The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
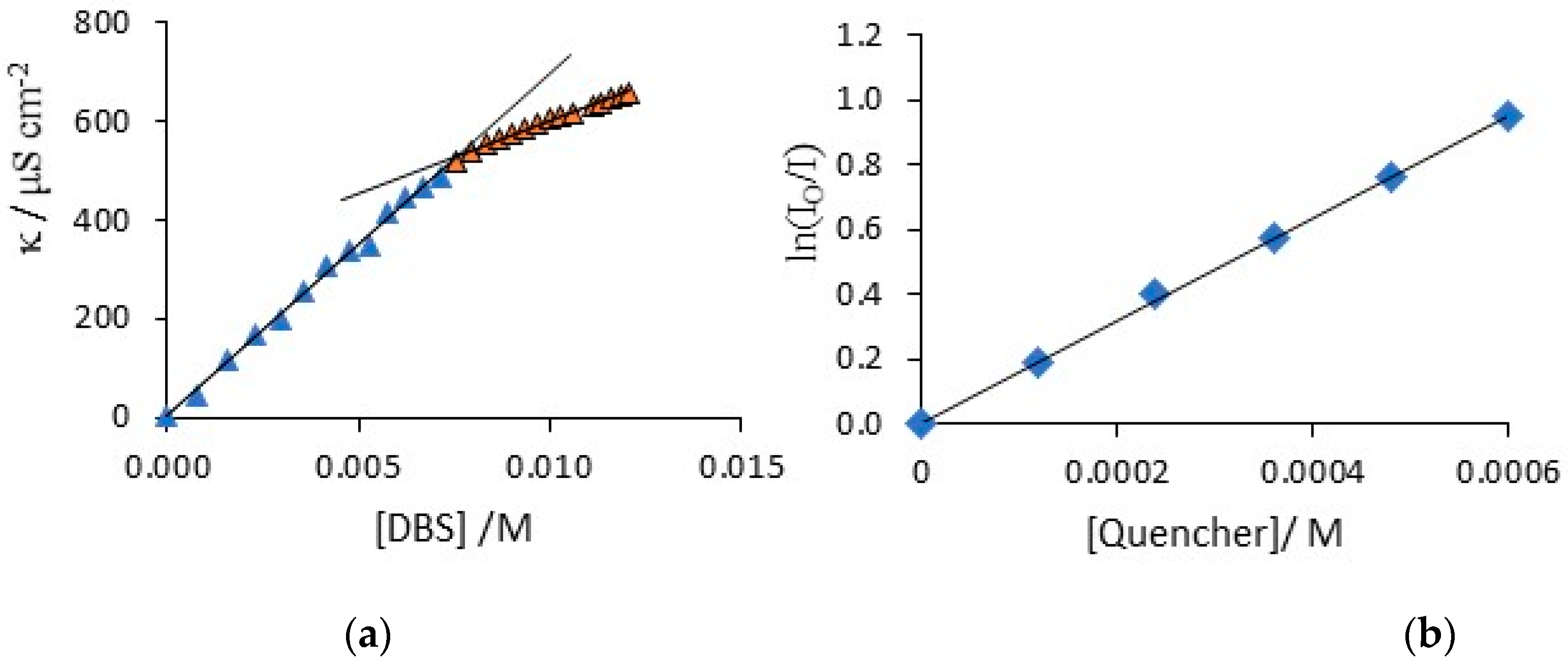
3.1. Characterisation of the DBS Solution and Functionalization of CNTs
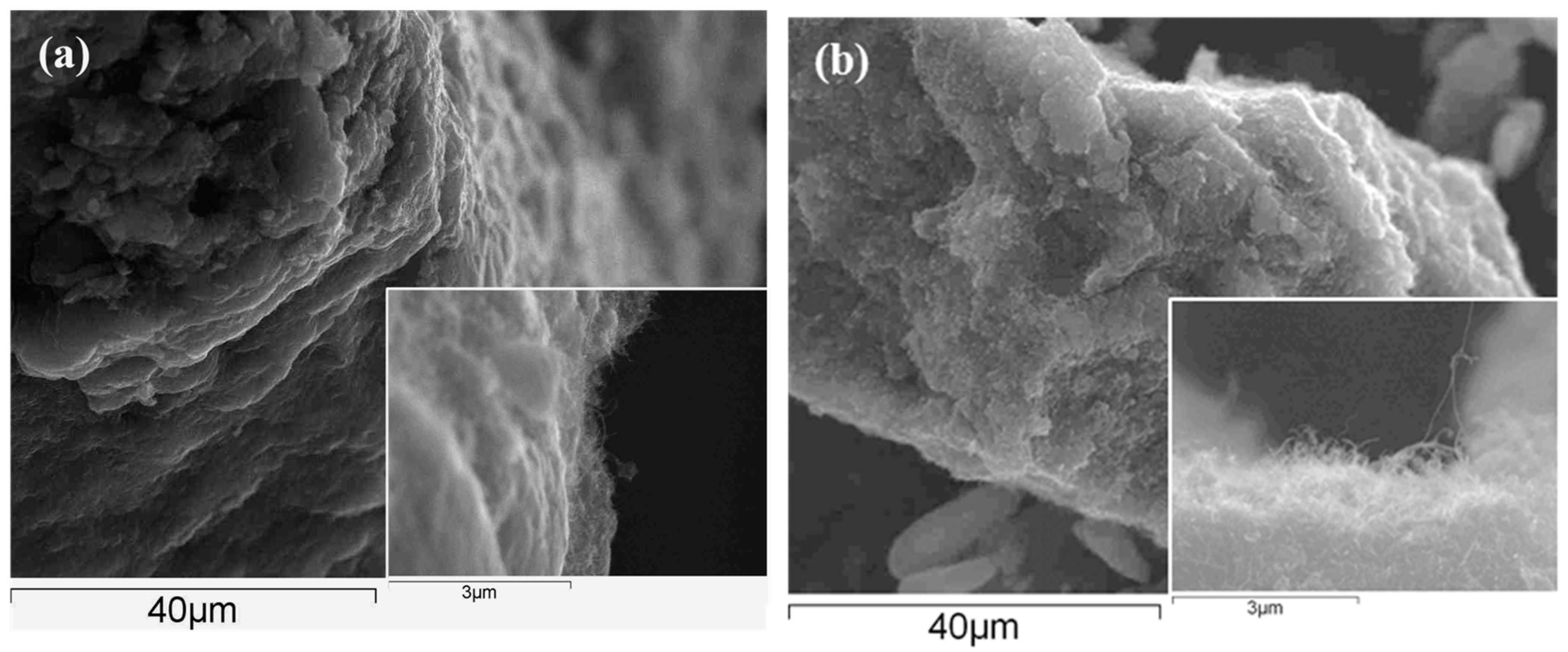
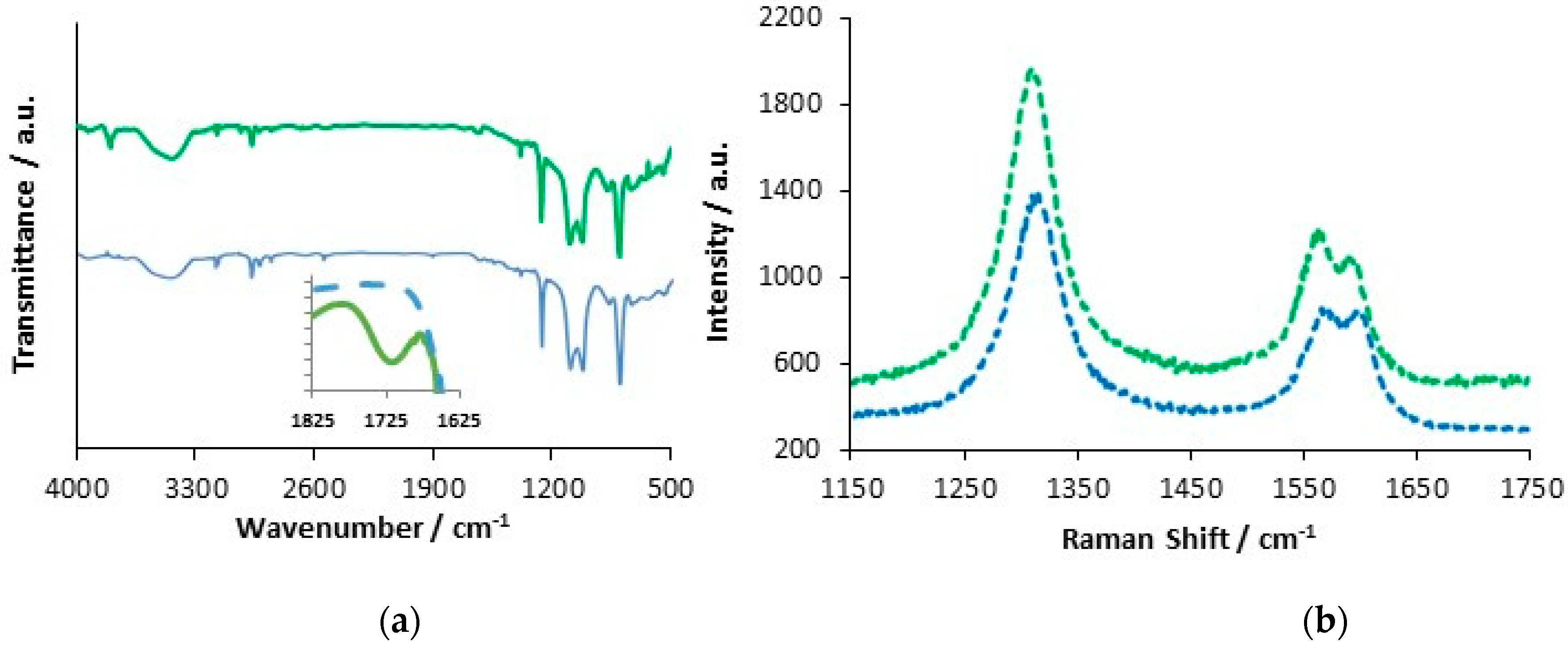
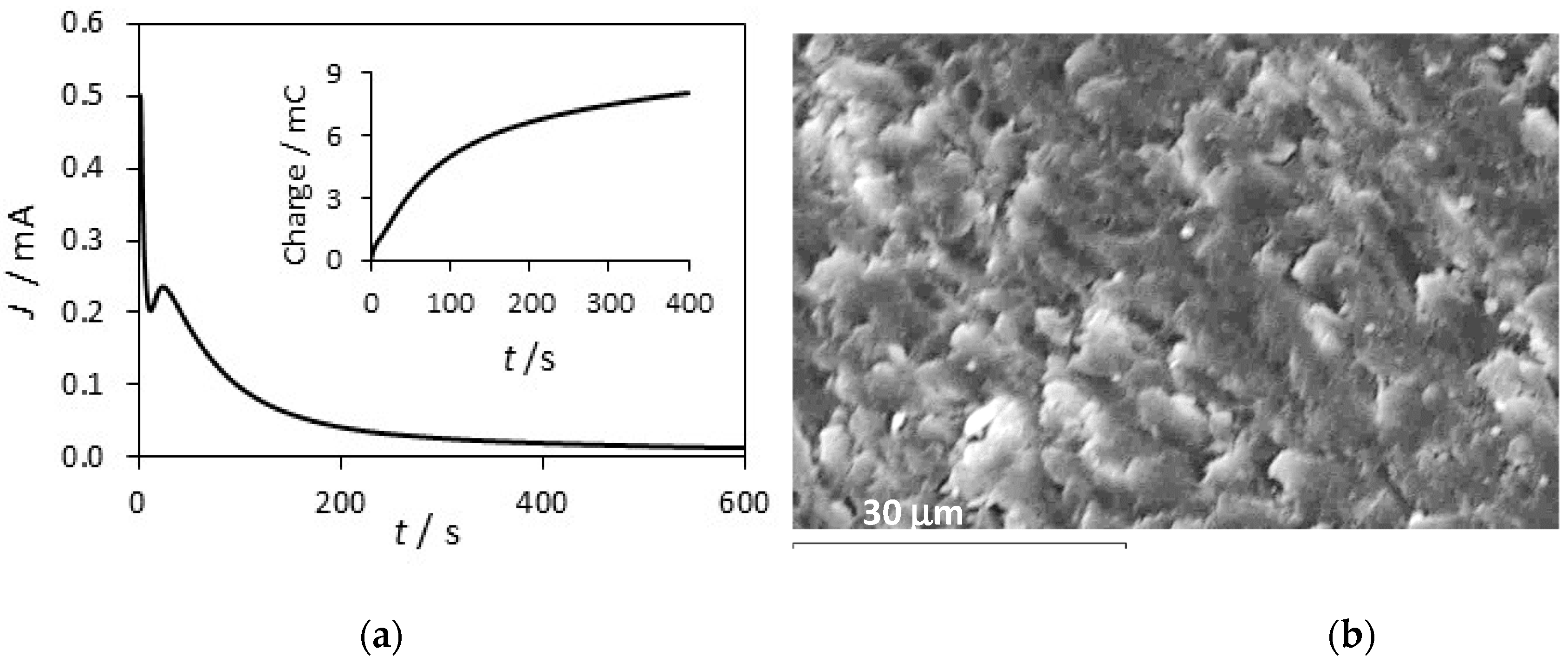
3.2. Formation PPy-DBS and PPy-DBSCNT
3.3. Corrosion Protection Properties
3.4. Role of CNT, DBS and Tartrate Pre-Layer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DeBerry, D.W. Modification of the Electrochemical and corrosion behavior of stainless steels with an electroactive coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Garcia, B.; Lamzoudi, A.; Pillier, F.; Nguyen, T.; Le, H.; Deslouis, C. Oxide/Polypyrrole composite films for corrosion protection of iron. J. Electrochem. Soc. 2002, 149, B560–B566. [Google Scholar] [CrossRef]
- Fenelon, A.M.; Breslin, C.B. The electrochemical synthesis of polypyrrole at a copper electrode: corrosion protection properties. Electrochim. Acta 2002, 47, 4467–4476. [Google Scholar] [CrossRef]
- Fenelon, A.M.; Breslin, C.B. The electropolymerization of pyrrole at a cuni electrode: Corrosion protection properties. Corros. Sci. 2003, 45, 2837–2850. [Google Scholar] [CrossRef]
- Nguyen, T.; Le, H.; Garcia, B.; Deslouis, C.; Xuan, Q.L. Corrosion protection and conducting polymers: Polypyrrole films on iron. Electrochim. Acta 2001, 46, 4259–4272. [Google Scholar] [CrossRef]
- El-Shazly, A.H.; Wazzan, A.A. Using polypyrrole coating for improving the corrosion resistance of steel buried in corrosive mediums. Int. J. Electrochem. Sci. 2012, 7, 1946–1957. [Google Scholar]
- Biallozor, S.; Kupniewska, A. Conducting polymers electrodeposited on active metals. Synth. Met. 2005, 155, 443–449. [Google Scholar] [CrossRef]
- Ladan, M.; Basirun, W.F.; Kazi, S.N.; Abdul Rahman, F. Corrosion protection of AISI 1018 steel using Co-doped TiO2/polypyrrole nanocomposites in 3.5% NaCl solution. Mater. Chem. Phys. 2017, 192, 361–373. [Google Scholar] [CrossRef]
- Haase, V.; Beck, F. Electrodeposition of N-Substituted polypyrroles on iron and the cipl strategy. Electrochim. Acta 1994, 39, 1195–1205. [Google Scholar] [CrossRef]
- Beck, F.; Michaelis, R.; Schloten, F.; Zinger, B. Filmforming electropolymerization of pyrrole on iron in aqueous oxalic acid. Electrochim. Acta 1994, 39, 229–234. [Google Scholar] [CrossRef]
- Fenelon, A.M.; Breslin, C.B. Corrosion protection properties afforded by an in situ electropolymerized polypyrrole layer on CuZn. J. Electrochem. Soc. 2003, 150, B540–B546. [Google Scholar] [CrossRef]
- Arabzadeh, H.; Shahidi, M.; Foroughi, M.M. Electrodeposited polypyrrole coatings on mild steel: Modeling the EIS data with a new equivalent circuit and the influence of scan rate and cycle number on the corrosion protection. J. Electroanal. Chem. 2017, 807, 162–173. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G. Cyclic voltammetric synthesis of poly(n–methyl pyrrole) on copper and effects of polymerisation parameters on corrosion performance. Ind. Eng. Chem. Res. 2012, 51, 5246–5255. [Google Scholar] [CrossRef]
- Redondo, M.I.; Sanchez de la Blanca, E.; Garcia, M.V.; Gonzalez-Tejera, M.J. Poly(N–methylpyrrole) electrodeposited on copper: Corrosion protection properties. Prog. Org. Coat. 2009, 65, 386–391. [Google Scholar] [CrossRef]
- Gvozdenovic, M.M.; Jugovic, B.Z.; Stevanovic, J.S.; Grgur, B.; Trisovic, T.L.; Jugovic, Z.S. Electrochemical synthesis and corrosion behavior of polyaniline-benzoate coating on copper. Synth. Met. 2011, 161, 1313–1318. [Google Scholar] [CrossRef]
- Shinde, V.; Sainkar, S.R.; Patil, P.P. Corrosion protective poly(o–toluidine) coatings on copper. Corros. Sci. 2005, 47, 1352–1369. [Google Scholar] [CrossRef]
- Cascalheira, A.C.; Aeiyach, S.; Lacaze, P.C.; Abrantes, L.M. Electrochemical synthesis and redox behaviour of polypyrrole coatings on copper in salicylate aqueous solution. Electrochim. Acta 2003, 48, 2523–2529. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.P. Electrodeposition of Poly(o–toluidine) on brass from aqueous salicylate solution and its corrosion protection performance. J. Appl. Polym. Sci. 2010, 118, 2084–2091. [Google Scholar] [CrossRef]
- Sharifirad, M.; Omrani, A.; Rostami, A.A.; Khoshroo, M. Electrodeposition and characterisation of polypyrrole films on copper. J. Electroanal. Chem. 2010, 645, 149–158. [Google Scholar] [CrossRef]
- Annabaldi, V.; Rooney, A.D.; Breslin, C.B. Corrosion protection of copper using polypyrrole electrosynthesised from a salicylate solution. Corros. Sci. 2012, 59, 179–185. [Google Scholar] [CrossRef]
- Redondo, M.I.; Breslin, C.B. Polypyrrole electrodeposited on copper from an aqueous phosphate solution: Corrosion protection properties. Corros. Sci. 2007, 49, 1765–1776. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Xu, B.; Guo, Y.; Chen, Y.; Yin, X.; Liu, Y. Corrosion behaviors and physical properties of polypyrrole-molybdate coating electropolymerized on carbon steel. Prog. Org. Coat. 2018, 122, 159–169. [Google Scholar] [CrossRef]
- El Jaouhari, A.; Ben Jadi, S.; Aouzal, Z.; Bouabdallaoui, M.; Bazzaoui, E.A.; Wang, R.; Bazzaoui, M. Comparison study between corrosion protection of polypyrrole synthesized on stainless steel from phthalate and saccharinate aqueous medium. Polym. Test. 2018, 67, 302–308. [Google Scholar] [CrossRef]
- Lei, Y.; Sheng, N.; Hyono, A.; Ueda, M.; Ohtsuka, T. Electrochemical synthesis of polypyrrole films on copper from phytic solution for corrosion protection. Corros. Sci. 2013, 76, 302–309. [Google Scholar] [CrossRef]
- Lei, Y.; Ohtsuka, T.; Sheng, N. Corrosion protection of copper by polypyrrole film studied by electrochemical impedance spectroscopy and the electrochemical quartz microbalance. Appl. Surf. Sci. 2015, 357, 1122–1132. [Google Scholar] [CrossRef]
- Fenelon, A.M.; Breslin, C.B. The formation of polypyrrole at iron from 1-Butyl-3-methylimidazolium hexafluorophosphate. J. Electrochem. Soc. 2005, 152, D6–D11. [Google Scholar] [CrossRef]
- Han, G.; Yuan, J.; Shi, G.; Wei, F. Electrodeposition of polypyrrole/multiwalled carbon nanotube composite films. Thin Solid Films 2005, 474, 64–69. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Zhao, J.; Cai, Z.; Ge, F. A free standing polypyrrole hybrid electrode supported by conducting silk fabric coated with PEDOT: PSS and MWCNTs for high-performance supercapacitor. Electrochim. Acta 2019, 317, 42–51. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen–doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Han, C.; Shi, R.; Zhou, D.; Li, H.; Xu, L.; Zhang, T.; Li, J.; Kang, F.; Wang, G.; Li, B. High-energy and high-power nonaqueous lithium-ion capacitors based on polypyrrole/carbon nanotube composites as pseudocapacitive cathodes. ACS Appl. Mater. Interfaces 2019, 11, 15646–15655. [Google Scholar] [CrossRef]
- Ionita, M.; Pruna, A. Polypyrrole/carbon nanotube composites: Molecular modelling and experimental investigation as anti–corrosive coatings. Prog. Org. Coat. 2011, 72, 647–652. [Google Scholar] [CrossRef]
- Ganash, A.A. Electrochemical synthesis and corrosion behaviour of polypyrrole and polypyrrole/carbon nanotube composite films. J. Compos. Mater. 2014, 48, 2215–2225. [Google Scholar] [CrossRef]
- Gergely, A.; Paszti, Z.; Hakkel, O.; Drotar, E.; Mihaly, J.; Kalman, E. Corrosion protection of cold-rolled steel with alkyd paint coatings composited with submicron-structure types polypyrrole-modified nano-zize alumina and carbon nanotubes. Mater. Sci. Eng. B 2012, 177, 1571–1582. [Google Scholar] [CrossRef]
- Carragher, U.; Breslin, C.B. Polypyrrole doped with dodecylbenzene sulfonate as a protective film for copper. Electrochim. Acta 2018, 291, 362–372. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Z.Z.; Luo, S.J.; Fu, S.F.; Wang, L.; Shi, X.S.; Wang, C.S.; Guo, R.B. Effects of different anionic surfactants on methane hydrate formation. Chem. Eng. Sci. 2015, 137, 896–903. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, K. Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: A conductometric study. J. Chem. Thermodyn. 2014, 71, 205–211. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Nagar, R.; Raman, V.; Rajalakshmi, N.; Dhathathreyan, K.S.; Ramaprabhu, S. Synthesis of graphene-multiwalled carbon nanotubes hybrid nanostructure by strengthened electrostatic interaction and its lithium ion battery application. J. Mater. Chem. 2012, 22, 9949–9956. [Google Scholar] [CrossRef]
- Branagan, D.; Breslin, C.B. Electrochemical detection of glucose at physiological pH using gold nanoparticles deposited on carbon nanotubes. Sens. Actuators B 2019, 282, 490–499. [Google Scholar] [CrossRef]
- Saito, T. Sensing of trace copper ion by a solid phase extraction–spectrophotometry using a poly (vinyl chloride) membrane containing bathocuproine. Talanta 1994, 41, 811–815. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre–tafel region of polarization curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Bay, L.; Mogensen, N.; Skaarup, S.; Summer-Larsen, P.; Jorgensen, M.; West, K. Polypyrrole doped with alky benzenesulfonates. Macromolecules 2002, 35, 9345–9351. [Google Scholar] [CrossRef]
- Prissanaroon, W.; Brack, N.; Pigram, P.J.; Liesegang, J.; Cardwell, T.J. Surface and electrochemical study of DBSA-doped polypyrrole films grown on stainless steel. Surf. Interface Anal. 2002, 33, 653–662. [Google Scholar] [CrossRef]
- Rohwerder, M.; Michalik, A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim. Acta 2007, 53, 1300–1313. [Google Scholar] [CrossRef]
- Ryan, E.; Breslin, C.B. Formation of polypyrrole with dexamethasone as a dopant: Its cation and anion exchange properties. J. Electroanal. Chem. 2018, 824, 188–194. [Google Scholar] [CrossRef]
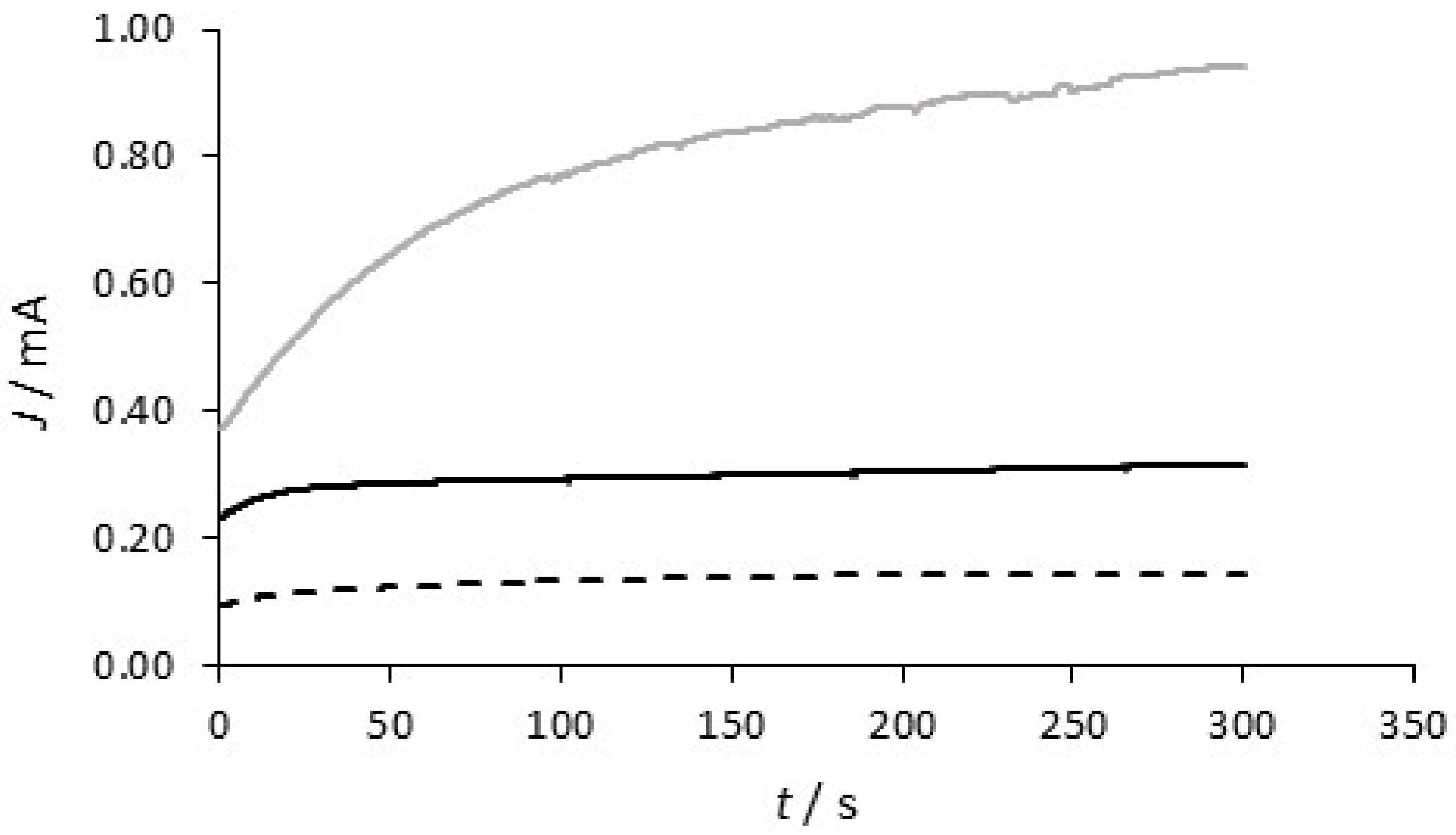
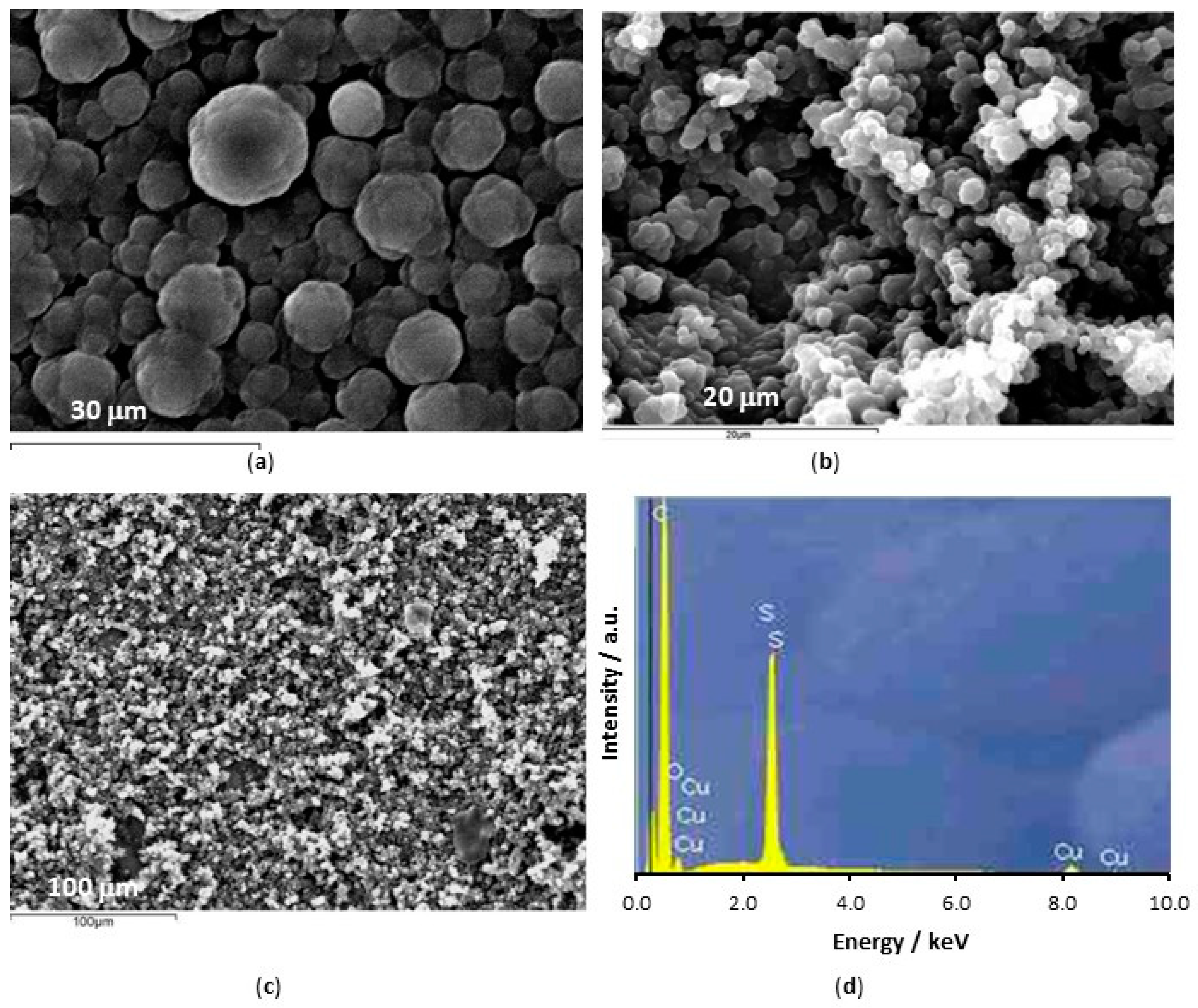
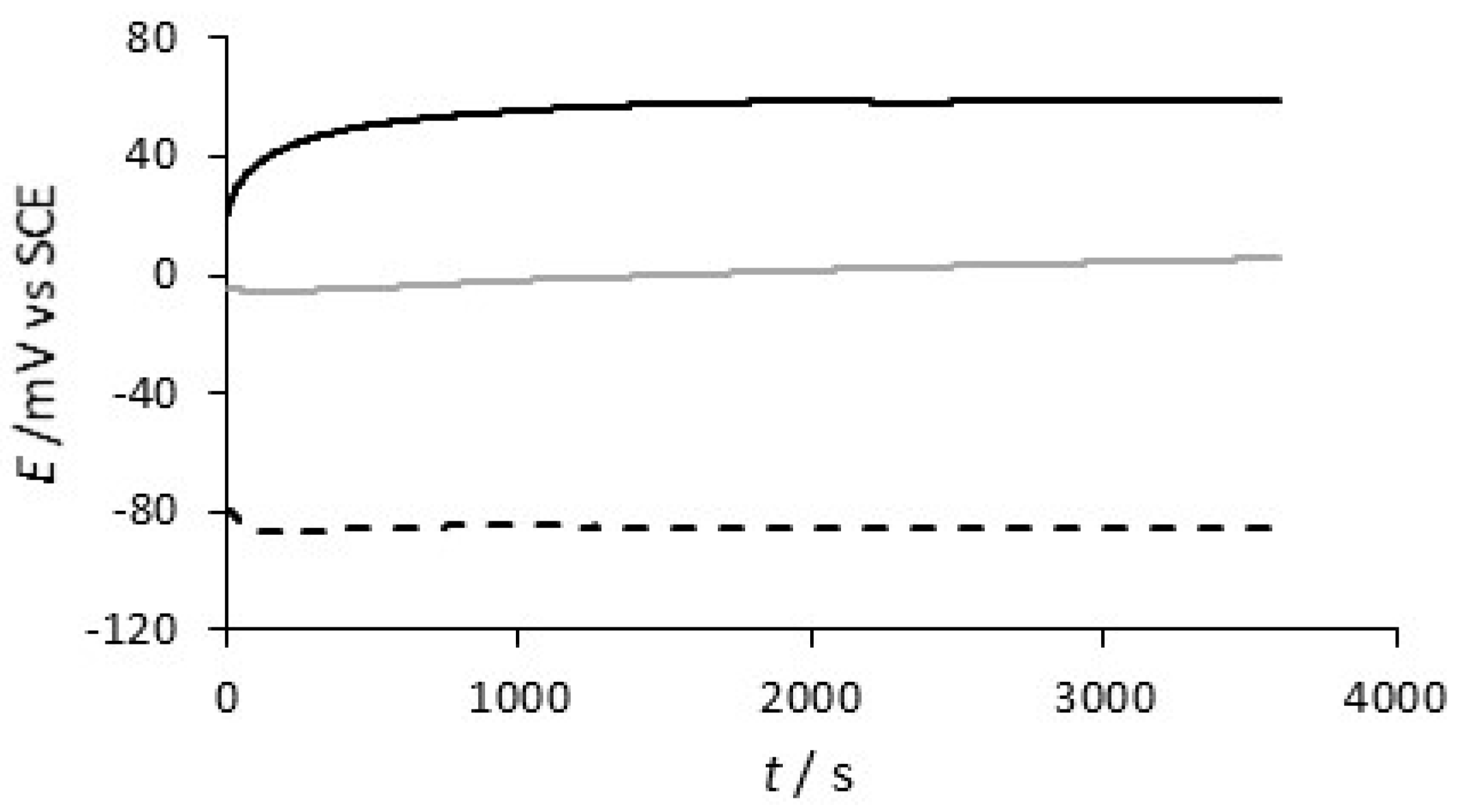
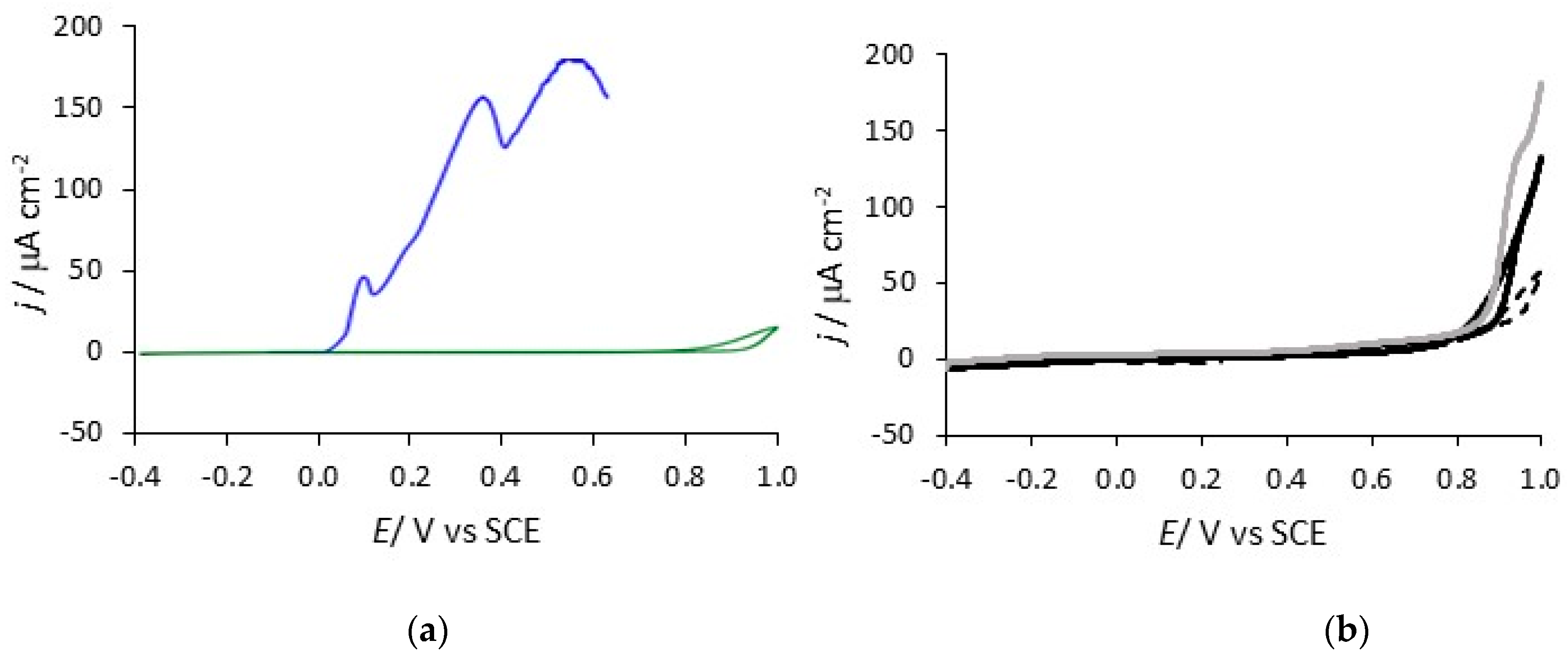

| System | Concentration/mM |
|---|---|
| Uncoated Cu | 4.95 ± 0.15 |
| PPy-Tar PPy-DBS (0.12 C) PPy-DBS (0.18 C) | 1.93 ± 0.17 0.62 ± 0.10 0.59 ± 0.10 |
| PPy-DBSCNT | 0.50 ± 0.10 |
| System | bc/mV Decade−1 | Ecorr V vs. SCE | Icorr µA cm−2 |
|---|---|---|---|
| Uncoated Cu | 95 | −0.235 ± 0.015 | 1.95 ± 0.03 |
| PPy-Tar | 52 | −0.080 ± 0.030 | 1.70 ± 0.04 |
| PPy-DBS (0.12 C) | 53 | −0.065 ± 0.017 | 0.12 ± 0.06 |
| PPy-DBS (0.18 C) | 55 | −0.060 ± 0.018 | 0.12 ± 0.05 |
| PPy-DBSCNT | 70 | −0.070 ± 0.016 | 0.05 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carragher, U.; Branagan, D.; Breslin, C.B. The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper. Materials 2019, 12, 2587. https://doi.org/10.3390/ma12162587
Carragher U, Branagan D, Breslin CB. The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper. Materials. 2019; 12(16):2587. https://doi.org/10.3390/ma12162587
Chicago/Turabian StyleCarragher, Ursula, David Branagan, and Carmel B. Breslin. 2019. "The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper" Materials 12, no. 16: 2587. https://doi.org/10.3390/ma12162587
APA StyleCarragher, U., Branagan, D., & Breslin, C. B. (2019). The Influence of Carbon Nanotubes on the Protective Properties of Polypyrrole Formed at Copper. Materials, 12(16), 2587. https://doi.org/10.3390/ma12162587






