Investigating Helium Bubble Nucleation and Growth through Simultaneous In-Situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
Specimens and Irradiation Treatment
3. Results
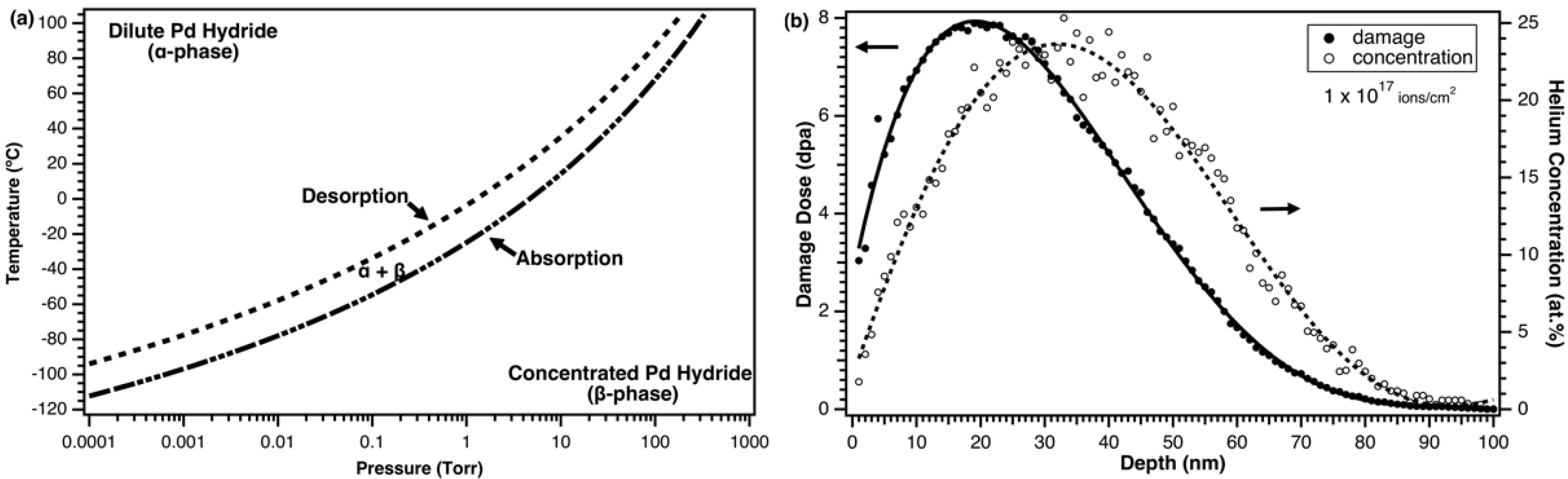
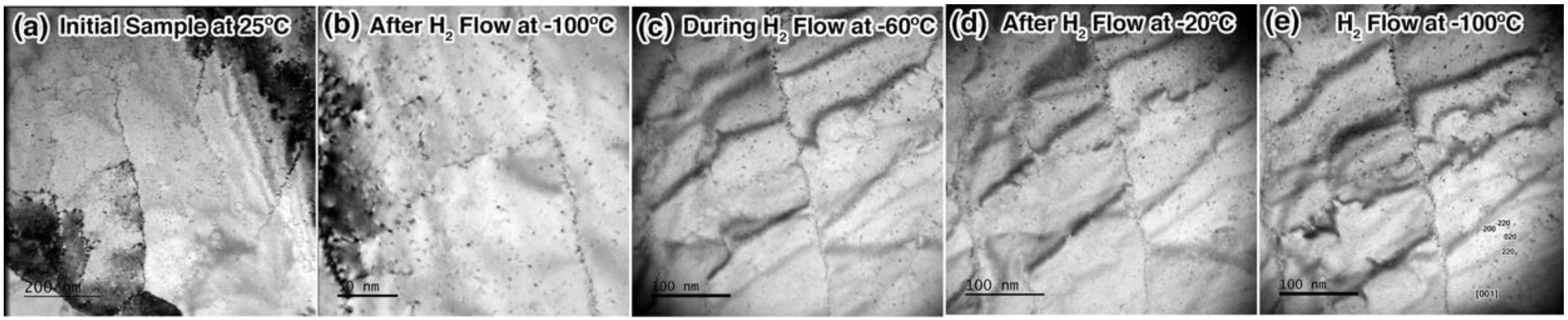
3.1. Exposure to H2 at Cryogenic Temperature
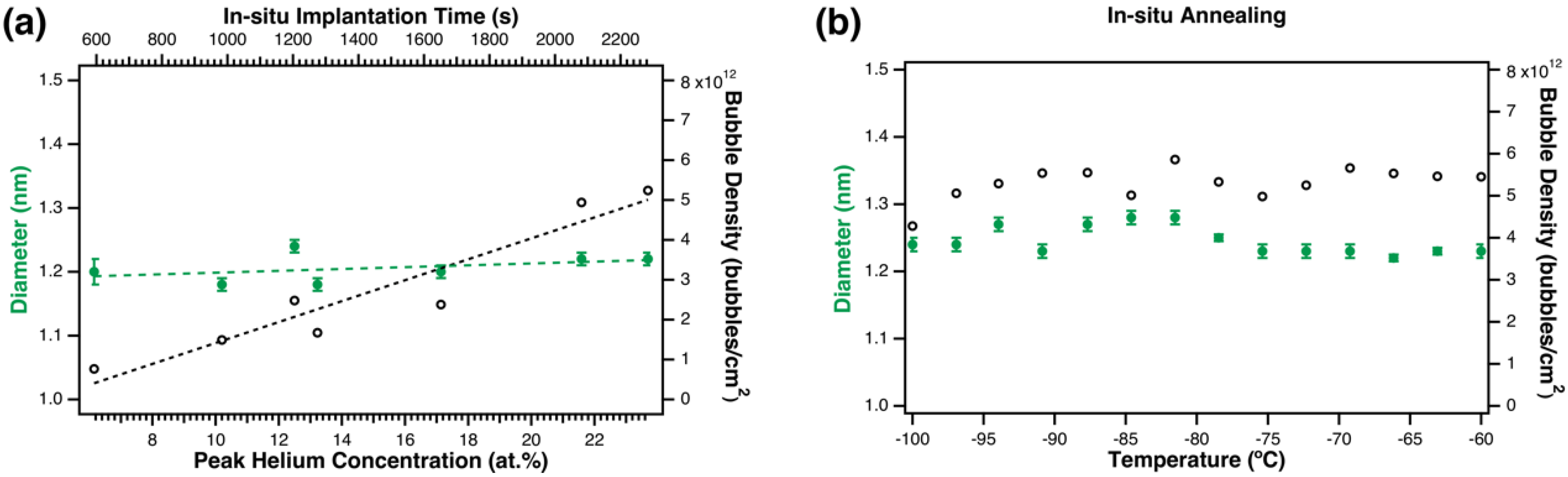
3.2. In-situ Helium Implantation and Annealing in H2
4. Discussion
4.1. Comparison with Data on He bubble Nucleation in Pd and PdT0.6
4.2. Comparison with Theory of He Bubble Nucleation and Growth at Cryogenic Temperatures
4.3. Hydrogen-helium Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, G.J.; Bauer, W. Helium Implantation Effects in Palladium at High Doses. Radiat. Eff. 1973, 17, 221–234. [Google Scholar] [CrossRef]
- Bauer, W.; Thomas, G.J. Helium Release and Electron-Microscopy of Helium-Implanted Palladium. J. Nucl. Mater. 1972, 42, 96–100. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The Role of Palladium in a Hydrogen Economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Manchester, F.D.; San-Martin, A.; Pitre, J.M. The H-Pd (Hydrogen-Palladium) System. J. Phase Equilibria 1994, 15, 62–83. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Oates, W.A. The Palladium-Hydrogen System. Annu. Rev. Mater. Sci. 1991, 21, 269–304. [Google Scholar] [CrossRef]
- Jewell, L.L.; Davis, B.H. Review of Absorption and Adsorption in the Hydrogen-Palladium System. Appl. Catal. A Gen. 2006, 310, 1–15. [Google Scholar] [CrossRef]
- Thiébaut, S.; Décamps, B.; Pénisson, J.M.; Limacher, B.; Percheron Guégan, A. TEM Study of the Aging of Palladium-Based Alloys During Tritium Storage. J. Nucl. Mater. 2000, 277, 217–225. [Google Scholar] [CrossRef]
- Thomas, G.J.; Mintz, J.M. Helium bubbles in palladium tritide. J. Nucl. Mater. 1983, 116, 336–338. [Google Scholar] [CrossRef]
- Fabre, A.; Decamps, B.; Finot, E.; Penisson, J.M.; Demoment, J.; Thiebaut, S.; Contreras, S.; Percheron-Guegan, A. On the correlation between mechanical and TEM studies of the aging of palladium during tritium storage. J. Nucl. Mater. 2005, 342, 101–107. [Google Scholar] [CrossRef]
- Hinks, J.A. Transmission Electron Microscopy with In-situ Ion Irradiation. J. Mater. Res. 2015, 30, 1214–1221. [Google Scholar] [CrossRef]
- Greaves, G.; Mir, A.H.; Harrison, R.W.; Tunes, M.A.; Donnelly, S.E.; Hinks, J.A. New Microscope and Ion Accelerators for Materials Investigations (MIAMI-2) system at the University of Huddersfield. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 931, 37–43. [Google Scholar] [CrossRef]
- Lasser, R.; Klatt, K.H. Solubility of Hydrogen Isotopes in Palladium. Phys. Rev. B 1983, 28, 748–758. [Google Scholar] [CrossRef]
- Lewis, F.A. The Hydrides of Palladium and Palladium Alloys: A Review of Recent Researches. Platin. Met. Rev. 1960, 4, 132. [Google Scholar]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM-The Stopping and Range of Ions in Matter. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Stoller, R.E.; Toloczko, M.B.; Was, G.S.; Certain, A.G.; Dwaraknath, S.; Garner, F.A. On the use of SRIM for computing radiation damage exposure. Nucl. Instrum. Methods Phys. Res. B 2013, 310, 75–80. [Google Scholar] [CrossRef]
- Jimenez, C.M.; Lowe, L.F.; Burke, E.A.; Sherman, C.H. Radiation Damage in Pd Produced by 1–3-MeV Electrons. Phys. Rev. 1967, 153, 735–739. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Roddatis, V.; Bongers, M.D.; Vink, R.; Burlaka, V.; Čížek, J.; Pundt, A. Insights into Hydrogen Gas Environment-Promoted Nanostructural Changes in Stressed and Relaxed Palladium by Environmental Transmission Electron Microscopy and Variable-Energy Positron Annihilation Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 5246–5253. [Google Scholar] [CrossRef]
- Trinkaus, H. Energetics and Formation Kinetics of Helium Bubbles in Metals. Radiat. Eff. 1983, 78, 1–4. [Google Scholar] [CrossRef]
- Abell, G.C.; Attalla, A. NMR Evidence for Solid-Fluid Transition near 250 K of He-3 Bubbles in Palladium Tritide. Phys. Rev. Lett. 1987, 59, 995–997. [Google Scholar] [CrossRef]
- Abell, G.C.; Cowgill, D.F. Low-Temperature He-3 NMR Studies in Aged Palladium Tritide. Phys. Rev. B 1991, 44, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Eremeev, S.V.; Lipnitskii, A.G.; Potekaev, A.I.; Chulkov, E.V. Activation Energy for Diffusion of Point Defects at the Surfaces of F.C.C. metals. Russ. Phys. J. 1997, 40, 584–589. [Google Scholar] [CrossRef]
- Wolfer, W.G. Radiation Effects in Plutonium. Los Alamos Sci. 2000, 26, 274–285. [Google Scholar]
- Wolfer, W.G. The Pressure for Dislocation Loop Punching by a Single Bubble. Philos. Mag. A 1988, 58, 285–297. [Google Scholar] [CrossRef]
- Wolfer, W.G. Dislocation Loop Punching in Bubble Arrays. Philos. Mag. A 1989, 59, 87–103. [Google Scholar] [CrossRef]
- Myers, S.M.; Baskes, M.I.; Birnbaum, H.K.; Corbett, J.W.; DeLeo, G.G.; Estreicher, S.K.; Haller, E.E.; Jena, P.; Johnson, N.M.; Kirchheim, R.; et al. Hydrogen Interactions with Defects in Crystalline Solids. Rev. Mod. Phys. 1992, 64, 559–617. [Google Scholar] [CrossRef]
- Norskov, J.K.; Besenbacher, F.; Bottiger, J.; Nielsen, B.B.; Pisarev, A.A. Interaction of Hydrogen with Defects in Metals: Interplay between Theory and Experiment. Phys. Rev. Lett. 1982, 49, 1420–1423. [Google Scholar] [CrossRef]
- Myers, S.M.; Wampler, W.R.; Besenbacher, F.; Robinson, S.L.; Moody, N.R. Ion Beam Studies of Hydrogen in Metals. Mater. Sci. Eng. 1985, 69, 397–409. [Google Scholar] [CrossRef]
- Besenbacher, F.; Nielsen, B.B.; Norskov, J.K.; Myers, S.M.; Nordlander, P. Interaction of Hydrogen Isotopes with Metals: Deuterium Trapped at Lattice Defects in Palladium. J. Fusion Energy 1990, 9, 257–261. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.A.; Briggs, S.; Greaves, G.; Monterrosa, A.; Aradi, E.; Sugar, J.D.; Robinson, D.B.; Hattar, K.; Hinks, J.A. Investigating Helium Bubble Nucleation and Growth through Simultaneous In-Situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy. Materials 2019, 12, 2618. https://doi.org/10.3390/ma12162618
Taylor CA, Briggs S, Greaves G, Monterrosa A, Aradi E, Sugar JD, Robinson DB, Hattar K, Hinks JA. Investigating Helium Bubble Nucleation and Growth through Simultaneous In-Situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy. Materials. 2019; 12(16):2618. https://doi.org/10.3390/ma12162618
Chicago/Turabian StyleTaylor, Caitlin A., Samuel Briggs, Graeme Greaves, Anthony Monterrosa, Emily Aradi, Joshua D. Sugar, David B. Robinson, Khalid Hattar, and Jonathan A. Hinks. 2019. "Investigating Helium Bubble Nucleation and Growth through Simultaneous In-Situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy" Materials 12, no. 16: 2618. https://doi.org/10.3390/ma12162618
APA StyleTaylor, C. A., Briggs, S., Greaves, G., Monterrosa, A., Aradi, E., Sugar, J. D., Robinson, D. B., Hattar, K., & Hinks, J. A. (2019). Investigating Helium Bubble Nucleation and Growth through Simultaneous In-Situ Cryogenic, Ion Implantation, and Environmental Transmission Electron Microscopy. Materials, 12(16), 2618. https://doi.org/10.3390/ma12162618




