Preparation of 6N,7N High-Purity Gallium by Crystallization: Process Optimization
Abstract
1. Introduction
2. Materials and Methods
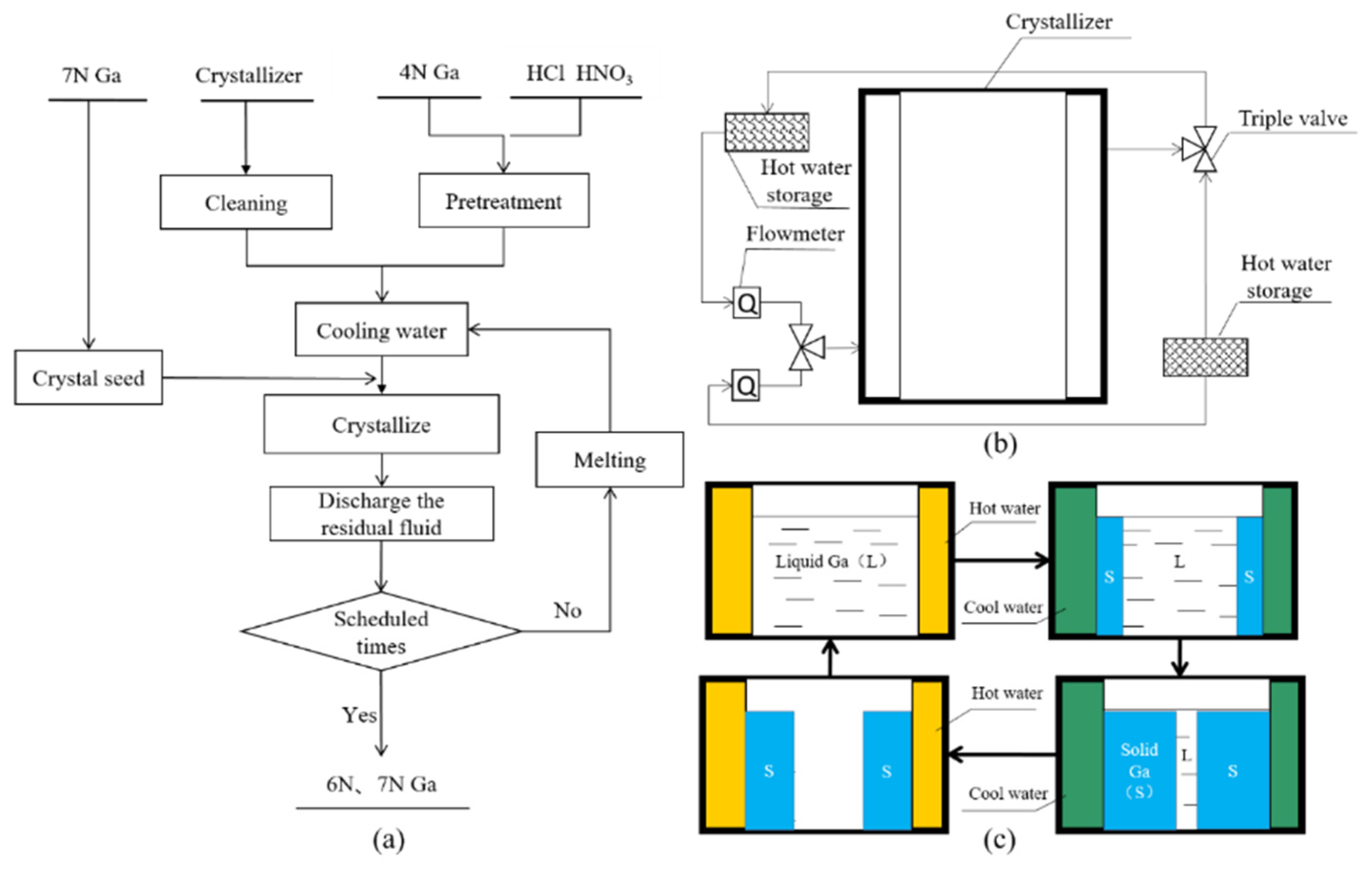
2.1. Process Design
2.2. Detection Method
3. Results and Discussion
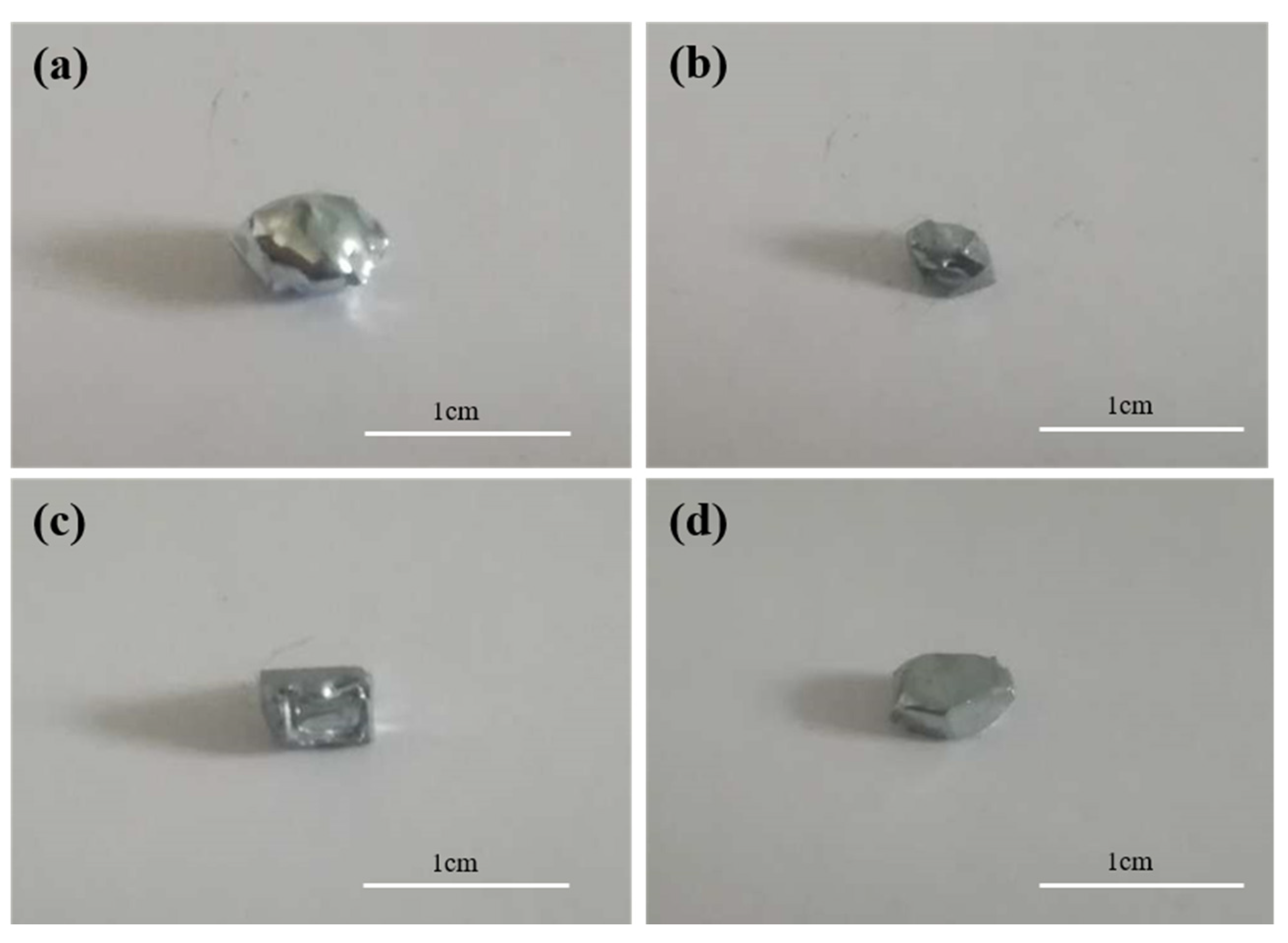
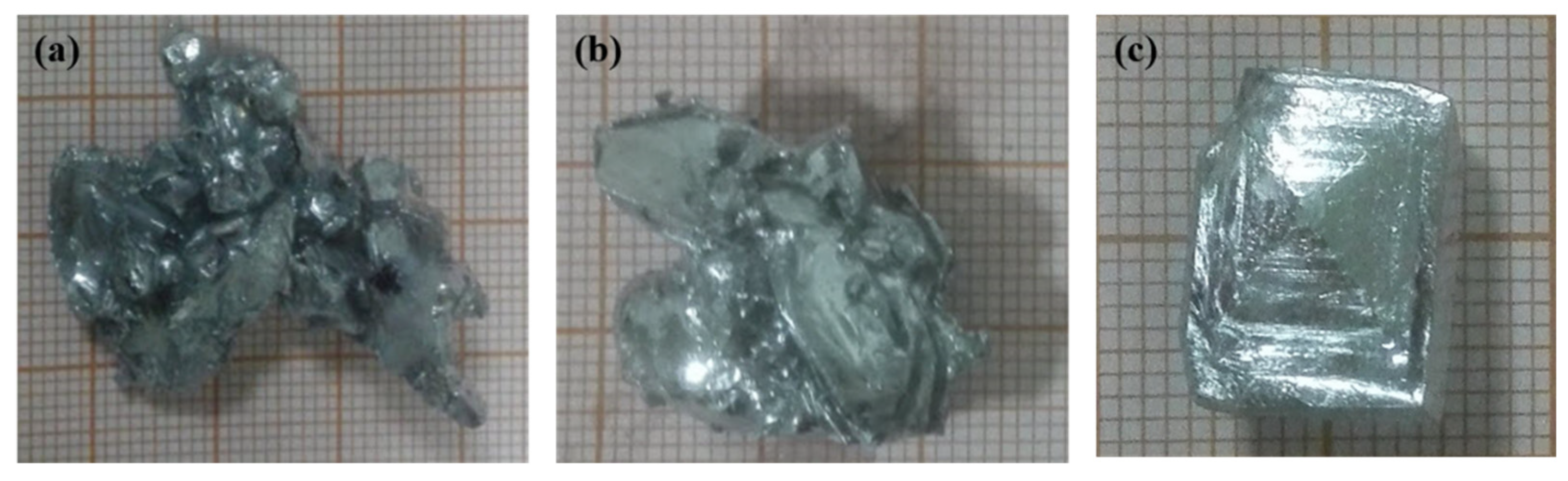
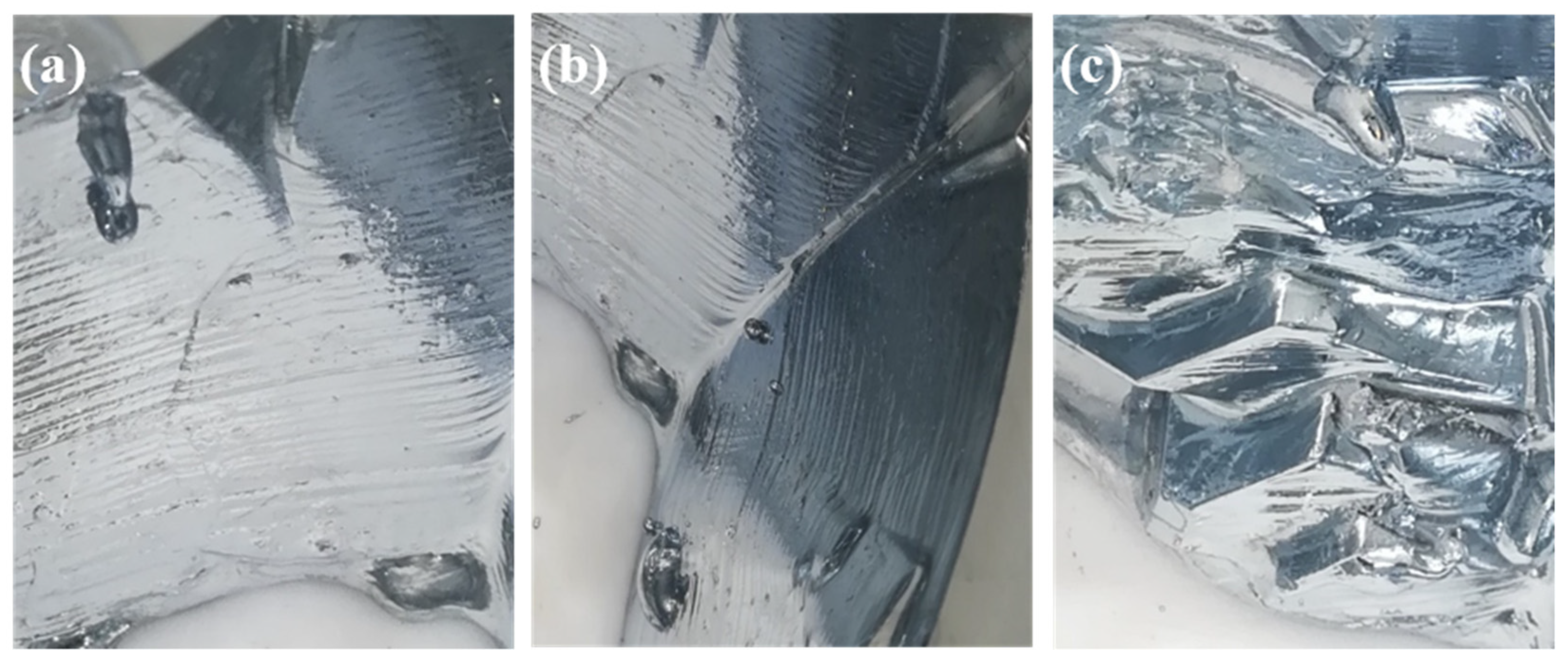
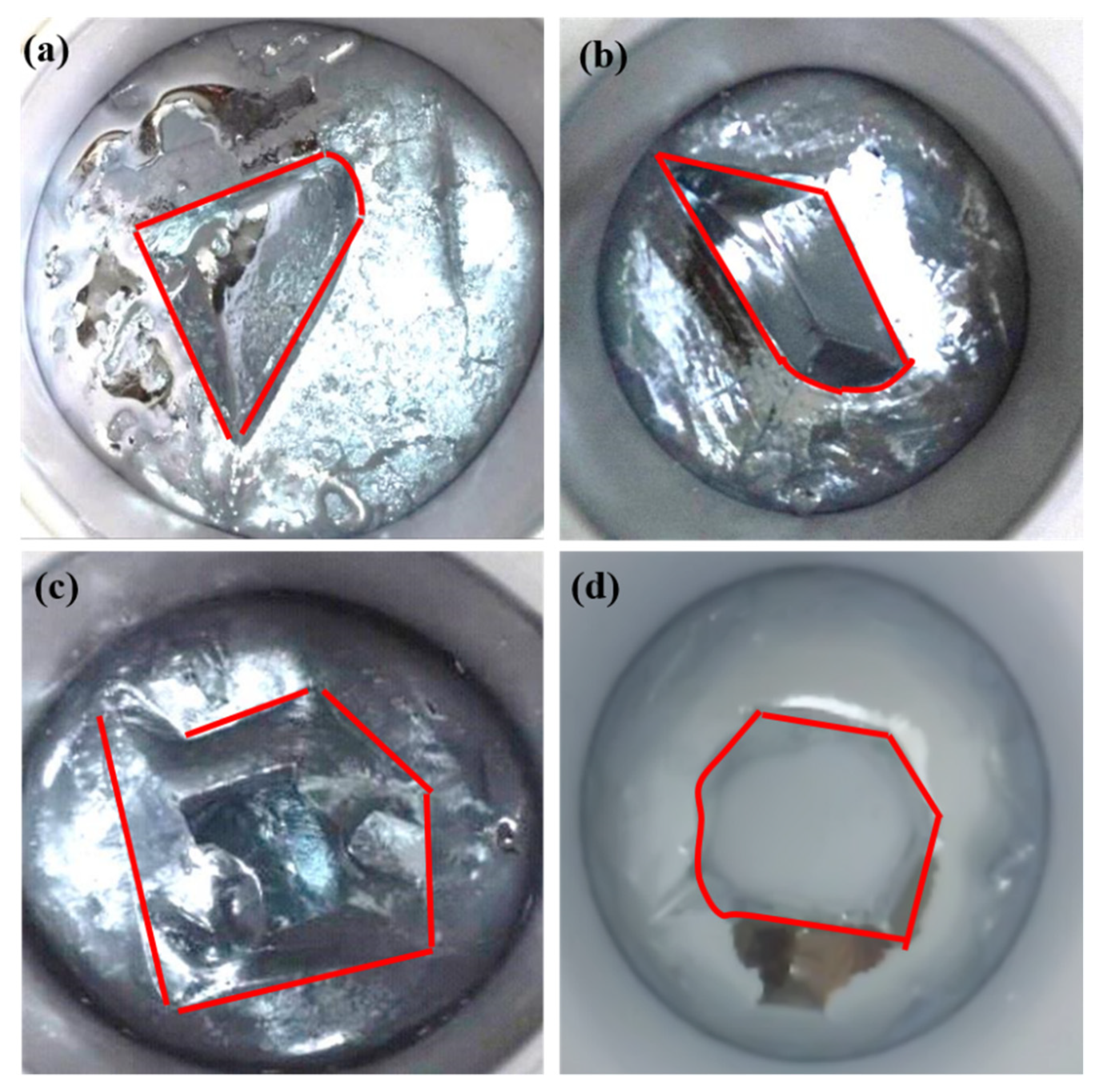
3.1. Effect of Cooling Temperature on Seed Morphology
3.2. Effect of Process Parameters on the Crystallization Process
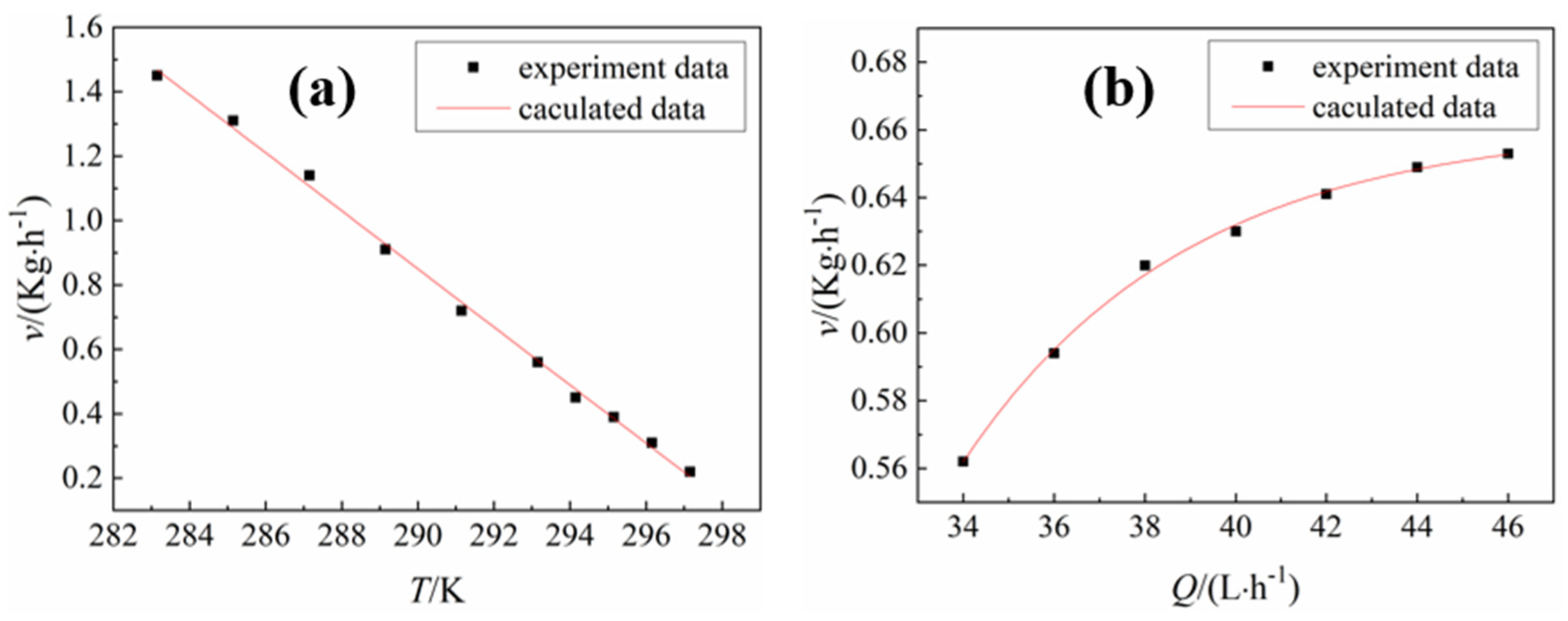
3.2.1. Effect of Temperature of Cooling Water on Crystallization Process
3.2.2. Effect of Cooling Water Flow on Crystallization Process
3.2.3. Effect of Seed Number on Crystallization Process
3.3. Effect of Process Parameters on Crystallization Rate
3.4. Analysis of Purification Results
4. Conclusions
- (1)
- The optimum process parameters for the crystallization purification of 4N raw material gallium are as follows: temperature of the seed preparation, 278 K; cooling water temperature, 293 K; cooling water flow, 40 L·h−1; the number of seed crystals added six 6;
- (2)
- The crystallization rate decreased linearly with increasing cooling water temperature and increased exponentially with increasing cooling water flow. The control formulas of the cooling water temperature T and flow Q on the crystallization rate v are, v(T) = −0.09T + 27 and , respectively;
- (3)
- The three proposed purification schemes effectively removed the impurity elements. When using Scheme I to purify the 4N crude gallium, high-purity gallium with a purity of 6N was obtained. When adopting Schemes II and III, 7N high-purity gallium was obtained. The purities of the high-purity gallium prepared by Schemes I, II, and III were 99.999987%, 99.9999958%, and 99.9999958%, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Chou, W.L.; Wang, C.T.; Yang, K.C.; Huang, Y.H. Removal of gallium (III) ions from acidic aqueous solution by supercritical carbon dioxide extraction in the green separation process. J. Hazard. Mater. 2008, 160, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H.J., Seal, R.R., II, Bradley, D.C., Eds.; US Geological Survey Reston: Reston, VA, USA, 2017.
- Tolcin, A.C. (Ed.) Mineral Commodity Summaries 2019; US Geological Survey Reston: Reston, VA, USA, 2019.
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125–126, 115–124. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Lundström, M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part I: Leaching and iron(III) removal. Hydrometallurgy 2017, 169, 564–570. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; US Geological Survey Reston: Reston, VA, USA, 2018.
- U.S. Geological Survey. Mineral Commodity Summaries 2015; US Geological Survey Reston: Reston, VA, USA, 2015.
- Meyer, S.; Wahl, S.; Molchanov, A.; Neckermann, K.; Möller, C.; Lauer, K.; Hagendorf, C. Influence of the feedstock purity on the solar cell efficiency. Sol. Energy Mater. Sol. Cells 2014, 130, 668–672. [Google Scholar] [CrossRef]
- Kenichi, T.; Nagayasu, Y.; Kishio, T. Electrolytic Refining Method for Gallium and Apparatus for Use in the Method. U.S. Patent 19,990,428,476, 2001. [Google Scholar]
- Kenichi, T.; Nagayasu, Y.; Kishio, T. Method for Electrolytically Refining Gallium and Deveice Therefor. JP10310331, 2000. [Google Scholar]
- Bollong Alan, B.I.; Bult Roelof, P.; Proux Gary, T. Method for the Zone Refining of Gallium. U.S. Patent 23371088A, 1989. [Google Scholar]
- Lee, M.S.; Ahn, J.G.; Oh, Y.J. Production of high-purity indium and gallium metals by vacuum refining. Mater. Trans. 2002, 43, 3195–3198. [Google Scholar] [CrossRef]
- Harper, J.G. Method of Purifying Galliun by Recrystallization. U.S. Patent 3,088,853, 7 May 1963. [Google Scholar]
- Pan, K.; Li, Y.; Zhao, Q.; Zhang, S. Simulation of Solidification Process of Metallic Gallium and Its Application in Preparing 99.99999% Pure Gallium. JOM 2019, 71, 737–743. [Google Scholar] [CrossRef]
- Pan, K.; Li, Y.; Zhang, J.; Zhao, Q. A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium. Materials 2018, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, K.; Li, Z.; Yan, C. Preparation of high-purity gallium by crystallization method. Xiyou Jinshu Chin. J. Rare Met. 2015. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Potolokov, N.A.; Gusev, A.V.; Fedorov, V.A. Determination of the Effective Segregation Coefficients of Metallic Impurities in Gallium during Directional Solidification. Inorg. Mater. 2002, 12, 1212–1215. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Potolokov, N.A.; Gusev, A.V.; Fedorov, V.A. Effect of Impurities on Crystal Growth of Gallium. Inorg. Mater. 2003, 39, 1267–1270. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Potolokov, N.A.; Gusev, A.V.; Fedorov, V.A. Segregation Behavior of Impurities in Gallium during Directional Solidification. Inorg. Mater. 2003, 5, 452–454. [Google Scholar] [CrossRef]

| Scheme Number | Recrystallization Number | Crystal Proportion % | |||
|---|---|---|---|---|---|
| First Time | Second Time | Third Time | Forth Time | ||
| I | 2 | 70 | 70 | / | / |
| II | 3 | 70 | 70 | 85 | / |
| III | 4 | 70 | 70 | 85 | 85 |
| Element | Mass Fraction (×10−6) | Removal Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| Raw Material | I | II | III | I | II | III | |
| Mg | 76 | 1.59 | 0.34 | 0.07 | 97.9 | 99.6 | 99.9 |
| Al | 1 | <0.1 | <0.1 | <0.1 | ≈100 | ≈100 | ≈100 |
| Cr | 40 | 2.66 | 0.94 | 0.34 | 93.3 | 97.6 | 99.2 |
| Fe | 15 | 1.94 | 0.93 | 0.45 | 87.1 | 93.8 | 97.0 |
| Cu | 107 | 1.32 | 0.22 | 0.04 | 98.8 | 99.8 | >99.9 |
| Zn | 24 | 2.42 | 1.05 | 0.45 | 89.9 | 95.6 | 98.2 |
| Pb | 56 | 2.32 | 0.68 | 0.20 | 95.9 | 98.8 | 99.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Pan, K.; Tan, X. Preparation of 6N,7N High-Purity Gallium by Crystallization: Process Optimization. Materials 2019, 12, 2549. https://doi.org/10.3390/ma12162549
Hou J, Pan K, Tan X. Preparation of 6N,7N High-Purity Gallium by Crystallization: Process Optimization. Materials. 2019; 12(16):2549. https://doi.org/10.3390/ma12162549
Chicago/Turabian StyleHou, Jianfeng, Kefeng Pan, and Xihan Tan. 2019. "Preparation of 6N,7N High-Purity Gallium by Crystallization: Process Optimization" Materials 12, no. 16: 2549. https://doi.org/10.3390/ma12162549
APA StyleHou, J., Pan, K., & Tan, X. (2019). Preparation of 6N,7N High-Purity Gallium by Crystallization: Process Optimization. Materials, 12(16), 2549. https://doi.org/10.3390/ma12162549




