Investigation of the Microcharacteristics of Asphalt Mastics under Dry–Wet and Freeze–Thaw Cycles in a Coastal Salt Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Properties
2.2. Experimental Work
2.2.1. Preparation of Asphalt Mastics
2.2.2. Salt “Dry–Wet and Freeze–Thaw (DW-FT)” Cycles Test
2.2.3. Fourier-Transform Infrared (FTIR) Test
2.2.4. Gel Permeation Chromatography (GPC) Test
2.2.5. Atomic Force Microscopy (AFM) Test
3. Results and Discussion
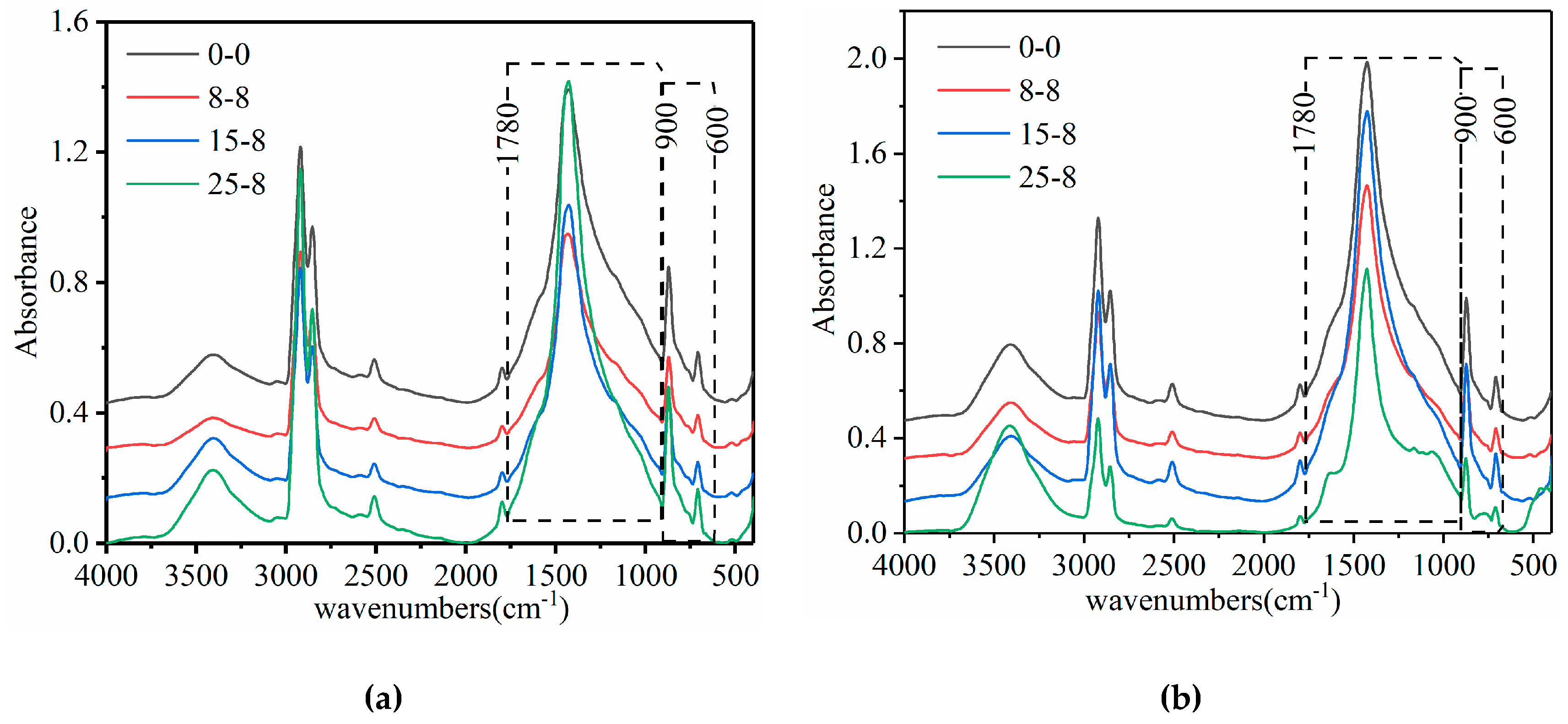
3.1. FTIR Test Results
3.1.1. FTIR Characteristics of the Asphalt Binder, Filler, and Mastic
3.1.2. Changes in the Spectra of the Mastics
3.1.3. Semiquantitative Analysis
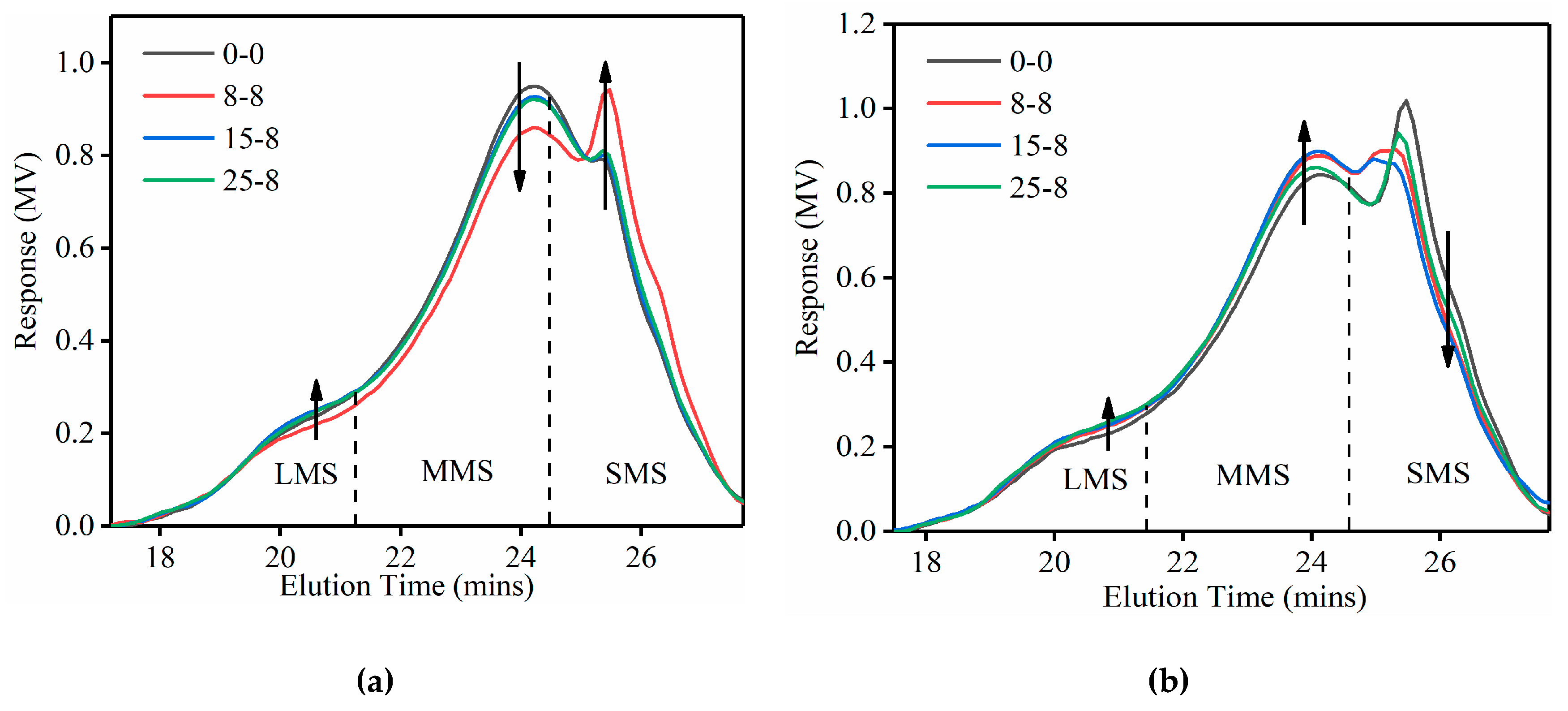
3.2. GPC Test Results
3.2.1. Molecular Size
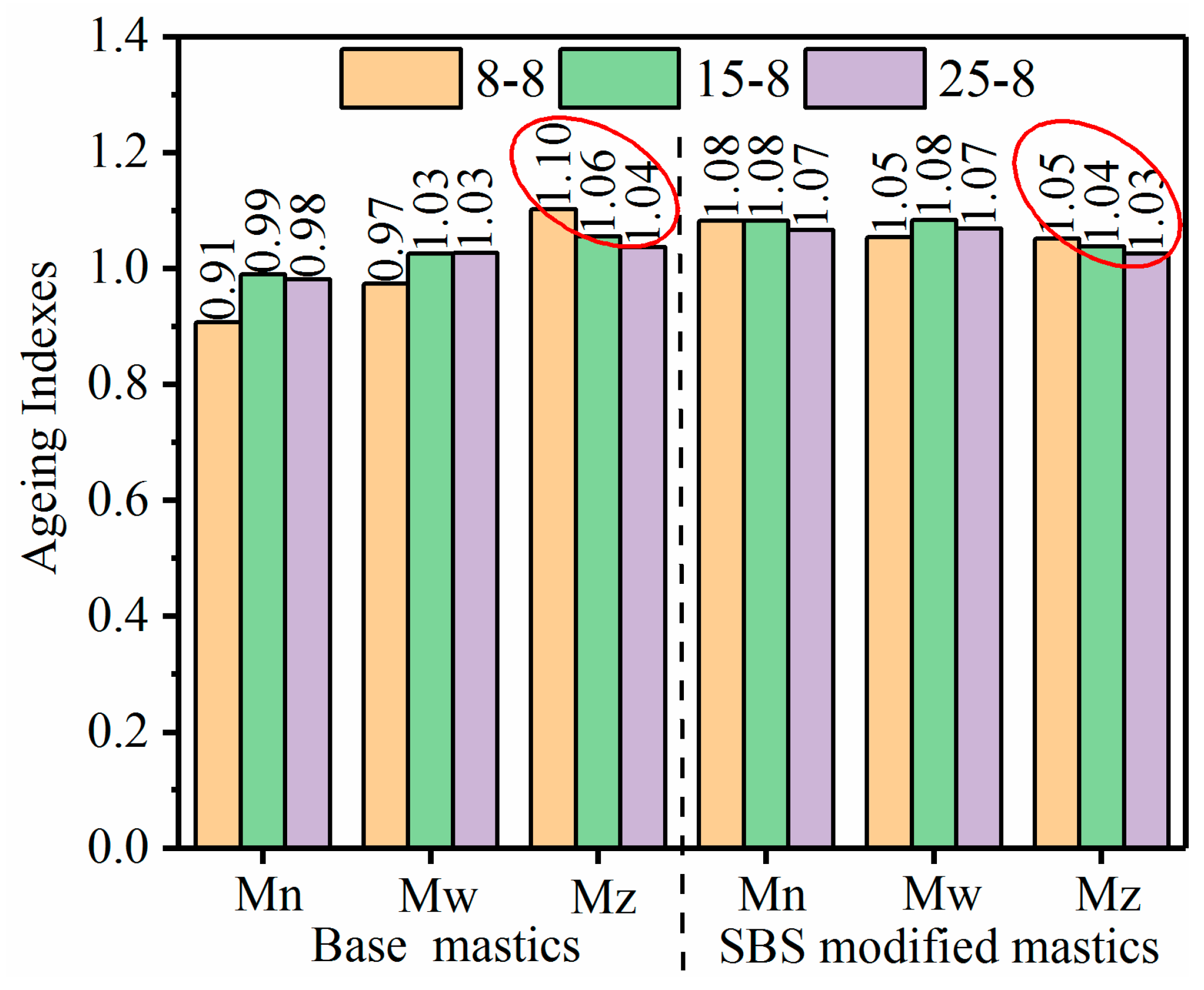
3.2.2. Molecular Weight
3.3. AFM Analysis
3.3.1. AFM Morphology
3.3.2. Microstructural Quantification
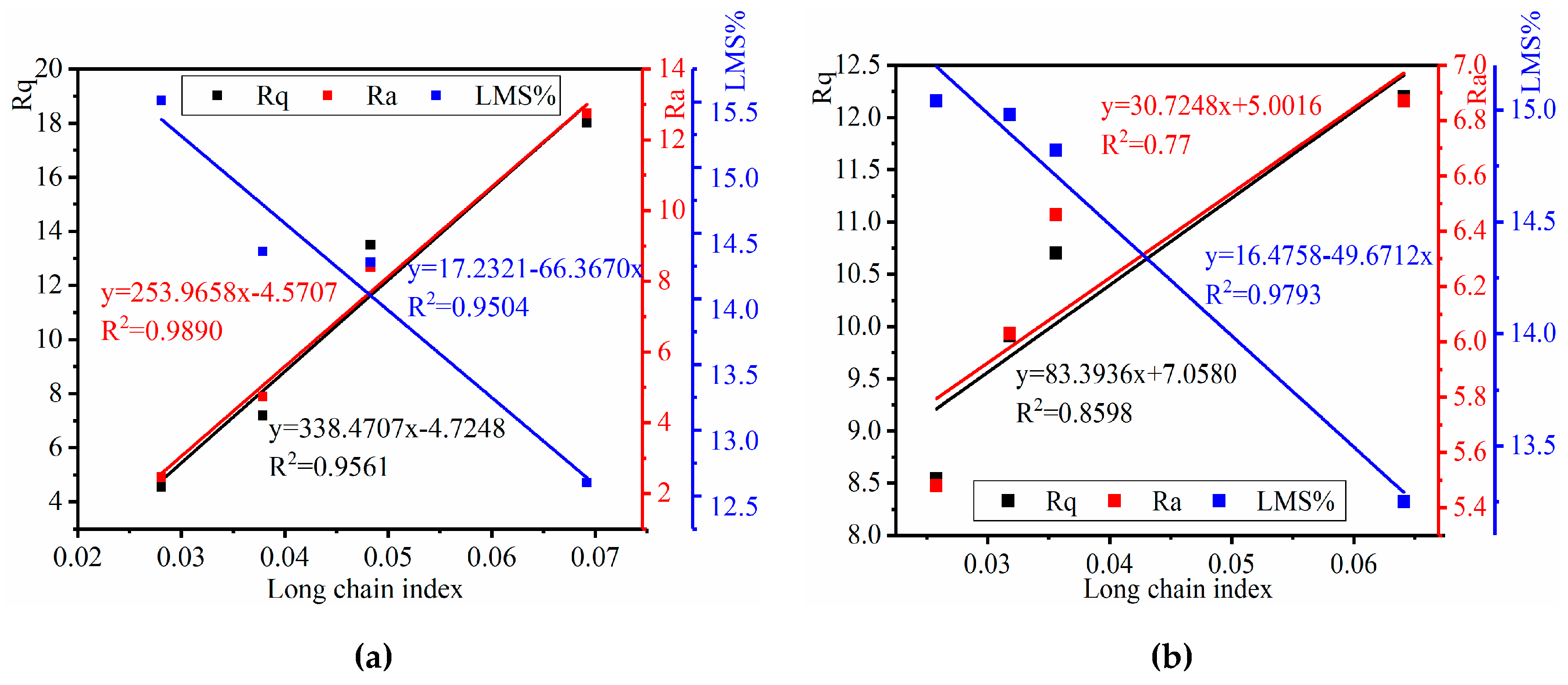
3.4. Correlation between the Chemical Composition and Microstructure Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, K. Marine Manual; Maritime Press: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Zhang, G.H. Research on Damage Mechanism and Service Life of Coastal Area Asphalt Pavement in North China. Ph.D. Thesis, Dalian University of Technology, Dalian, China, May 2013. [Google Scholar]
- He, X.B. Seawater salt spray on harm and preventive measures of asphalt pavement. Fujian Traffic Sci. Technol. 2009, 2, 33–35. (In Chinese) [Google Scholar]
- Zhao, L.H.; Zeng, Y.Y.; Chao, J.C. Research on impact of salt mist-temperature coupling actions on performance of asphalt mixture. Highway 2018, 6, 253–257. (In Chinese) [Google Scholar]
- Obika, B.; Freer-Hewish, R.J.; Fookes, G.P. Soluble salt damage to thin bituminous road and runway surfaces. Q. J. Eng. Geol. Hydroge. 1989, 22, 59–73. [Google Scholar] [CrossRef]
- Juli-Gándara, L.; Vega-Zamanillo, Á.; Calzada-Pérez, M.Á. Sodium chloride effect in the mechanical properties of the bituminous mixtures. Cold Reg. Sci. Technol. 2019, 164, 1–6. [Google Scholar] [CrossRef]
- Starck, P.; Lofgren, B. Influence of de-icing agents on the viscoelastic properties of asphalt mastics. J. Mater. Sci. 2007, 42, 676–685. [Google Scholar] [CrossRef]
- Hassan, Y.; El Halim, A.O.A.; Razaqpur, A.G.; Bekheet, W.; Farha, M.H. Effects of runway deicers on pavement materials and mixes: Comparison with road salt. Transp. Eng. 2002, 128, 385–391. [Google Scholar] [CrossRef]
- Mc Cutchon, J.R.; Joseph, A.P.; Van Valkenburg, J. Assessment of the Effect of Urea Deicing Solution on Asphaltic Concrete Pavements; Project No. 999821; Public Works and Government Services Canada: Hull, QC, Canada, 1992.
- Salt Institute Honors Safe, Environmentally Sensitive, Storage of Road Salt; Salt Institute: Alexandria, VA, USA, September 2011.
- Shi, X.M.; Akin, M.; Pan, T.Y.; Fay, L.; Liu, Y.J.; Yang, Z.X. Deicer impacts on pavement materials: Introduction and recent developments. Open Civil Eng. J. 2009, 3, 16–27. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhang, Y.; Shi, X.M. Impact of nano-clay and carbon microfiber in combating the deterioration of asphalt concrete by non-chloride deicers. Constr. Build. Mater. 2018, 160, 514–525. [Google Scholar] [CrossRef]
- Xiong, R.; Guan, B.W.; Sheng, Y.P. Anti-fatigue property of brucite fiber reinforced asphalt mixture under sulfate and dry-wet circle corrosion environment. J. Wuhan Univ. Technol. 2014, 36, 45–51. (In Chinese) [Google Scholar]
- Xiong, R.; Chen, S.F.; Guan, B.W.; Cong, P.L.; Ma, L.L. Research on performance of asphalt concrete under the action of freeze-thaw and corrosion. J. Wuhan Univ. Technol. 2011, 33, 72–76. (In Chinese) [Google Scholar]
- Cui, Y.N.; Sun, G.N.; Han, J.W.; Zhao, L. Microstructure and high-and-low temperature performance of two kinds of asphalt under salt freezing cycle. AMCS. 2017, 34, 906–914. (In Chinese) [Google Scholar]
- Han, J.W.; Cui, Y.N.; Li, Z.; Li, C.; Liu, Z.Z. Research on the microstructure and performance of asphalt after the salt freezing cycle. Appl. Mech. Mater. 2014, 575, 238–244. [Google Scholar] [CrossRef]
- Glover, C.J.; Bullin, J.A.; Button, J.W.; Davison, R.R.; Donaldson, G.R.; Hlavinka, M.W.; Philip, C.V. Characterization of asphalt using gel permeation chromatography and other methods. Bitum. Pavements 1987.
- Fini, E.H.; Kalberer, E.W.; Shahbazi, A.; Basti, M.; Zhanping You, Z.P.; Ozer, H.; Aurangzeb, Q. Chemical characterization of bio-binder from swine manure: Sustainable modifier for asphalt binder. J. Mater.Civil Eng. 2011, 23, 1506–1513. [Google Scholar] [CrossRef]
- Huang, S.C.; Robertson, R.E.; Branthaver, J.F.; Claine Petersen, J. Impact of lime modification of asphalt and freeze-thaw cycling on the asphalt-aggregate interaction and moisture resistance to moisture damage. J. Mater. Civil Eng. 2005, 17, 711–718. [Google Scholar] [CrossRef]
- Zhao, S.; Bowers, B.; Huang, B.; Shu, X. Characterizing rheological properties of binder and blending efficiency of asphalt paving mixtures containing RAS through GPC. J. Mater. Civil Eng. 2014, 26, 941–946. [Google Scholar] [CrossRef]
- Jäger, A.; Lackner, R.; Eisenmenger-Sittner, C.; Blab, R. Identification of microstructural components of bitumen by means of atomic force microscopy (AFM). PAMM. 2004, 1, 400–401. [Google Scholar] [CrossRef]
- Masson, J.F.; Leblond, V.; Margeson, J. Bitumen morphologies by phase Detection atomic force microscopy. J. Microsc. 2006, 1, 17–29. [Google Scholar] [CrossRef]
- Zhang, H.L.; Shi, C.J.; Yu, J.Y.; Shen, J.N. Modification and its mechanism of different asphalts by polyphosphoric acid. J. Build. Mater. 2013, 2, 255–260. [Google Scholar]
- Asa, L.L.; Viveca, W.; Bjorn, B. Adhesive surface characteristics of bitumen binders investigated by atomic force microscopy. Fuel 2013, 2, 248–256. [Google Scholar]
- Zhu, C.Z.; Zhang, H.L.; Zhang, D.M.; Chen, Z.H. Influence of base asphalt and SBS modifier on the weathering aging behaviors of SBS modified asphalt. J. Mater. Civil Eng. 2018, 3, 1–10. [Google Scholar] [CrossRef]
- JTG E20-2011. Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering; People’s Transportation Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- JTG F40-2004. Technical Specifications for Construction of Highway Asphalt Pavement; People’s Transportation Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Kennedy, T.W.; Huber, G.A.; Harrigan, E.T.; Cominsky, R.J.; Hughes, C.S.; Von Quintus, H.; Moulthrop, J.S. Superior performing asphalt pavement (Superpave): The product of the SHRP asphalt research council. In Proceedings of the 73rd TRB Annual Meeting, Washington, DC, USA, July 1994. [Google Scholar]
- Cardone, F.; Frigio, F.; Ferrotti, G.; Canestrari, F. Influence of mineral fillers on the rheological response of polymer-modified bitumen and mastics. J. Traffic Transp. Eng. 2015, 2, 373–381. [Google Scholar] [CrossRef]
- Fagerlund, G. Scaling, absorption and dilation of cement mortars exposed to freezing and thawing in NaCl solution. In Freeze—Thaw Durability of Concrete; E&FN Spon: London, UK, 1992. [Google Scholar]
- Jacobsen, S.; Sellevold, E.J. Frost/salt scaling and ice formation of concrete: Effect of curing temperature and silica fume on normal and high strength concrete. Proceedings of TRB Annual Meeting, London, UK, 29 April 1997. [Google Scholar]
- Talamucci, F. Freezing processes in porous media: Formation of ice lenses, swelling of the soil. Math. Comput. Model. 2003, 37, 595–602. [Google Scholar] [CrossRef]
- Padilla, F.; Villeneuve, J.P.; Stein, J. Simulation and analysis of frost heaving in subsoils and granular fills of roads. Cold Reg. Sci. Technol. 1997, 25, 89–99. [Google Scholar] [CrossRef]
- Feng, D.; Yi, J.; Wang, D.; Chen, L. Impact of salt and freeze–thaw cycles on performance of asphalt mixtures in coastal frozen region of China. Cold Reg. Sci. Technol. 2010, 62, 34–41. [Google Scholar] [CrossRef]
- AASHTO T 283. Resistance of Compacted Asphalt Mixtures to Moisture-Induced Damage; AASHTO: Washington, DC, USA, 1991. [Google Scholar]
- Goh, S.; Akin, M.; You, Z.; Shi, X. Effect of deicing solutions on the tensile strength of micro-or nano-modified asphalt mixture. Constr. Build. Mater. 2011, 5, 195–200. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, J. Influence of coastal area salinity on road surface resistance to permanent deformation performance. J. Da Lian Jiao Tong Univ. 2016, 37, 69–72. (In Chinese) [Google Scholar]
- Zhang, K.; Zhang, Z.Q. Deterioration of mechanical properties of asphalt mixture in salty and humid environment. J. South China Univ. Technol. (Natural Sci. ed.) 2015, 43, 106–112. (In Chinese) [Google Scholar]
- Mansour, E. Semi-quantitative analysis for FTIR spectra of Al2O3-PbO-B2O3-SiO2 glasses. J. Non-Cryst. Solids 2012, 358, 0–460. [Google Scholar] [CrossRef]
- Moraes, R.; Bahia, H.U. Effect of mineral filler on changes in molecular size distribution of asphalts during oxidative ageing. Road Mater. Pavement 2015, 16, 55–72. [Google Scholar] [CrossRef]
- Liu, B.; Shen, J.N.; Song, X. Changes in nanoscaled mechanical and rheological properties of asphalt binders caused by aging. J. Nanomater. 2015, 961924. [Google Scholar] [CrossRef]
- Gong, M.H. Research on micro-healing mechanism of asphalt based on atomic force microscopy and quantitative analysis. Ph.D. Thesis, Southeast University, Jiangsu China, May 2014. (In Chinese). [Google Scholar]
- Weng, S.F.; Xu, Y.Z. Fourier Transform Infrared Spectroscopy Analysis, 3rd ed.; Chemical Industry Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Singh, B.; Saboo, N.; Kumar, P. Use of Fourier transform infrared spectroscopy to study ageing characteristics of asphalt binders. Petrol. Sci. Technol. 2017, 35, 1648–1654. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, X.N. Microscopic analysis on the aging mechanism of asphalt. Bull. Chin. Ceram. Soc. 2014, 33, 1275–1281. (In Chinese) [Google Scholar]
- Lórenz-Fonfría, V.A.; Padrós, E. Curve-fitting of Fourier manipulated spectra comprising apodization, smoothing, derivation and deconvolution. Spectrochim. Acta. A 2004, 60, 2703–2710. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain problems in least squares. Quart. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Indust. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Adachi, D.; Sato, H.; Ozaki, Y. Usefulness of a curve fitting method in the analysis of overlapping overtones and combinations of CH stretching modes. J. Near Infrared Spec. 2002, 10, 85–91. [Google Scholar] [CrossRef]
- Lórenz-Fonfría, V.A.; Padrós, E. Curve-fitting overlapped bands: Quantification and improvement of curve-fitting robustness in the presence of errors in the model and in the data. Analyst 2004, 129, 1243–1250. [Google Scholar] [CrossRef]
- Parente, M.; Makarewicz, H.D.; Bishop, J.L. Decomposition of mineral absorption bands using nonlinear least squares curve fitting: Application to Martian meteorites and CRISM data. Planet Space Sci. 2011, 59, 423–442. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Li, W. Resolution of overlapping spectra by curve-fitting. Anal. Chim. Acta 2005, 538, 383–389. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hsu, C.H.; Sheu, M.T. Curve-fitting FTIR studies of loratadine/hydroxypropyl-beta-cyclodextrin inclusion complex induced by co-grinding process. J. Pharmaceut. Biomed. 2010, 53, 799–803. [Google Scholar] [CrossRef]
- Morris, A.M.; Watzky, M.A.; Finke, R.G. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. BBA-Proteins Proteom. 2009, 1794, 375–397. [Google Scholar] [CrossRef]
- Liu, C.H.; Ng, H.T.; Ng, P.C.; Tsai, K.Y.; Lin, S.J.; Chen, J.H. A novel curve-fitting procedure for determining proximity effect parameters in electron beam lithography. In Proceedings of the SPIE Lithography Asia 2008, Taipei, Taiwan, 4–6 November 2008. [Google Scholar]
- Feng, Z.G.; Yu, J.Y.; Zhang, H.L.; Kuang, D.L.; Xue, L.H. Effect of ultraviolet aging on rheology, chemistry and morphology of ultraviolet absorber modified bitumen. Mater. Struct. 2013, 46, 1123–1132. [Google Scholar] [CrossRef]
- Feng, Z.G.; Bian, H.J.; Li, X.J.; Yu, J.Y. FTIR analysis of UV aging on bitumen and its fractions. Mater. Struct. 2016, 49, 1381–1389. [Google Scholar] [CrossRef]
- Sastry, M.I.S.; Raman, N.S.; Jain, N.; Mukherjee, S.; Kagdiyal, V.; Singhal, S.K.; Sarpal, A.S. Determination of wax appearance temperature and wax dissolution temperature of waxy crude oils by temperature programmed. IR Spectroscopy 2010, 20100930. [Google Scholar]
- Becker, Y.; Mendez, M.P.; Rodriguez, Y. Polymer modified asphalt. Vision Tecnol. 2001, 9, 12. [Google Scholar]
- Bowers, B.F. Investigation of Asphalt Pavement Mixture Blending Utilizing Analytical Chemistry Techniques. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, December 2013. [Google Scholar]
- Jennings, P.W. High Pressure Liquid Chromatography as A Method of Measuring Asphalt Composition; Amazon: Seattle, WA, USA, 1980. [Google Scholar]
- Ma, T.; Wang, H.; He, L.; Zhao, Y.L.; Huang, X.M.; Chen, J. Property characterization of asphalt binders and mixtures modified by different crumb rubbers. Mater. Civil Eng. 2017, 29, 04017036. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, K.; Doh, Y.S.; Amirkhanian, S.N. Estimation of RAP’s binder viscosity using GPC without binder recovery. Mater. Civil Eng. 2006, 18, 561–567. [Google Scholar] [CrossRef]
- Bowers, B.F.; Huang, B.; Shu, X.; Miller, B.C. Investigation of Reclaimed Asphalt Pavement blending efficiency through GPC and FTIR. Constr. Build. Mater. 2014, 50, 517–523. [Google Scholar] [CrossRef]
- Mogawer, W.; Bennert, T.; Danie, l J.S.; Bonaquist, R.; Austerman, A.; Booshehrian, A. Performance characteristics of plant produced high RAP mixtures. Road Mater. Pavement 2012, 13, 183–208. [Google Scholar] [CrossRef]
- Doh, Y.S.; Amirkhanian, S.N.; Kim, K.W. Analysis of unbalanced binder oxidation level in recycled asphalt mixture using GPC. Constr. Build. Mater. 2008, 22, 1253–1260. [Google Scholar] [CrossRef]
- Lee, S.J.; Amirkhanian, S.N.; Shatanawi, K. Effects of reaction time on physical and chemical properties of rubber-modified binders. In Proceedings of the International Rubber Conference, Lyon, France, 13–16 June 2006. [Google Scholar]
- Loeber, L.; Sutton, O.; Morel, J.; Valleton, J.M.; Muller, G. New direct observations of asphalt and asphalt binders by scanning electron microscopy and atomic force microscopy. J. Microsc. 1996, 182, 32–39. [Google Scholar] [CrossRef]
- Allen, R.G. Properties in Asphalt using Atomic Force Microscopy; Texas University: Austin, TX, USA, 2011. [Google Scholar]
- Branthaver, J.F.; Petersen, J.C.; Robertson, R.E.; Duvall, J.J.; Kim, S.S.; Harnsberger, P.M.; Mill, T.; Ensley, E.K.; Barbour, F.A.; Scharbron, J.F. Binder characterization and evaluation, volume 2: Chemistry. In Proceedings of the 72nd TRB Annual Meeting, Washington, DC, USA, March 1993. [Google Scholar]












| Properties | AH-70 base Binders | Styrene-Butadiene-Styrene (SBS)-I-D Modified Binders | |||
|---|---|---|---|---|---|
| Standard Values | Test Results | Standard Values | Test Results | ||
| Penetration (25 °C, 100 g, 5 s), 0.1 mm | 60–80 | 74 | 40–60 | 55 | |
| Softening point, °C | 44–57 | 47.3 | ≥60 | 86.6 | |
| Ductility (5 °C, 5 cm/min), cm | ≥100 | >150 | ≥20 | 31.5 | |
| Wax content, % | ≯3.0 | 1.1 | - | - | |
| RTFOT | Mass loss, % | ≤0.8 | 0.01 | ≤1 | 0.08 |
| Penetration ratio (25 °C), % | ≥55 | 68.9 | ≥65 | 71.6 | |
| Ductility (5 °C, 5 cm/min), cm | ≥30 | 68.4 | ≥15 | 17.3 | |
| Properties | Test Results | Standard Values | Test Method | |
|---|---|---|---|---|
| Apparent density, g/cm3 | 2.798 | ≥2.50 | T0352 | |
| Water content, % | 0.49 | ≤1 | T0332 | |
| Through the percentage of sieves, % | <0.6 mm | 100 | 100 | T0351 |
| <0.15 mm | 95.23 | 90~100 | ||
| <0.075 mm | 85.91 | 75~100 | ||
| Hydrophilic coefficient | 0.78 | <1 | T0353 | |
| Wave Numbers (cm−1) | Explanation |
|---|---|
| 3419/1645 | Hydroxy (−OH) asymmetric stretch and angular vibration of water molecules |
| 2922/2854/2853 | Methy(−CH2−) symmetry vibration of the aliphatic long chain (saturated) |
| 2509/2512 | combination frequency of antisymmetric and symmetric stretching vibrations |
| 1795/1799 | weak symmetrical stretching vibration and in-plane bending vibration |
| 1602 | Asymmetric ring vibration of the benzene ring and carboxyl group |
| 1460/1456/1431/1425/1427 | Symmetric bending vibration of −CH2 groups and aliphatic long chains (saturated) |
| 1377/1375 | Methy l(−CH3) umbrella vibration and aliphatic branched chain (saturated) |
| 1161 | Aliphatic sulfonic acid (SO2) symmetric stretching |
| 1032 | Sulfoxide (S=O) stretching vibration |
| 967 | Butadiene (SBS) stretching vibration |
| 876/712 | out-of-plane bending vibration and in-plane bending vibration |
| 870/812 | Stretching vibration of benzene ring |
| 723 | Covibration of the methylene segment (−CH2−)n (n ≥ 4) |
| Numbers | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Base and SBS-modified mastics | 870 | 812 | 723 | 710 |
| Numbers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Base mastics | 1645 | 1600 | 1533 | 1460 | 1420 | 1377 | 1300 | 1161 | 1080 | 1032 |
| SBS-modifiedmastics | 1645 | 1600 | 1533 | 1460 | 1420 | 1377 | 1300 | 1161 | 1032 | 967 |
| Samples | Aromatic Index | Branched Aliphatic Index | Sulfoxide Index | Long Chain Alkane Index | Butadiene Index | |
|---|---|---|---|---|---|---|
| Base mastics | 0–0 | 0.0284 | 0.5904 | 0.0008 | 0.0692 | - |
| 8–8 | 0.0300 | 0.5169 | 0.0068 | 0.0483 | - | |
| 15–8 | 0.0302 | 0.3629 | 0.0145 | 0.0379 | - | |
| 25–8 | 0.0317 | 0.4119 | 0.0382 | 0.0281 | - | |
| SBS-modified mastics | 0–0 | 0.0393 | 0.2840 | 0.0006 | 0.0641 | 0.0208 |
| 8–8 | 0.0230 | 0.2756 | 0.0034 | 0.0356 | 0.0119 | |
| 15–8 | 0.0296 | 0.2072 | 0.0107 | 0.0318 | 0.0089 | |
| 25–8 | 0.0349 | 0.2287 | 0.0246 | 0.0258 | 0.0052 | |
| Test Conditions | 0–0 | 8–8 | 15–8 | 25–8 |
|---|---|---|---|---|
| Base mastics | 128.56 | 96.69 | 78.34 | 59.41 |
| SBS-modified mastics | 80 | 51.37 | 49.87 | 43.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Huang, Z. Investigation of the Microcharacteristics of Asphalt Mastics under Dry–Wet and Freeze–Thaw Cycles in a Coastal Salt Environment. Materials 2019, 12, 2627. https://doi.org/10.3390/ma12162627
Zhang Q, Huang Z. Investigation of the Microcharacteristics of Asphalt Mastics under Dry–Wet and Freeze–Thaw Cycles in a Coastal Salt Environment. Materials. 2019; 12(16):2627. https://doi.org/10.3390/ma12162627
Chicago/Turabian StyleZhang, Qinling, and Zhiyi Huang. 2019. "Investigation of the Microcharacteristics of Asphalt Mastics under Dry–Wet and Freeze–Thaw Cycles in a Coastal Salt Environment" Materials 12, no. 16: 2627. https://doi.org/10.3390/ma12162627
APA StyleZhang, Q., & Huang, Z. (2019). Investigation of the Microcharacteristics of Asphalt Mastics under Dry–Wet and Freeze–Thaw Cycles in a Coastal Salt Environment. Materials, 12(16), 2627. https://doi.org/10.3390/ma12162627




