % DACT-II: CBP/BAlq/Liq/Al | 40 | - | - | - | - | 29.6 | [84] |
| DPA-TRZ | ITO/α-NPD/6 wt % DPA-TRZ: mCBP/TPBi/LiF/Al | 15 | 548 | - | - | - | 13.8 | [91] |
| TrzIAc | 20 wt % TrzIAc: mCP and TPBI | 25 | 511 | 0.33, 0.57 | - | - | 20.9 | [61] |
| 3ACR-TRZ | ITO/PEDOT: PSS/16 wt % 3ACR-TRZ: CBP/BmPyPhB/Liq/Al | 55 | 520 | - | - | - | 18.6 | [93] |
| BCzTrzDBF | ITO/DNTPD/BPBPA/PCzAc/5 wt % BCzTrzDBF: mCBPTrz/DBFTrz/ZADN/LiF/Al | 30 | 503 | 0.24, 0.52 | 59.6 | 35.1 | 20.1 | [95] |
| TCzTrzDBF | ITO/DNTPD/BPBPA/PCzAc/5 wt % TCzTrzDBF: mCBPTrz/DBFTrz/ZADN/LiF/Al | 30 | 511 | 0.27, 0.57 | 74.8 | 44.7 | 23.5 | [95] |
| IDCzTrzDBF | ITO/DNTPD/BPBPA/PCzAc/5 wt % IDCzTrzDBF: mCBPTrz/DBFTrz/ZADN/LiF/Al | 30 | 500 | 0.22, 0.48 | 33.6 | 19.3 | 12.2 | [95] |
| BFAcTrz | ITO/PEDOT: PSS/TAPC/mCP/30 wt % BFAcTrz: DPEPO/TSPOI/TPBi/LiF/Al | 25 | 506 | 0.25, 0.51 | 58.7 | 52.7 | 20.4 | [94] |
| BTAcTrz | ITO/PEDOT: PSS/TAPC/mCP/50 wt % BTAcTrz: DPEPO/TSPO1/TPBi/LiF/Al | 25 | 526 | 0.35, 0.57 | 68.9 | 58.5 | 21.8 | [94] |
| TmCzTrz | ITO/PEDOT: PSS/TAPC/mCP/30 wt % TmCzTrz: DPEPO/TSPO1/TPBI/LiF/Al | 25 | 500 | 0.25, 0.50 | 18.6 | 52.1 | 25.5 | [92] |
| DCzmCzTrz | ITO/PEDOT: PSS/TAPC/mCP/20 wt % DCzmCzTrz: DPEPO/TSPO1/TPBI/LiF/Al | 25 | 496 | 0.23, 0.46 | 16.8 | 42.4 | 21.3 | [92] |
| TRZ-DDPAc | ITO/HATCN/TAPC/DCDPA/30 wt % TRZ-DDPAc: DBFPO/TPBi/LiF/Al | 25 | 509 | 0.25, 0.52 | 62.8 | 56.3 | 27.3 | [83] |
| TRZ-DDMAc | ITO/HATCN/TAPC/DCDPA/20 wt % TRZ-DDMAc: PPBI/TPBi/LiF/Al | 25 | 511 | 0.26, 0.54 | 43.2 | 33.7 | 17.6 | [83] |
| 2Cz3Trz | ITO/DNTPD/BPBPA/PCZAC/10 wt % 2Cz3Trz: CzTrz/CzTrz/ZADN/LiF/Al | 30 | 521 | 0.30, 0.56 | 55.8 | 28.3 | 17.9 | [96] |
| 3Cz2Trz | ITO/DNTPD/BPBPA/PCZAC/10 wt % 3Cz2Trz: CzTrz/CzTrz/ZADN/LiF/Al | 30 | 512 | 0.26, 0.50 | 42.9 | 21.8 | 15.0 | [96] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braveenth, R.; Chai, K.Y. Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Materials 2019, 12, 2646. https://doi.org/10.3390/ma12162646
Braveenth R, Chai KY. Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Materials. 2019; 12(16):2646. https://doi.org/10.3390/ma12162646
Chicago/Turabian StyleBraveenth, Ramanaskanda, and Kyu Yun Chai. 2019. "Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes" Materials 12, no. 16: 2646. https://doi.org/10.3390/ma12162646
APA StyleBraveenth, R., & Chai, K. Y. (2019). Triazine-Acceptor-Based Green Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Materials, 12(16), 2646. https://doi.org/10.3390/ma12162646





