Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites
Abstract
:1. Introduction
2. Materials and Methods
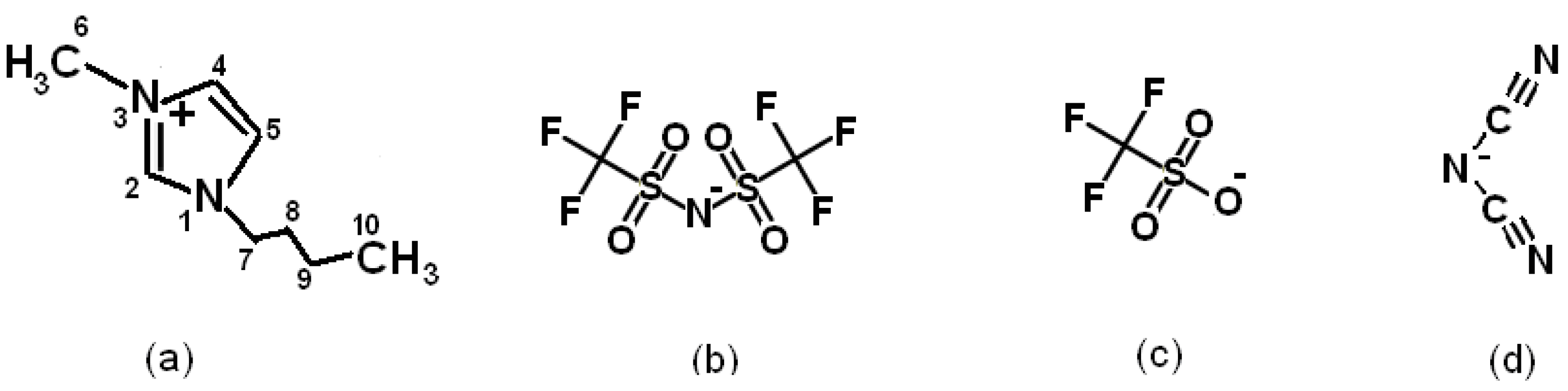
2.1. Materials
2.2. Methods and Apparatus
2.2.1. Dynamic Light scattering (DLS)
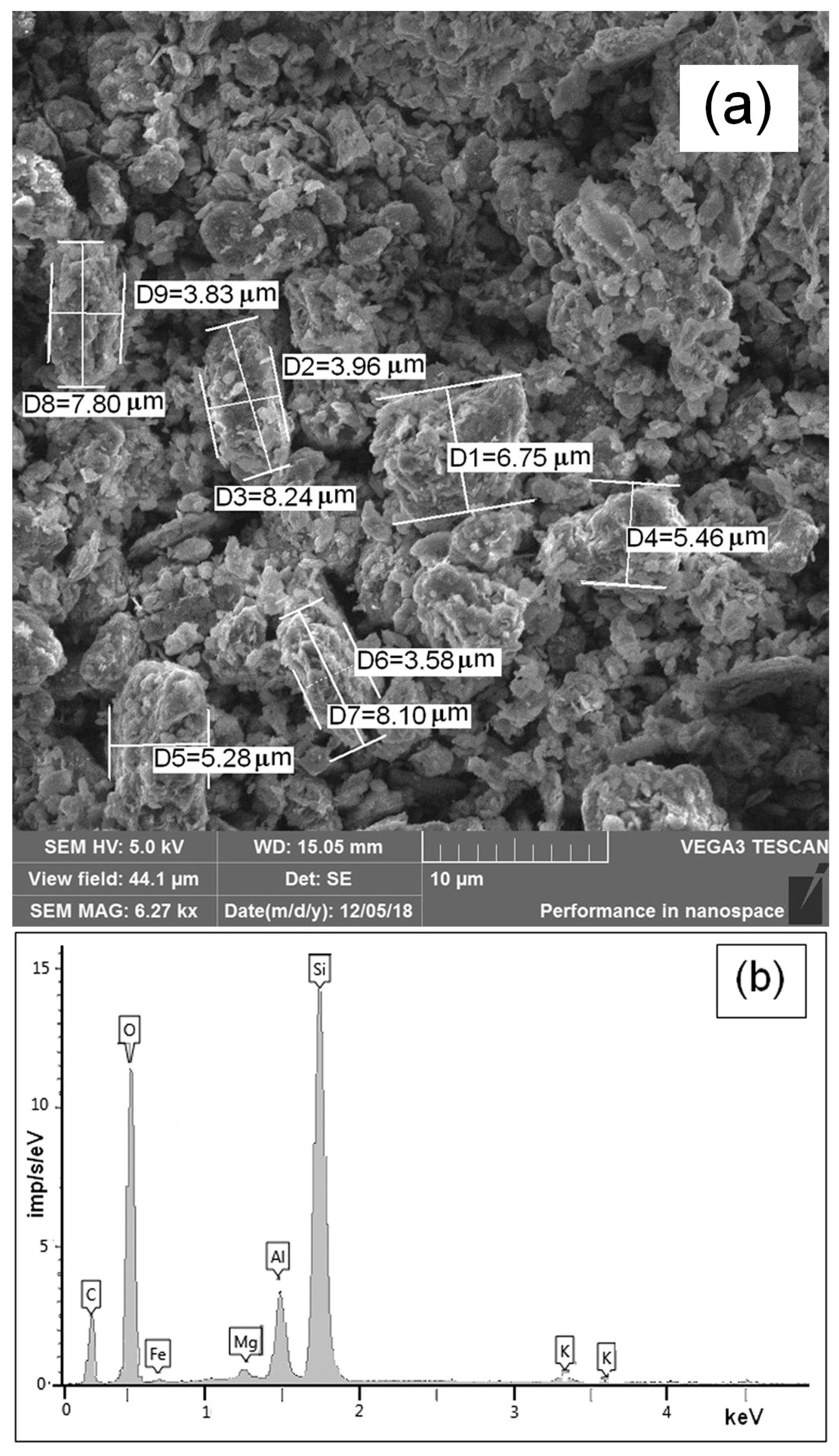
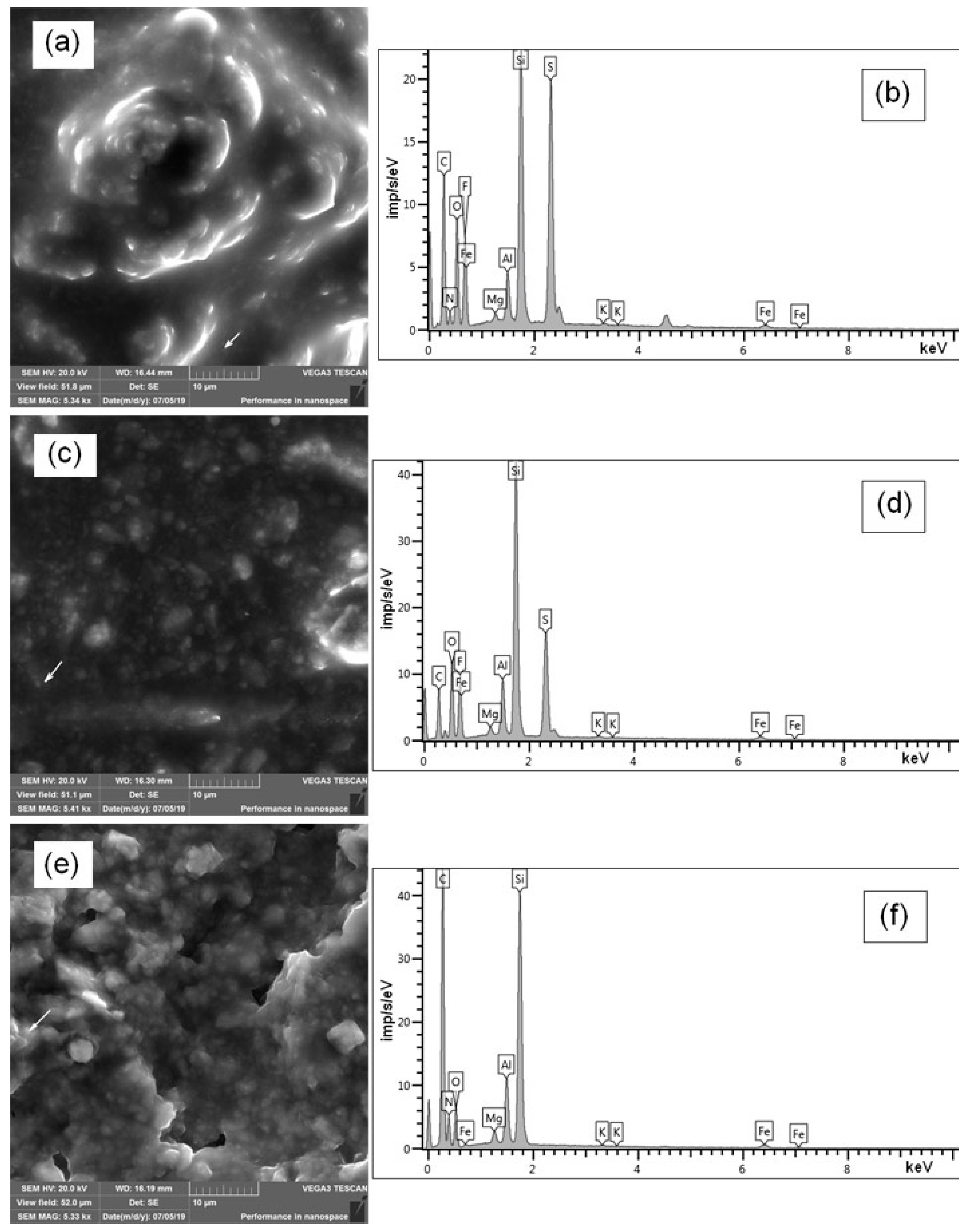
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Nitrogen Vapor Adsorption–Desorption
2.2.4. X-Ray Diffraction (XRD)
2.2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.6. Thermogravimetric Measurements
2.2.7. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Characterization of Montmorillonite K10 Powder
3.2. Structure and Properties of the Montmorillonite K10/IL Composite Materials
3.2.1. Surface Morphology and Composition
3.2.2. Crystal Structure of the Montmorillonite K10/IL Composites
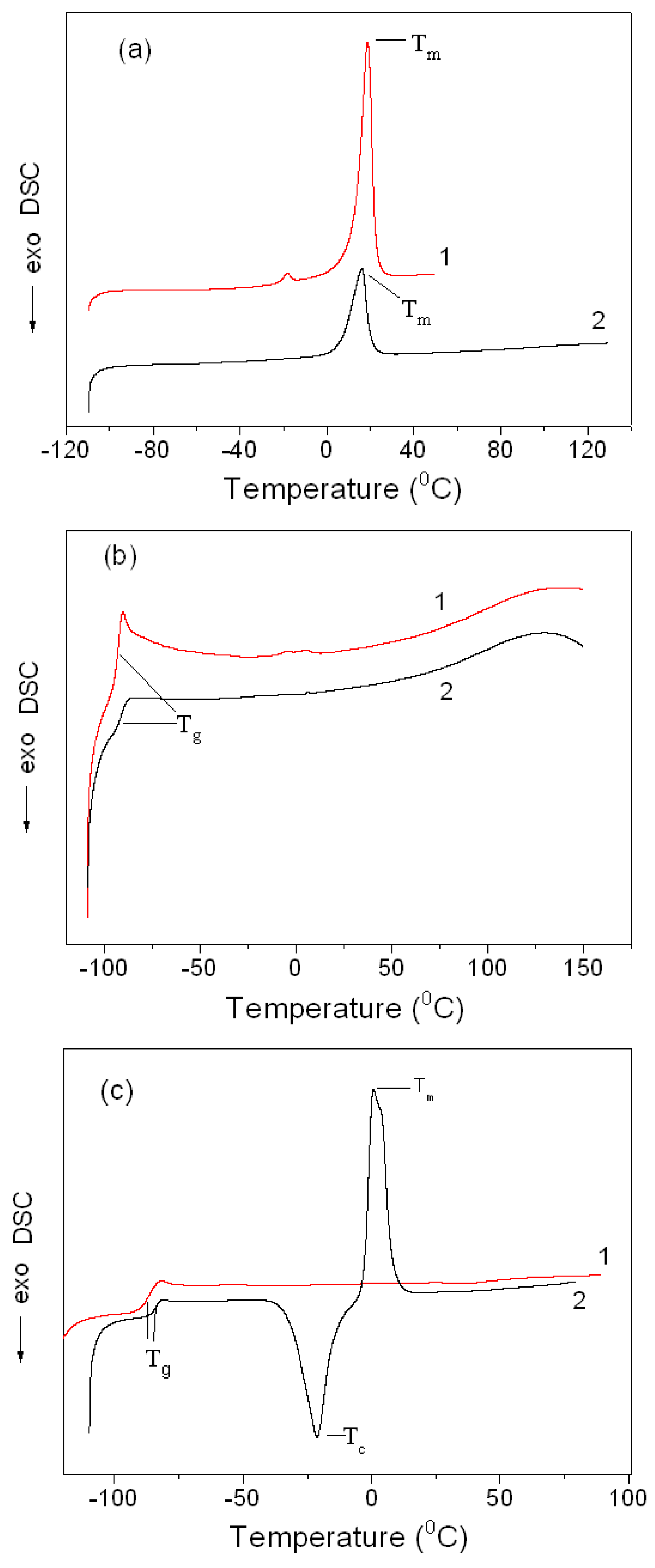
3.2.3. Phase Transitions in the Montmorillonite K10/IL Composites
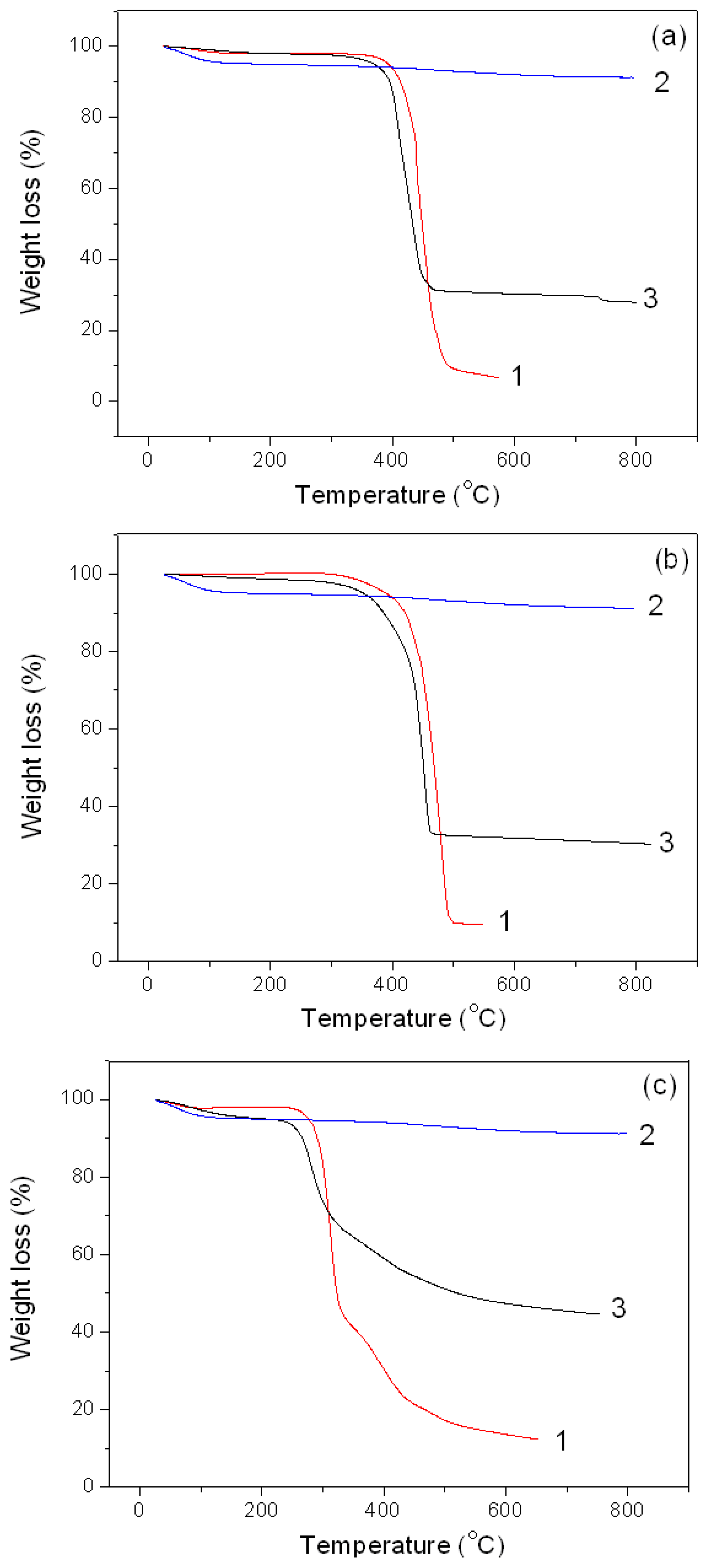
3.2.4. Thermal Degradation of the Montmorillonite K10/IL Composites
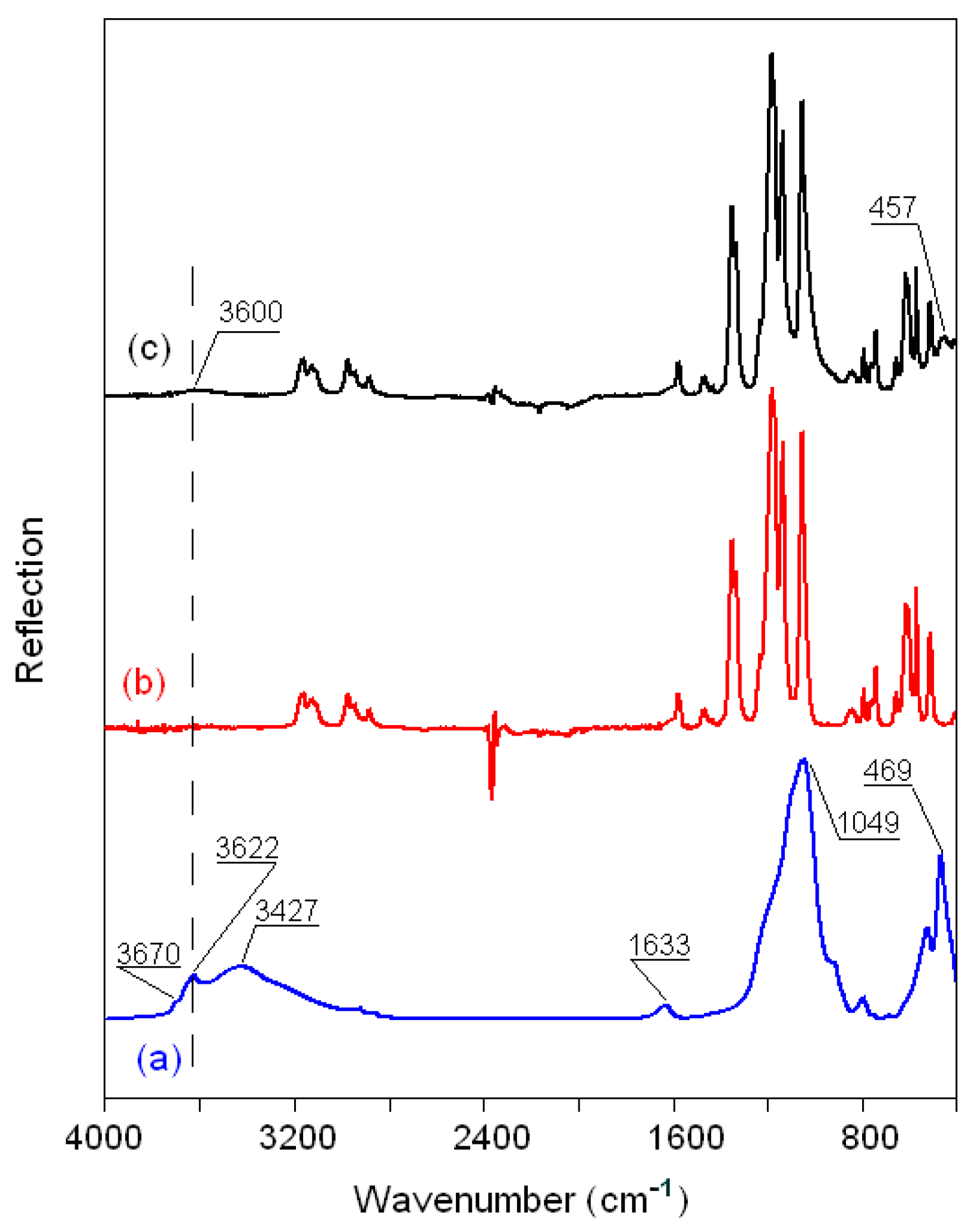
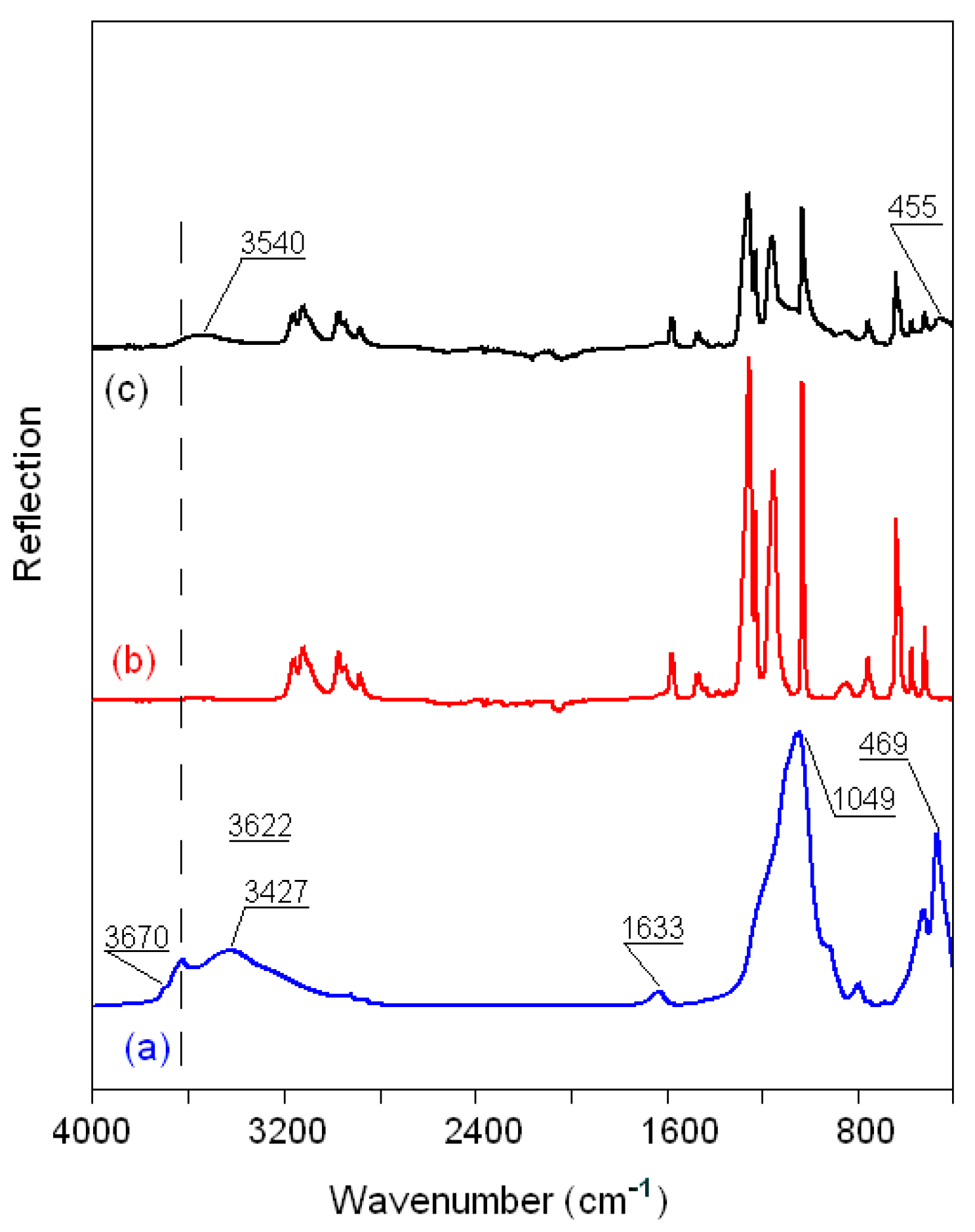
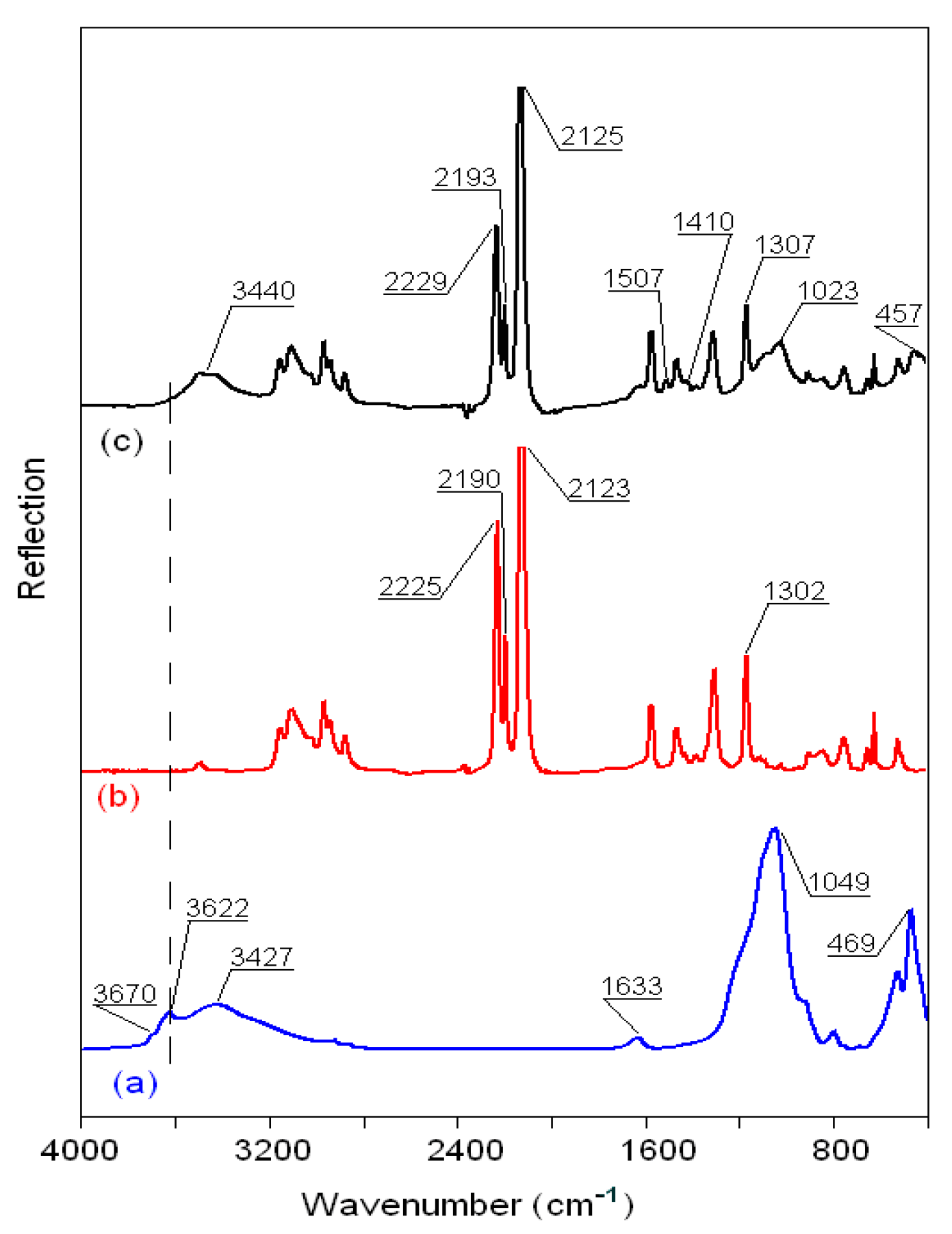
3.2.5. FTIR Spectra of the Montmorillonite K10/IL Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Fuji, M. Study on intercalation of ionic liquid into montmorillonite and its property evaluation. Mater. Chem. Phys. 2012, 135, 681–686. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Rodionova, A.N.; Bagrovskaya, N.A.; Agafonov, A.V. Synthesis, structure, and properties of a bentonite–magnetite composite. Prot. Met. Phys. Chem. Surf. 2016, 52, 819–824. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Wang, C. Investigation of electrochemical performance of montmorillonite clay as Li-ion battery electrode. Sustain. Mater. Technol. 2019, 19, e00086. [Google Scholar] [CrossRef]
- Butman, M.F.; Ovchinnikov, N.L.; Karasev, N.S.; Kochkina, N.E.; Agafonov, A.V.; Vinogradov, A.V. Photocatalytic and adsorption properties of TiO2-pillared montmorillonite obtained by hydrothermally activated intercalation of titanium polyhydroxocomplexes. Beilstein J. Nanotechnol. 2018, 9, 364–378. [Google Scholar] [CrossRef]
- Bouazizi, N.; Barrimo, D.; Nousir, S.; Ben Slama, R.; Shiao, T.C.; Roy, R.; Azzouz, A. Metal-loaded polyol-montmorillonite with improved affinity towards hydrogen. J. Energy Inst. 2018, 91, 110–119. [Google Scholar] [CrossRef]
- Bouazizi, N.; Barrimo, D.; Nousir, S.; Ben Slama, R.; Roy, R.; Azzouz, A. Montmorillonite-supported Pd0, Fe0, Cu0 and Ag0 nanoparticles: Properties and affinity towards CO2. Appl. Surf. Sci. 2017, 402, 314–322. [Google Scholar] [CrossRef]
- Fontana, J.P.; Camilo, F.F.; Bizeto, M.A.; Faez, R. Evaluation of the role of an ionic liquid as organophilization agent into montmorillonite for NBR rubber nanocomposite production. Appl. Clay Sci. 2013, 83–84, 203–209. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Detellier, C. Clay minerals—ionic liquids, nanoarchitectures, and applications. Adv. Funct. Mater. 2017, 28, 1703845. [Google Scholar] [CrossRef]
- Montano, D.F.; Casanova, H.; Cardona, W.I.; Giraldo, L.F. Functionalization of montmorillonite with ionic liquids based on 1-alkyl-3-methylimidazolium: Effect of anion and length chain. Mater. Chem. Phys. 2017, 198, 386–392. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, P.; Sun, C.; Yuanjiang Pan, Y. Application of ionic liquids in separation and analysis of carbohydrates: State of the art and future trends. TrAC—Trend. Anal. Chem. 2019, 111, 148–162. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Wishart, J.F. Energy applications of ionic liquids. Energy Environ. Sci. 2009, 2, 956–961. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Towards understanding the structure and capacitance of electrical double layer in ionic liquids. Electrochim. Acta 2008, 53, 6835–6840. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquid near a charged wall: Structure and capacitance of electrical double layer. J. Phys. Chem. B 2008, 112, 11868–11872. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Detellier, C. Characterization and applications of kaolinite robustly grafted by an ionic liquid with naphthyl functionality. Materials 2017, 10, 1006. [Google Scholar] [CrossRef]
- Selvam, T.; MacHoke, A.; Schwieger, W. Supported ionic liquids on non-porous and porous inorganic materials—A topical review. Appl. Catal. A-Gen. 2012, 445–446, 92–101. [Google Scholar] [CrossRef]
- Fiscal-Ladino, J.A.; Obando-Ceballos, M.; Rosero-Moreano, M.; Montano, D.F.; Cardona, W.; Giraldo, L.F.; Ritcher, P. Ionic liquids intercalated in montmorillonite as the sorptive phase for the extraction of low-polarity organic compounds from water by rotating-disk sorptive extraction. Anal. Chim. Acta 2017, 953, 23–31. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Hayashi, Y.; Fuji, M. Study of intercalation compounds using ionic liquids into montmorillonite and their thermal stability. Solid State Ion. 2013, 241, 53–61. [Google Scholar] [CrossRef]
- Jha, A.; Garade, A.C.; Shirai, M.; Rode, C.V. Metal cation-exchanged montmorillonite clay as catalysts for hydroxyalkylation reaction. Appl. Clay Sci. 2013, 74, 141–146. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, N.V.K.; Brennecke, J.F. Termophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic and molar volumes of room temperature ionic liquids. J. Mol. Liq. 2015, 209, 289–293. [Google Scholar] [CrossRef]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen bonding in ionic liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef]
- Deng, M.J.; Chen, P.Y.; Leong, T.-I.; Sun, I.-W.; Chang, J.K.; Tsai, W.T. Dicyanamide anion based ionic liquids for electrodeposition of metals. Electrochem. Commun. 2008, 10, 213–216. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C. Energy applications of ionic liquids. Energ. Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, T.-C.; Lin, J.-Y.; Chang, J.-K.; Tseng, C.-M. Corrosion properties of metals in dicyanamide-based ionic liquids. Corros. Sci. 2014, 78, 81–88. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Electrochemical energy storage in montmorillonite K10 clay based composite as supercapacitor using ionic liquid electrolyte. J. Colloid Interf. Sci. 2016, 464, 73–82. [Google Scholar] [CrossRef]
- He, H.; Guo, J.; Xie, X.; Lin, H.; Li, L. A microstructural study of acid-activated montmorillonite from Choushan, China. Clay Miner. 2002, 37, 337–344. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.G.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical Properties and Structures of Room-Temperature Ionic Liquids. 3. Variation of Cationic Structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K.M. Amine functionalized K10 montmorillonite: A solid acid–base catalyst for the Knoevenagel condensation reaction. Dalton Trans. 2013, 42, 5122–5129. [Google Scholar] [CrossRef]
- Tabernero, V.; Camejo, C.; Terreros, P.; Alba, M.D.; Cuenca, T. Silicoaluminates as “support activator” systems in olefin polymerization processes. Materials 2010, 3, 1015–1030. [Google Scholar] [CrossRef]
- Ramenskaya, L.M.; Grishina, E.P.; Kudryakova, N.O. Physicochemical features of short-chain 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)-imide ionic liquids containing equilibrium water absorbed from air. J. Mol. Liq. 2018, 272, 759–765. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar] [CrossRef]
- Grishina, E.P.; Ramenskaya, L.M.; Gruzdev, M.S.; Kraeva, O.V. Water effect on physicochemical properties of 1-butyl-3-methylimidazolium based ionic liquids with inorganic anions. J. Mol. Liq. 2013, 177, 267–272. [Google Scholar] [CrossRef]
- Tonle, I.K.; Letaief, V.; Ngamenic, E.; Detellier, C. Nanohybrid materials from the grafting of imidazolium cations on the interlayer surfaces of kaolinite. Application as electrode modifier. J. Mater. Chem. 2009, 19, 5996–6003. [Google Scholar] [CrossRef]
- Ahmed, A.; Chaker, Y.; Belarbi, E.H.; Abbas, O.; Chotard, J.N.; Abassi, H.B.; Van Nhien, A.N.; Hadri, M.E.; Bresson, S. XRD and ATR/FTIR investigations of various montmorillonite clays modified by monocationic and dicationic imidazolium ionic liquids. J. Mol. Struct. 2018, 1173, 653–664. [Google Scholar] [CrossRef]
- Kim, N.H.; Malhotra, S.V.; Xanthos, M. Modification of cationic nanoclays with ionic liquids. Micropor. Mesopor. Mat. 2006, 96, 29–35. [Google Scholar] [CrossRef]
- Vitucci, F.M.; Trequattrini, F.; Palumbo, O.; Brubach, J.-B.; Roy, P.; Paolone, A. Infrared spectra of bis(trifluoromethanesulfonyl)imide based ionic liquids: Experiments and DFT simulations. Vib. Spectrosc. 2014, 74, 81–87. [Google Scholar] [CrossRef]
- Heimer, N.E.; Del Sesto, R.E.; Meng, R.E.; Wilkes, J.S.; Carper, W.R. Vibrational spectra of imidazolium tetrafluoroborate ionic liquids. J. Mol. Liq. 2006, 124, 84–95. [Google Scholar] [CrossRef]
- Ferry, A.; Edman, L.; Forsyth, M.; MacFarlane, D.R.; Sun, J. NMR and Raman studies of a novel fast-ion-conducting polymer-in-salt electrolyte based on LiCF3SO3 and PAN. Electrochim. Acta 2000, 45, 1237–1242. [Google Scholar] [CrossRef]
- Cammarata, L.; Kazarin, S.G.; Salter, P.A.; Welton, T. Molecular states of water in room temperature ionic liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef]

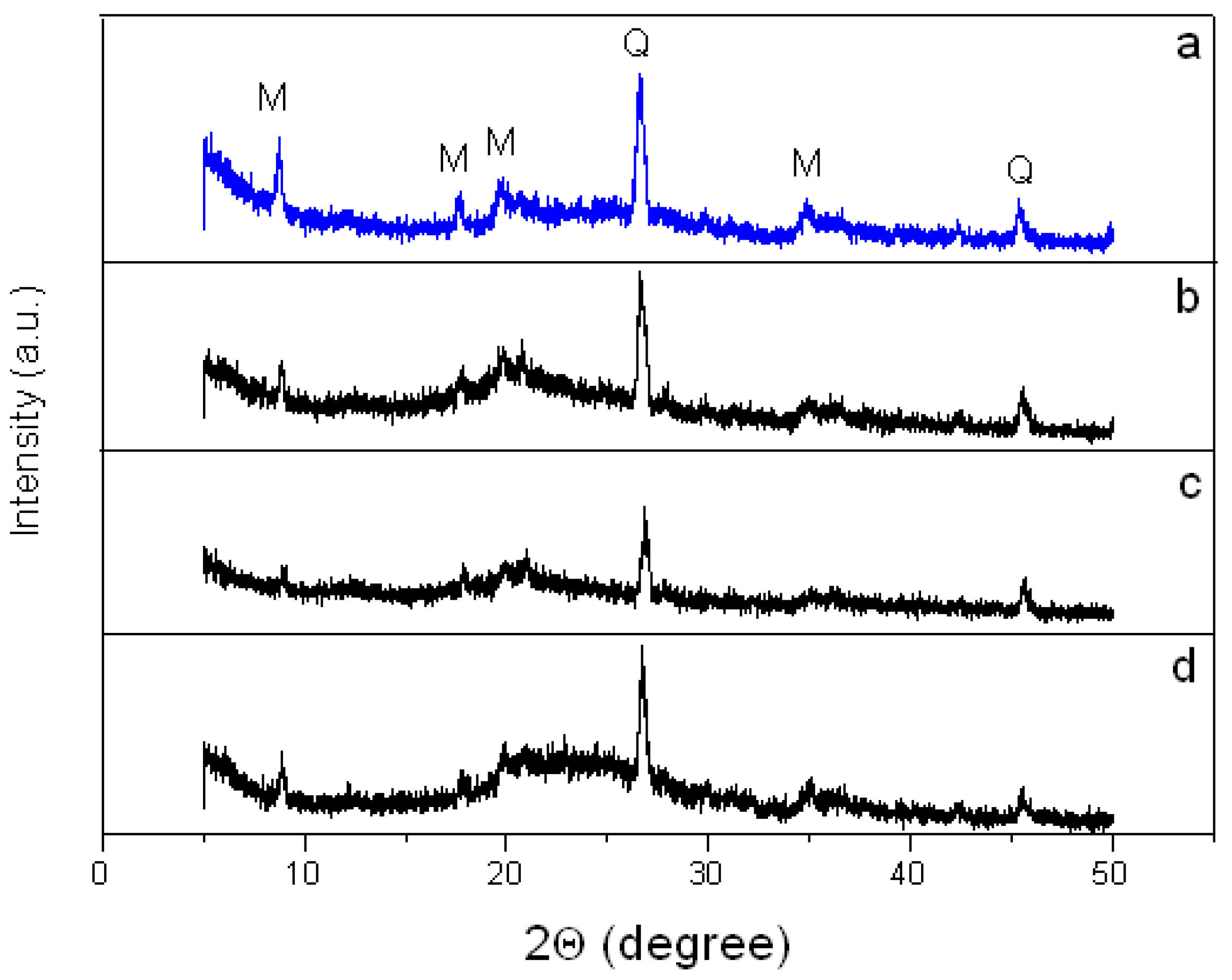
| Reflection Index | MMT K10 | MMT/BMImOTf | MMT/BMImNTf2 | MMT/BMImDCA | ||||
|---|---|---|---|---|---|---|---|---|
| 2θ, deg | d, nm | 2θ, deg | d, nm | 2θ, deg | d, nm | 2θ, deg | d, nm | |
| (001) | 8.74 | 1.011 | 8.88 | 0.995 | 8.91 | 0.992 | 8.87 | 0.996 |
| (002) | 17.67 | 0.502 | 17.84 | 0.497 | 17.87 | 0.496 | 17.76 | 0.499 |
| (110) | 19.65 | 0.451 | 19.64 | 0.452 | 19.85 | 0.447 | 19.95 | 0.445 |
| (101) | 26.54 | 0.336 | 26.60 | 0.335 | 26.84 | 0.332 | 26.76 | 0.333 |
| (220) | 34.85 | 0.257 | 34.94 | 0.257 | 35.0 | 0.256 | 34.96 | 0.256 |
| (112) | 45.38 | 0.200 | 45.55 | 0.199 | 45.68 | 0.198 | 45.53 | 0.199 |
| Sample | Tg, °C | Tc, °C | Tm, °C |
|---|---|---|---|
| BMImOTf | 18.7 | ||
| MMT K10/BMImOTf | 16.2 | ||
| BMIm DCA | −92.7 | ||
| MMT K10/BMImDCA | −91.6 | ||
| BMImNTf2 | −86.3–−86.8 a | −48.6 b–−46.3 a,b | −6.5 b–−6.5 a,b |
| MMT K10/BMImNTf2 | −83.5 | −19.3 | 0.7 |
| Parameter | BMImOTf | MMT K10/BMImOTf | BMImNTf | MMT K10/BMImNTf2 | BMImDCA | MMT K10/BMImDCA |
|---|---|---|---|---|---|---|
| First stage | ||||||
| T1, °C | 54.3 | 61.9 | - | 53.3 | 40.1 | 52.2 |
| T2, °C | 110.1 | 164.2 | - | 119.0 | 80.6 | 130.2 |
| Δm1, % | 1.9 | 2.1 | - | 1.3 | 1,9 | 4.9 |
| Second stage | ||||||
| T1, °C | 427.7 | 392.8 | 441.1 | 425.2 | 293.7 | 260.7 |
| Td, °C | 440.9 | 408.9 | 481.5 | 453.8 | 313.5 | 280.4 |
| T2, °C | 462.8 | 449.5 | 500.1 | 460.4 | 324.1 | 303.7 |
| Δm2, % | 91.6 | 67.7 | 90.4 | 68.6 | 57.4 | 30.9 |
| Third stage | ||||||
| T1, °C | - | - | - | - | 372.1 | 374.9 |
| Td, °C | - | - | - | - | 393.0 | 385.8 |
| T2, °C | - | - | - | - | 428.4 | 482.0 |
| Δm3, % | - | - | - | - | 28.4 | 20.1 |
| Wavenumber (cm−1) | Assignment [40,41,42,43,44,45] |
|---|---|
| Clay 3630–3700 | νSi–OH |
| 3300–3500 | νOH (H2O) |
| 1633 | δOH (H2O) |
| 1049 | νSiO (Si–O–Si) |
| 620 | νAlO (Al–O–Al) |
| 529–469 | νMgO (Mg–O–Mg) |
| Ionic liquid | |
| 3000–32000 | νasH(C4,5)H, νsH(C4,5)H, νC2H |
| 2800–3000 | νCH3, νCH2, νNH |
| 2100–2300 | νs(N≡C) |
| 1150–1600 a | νCH2(N), νCH3(N), ring CH3 and νCN, νCCCC, δasip ring and δCCCC, δCH3(N)CN |
| 1000–1200 b | νsSO3, νsCF3 |
| 1100–1200 b | νasCF3 |
| 1000–1050 b | νsSO3 |
| 1300-137 °C | νasSO2 |
| 1110-125 °C | νsCF3, νasCF3, νsSO2 |
| 1000-180 °C | νaSNS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, O.; Noskov, A.; Grishina, E.; Ramenskaya, L.; Kudryakova, N.; Ivanov, V.; Agafonov, A. Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites. Materials 2019, 12, 2578. https://doi.org/10.3390/ma12162578
Alekseeva O, Noskov A, Grishina E, Ramenskaya L, Kudryakova N, Ivanov V, Agafonov A. Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites. Materials. 2019; 12(16):2578. https://doi.org/10.3390/ma12162578
Chicago/Turabian StyleAlekseeva, Olga, Andrew Noskov, Elena Grishina, Lyudmila Ramenskaya, Nadezhda Kudryakova, Vladimir Ivanov, and Alexander Agafonov. 2019. "Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites" Materials 12, no. 16: 2578. https://doi.org/10.3390/ma12162578
APA StyleAlekseeva, O., Noskov, A., Grishina, E., Ramenskaya, L., Kudryakova, N., Ivanov, V., & Agafonov, A. (2019). Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites. Materials, 12(16), 2578. https://doi.org/10.3390/ma12162578






