Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing
Abstract
1. Introduction
2. Experimental Investigation
2.1. Materials
2.2. Experimental Pogram
2.3. Specimen Preparation and Testing Methods
3. Results and Discussion
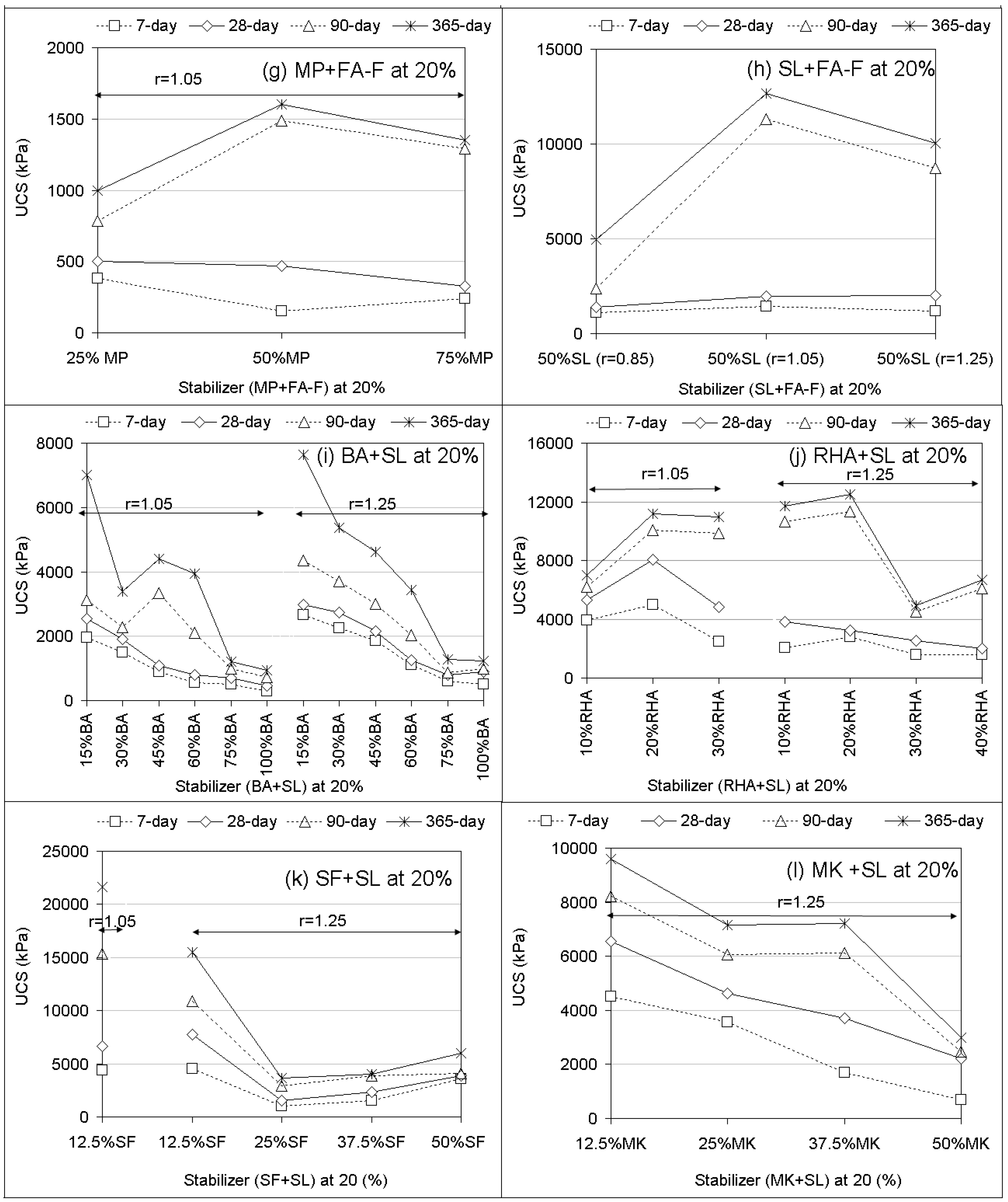
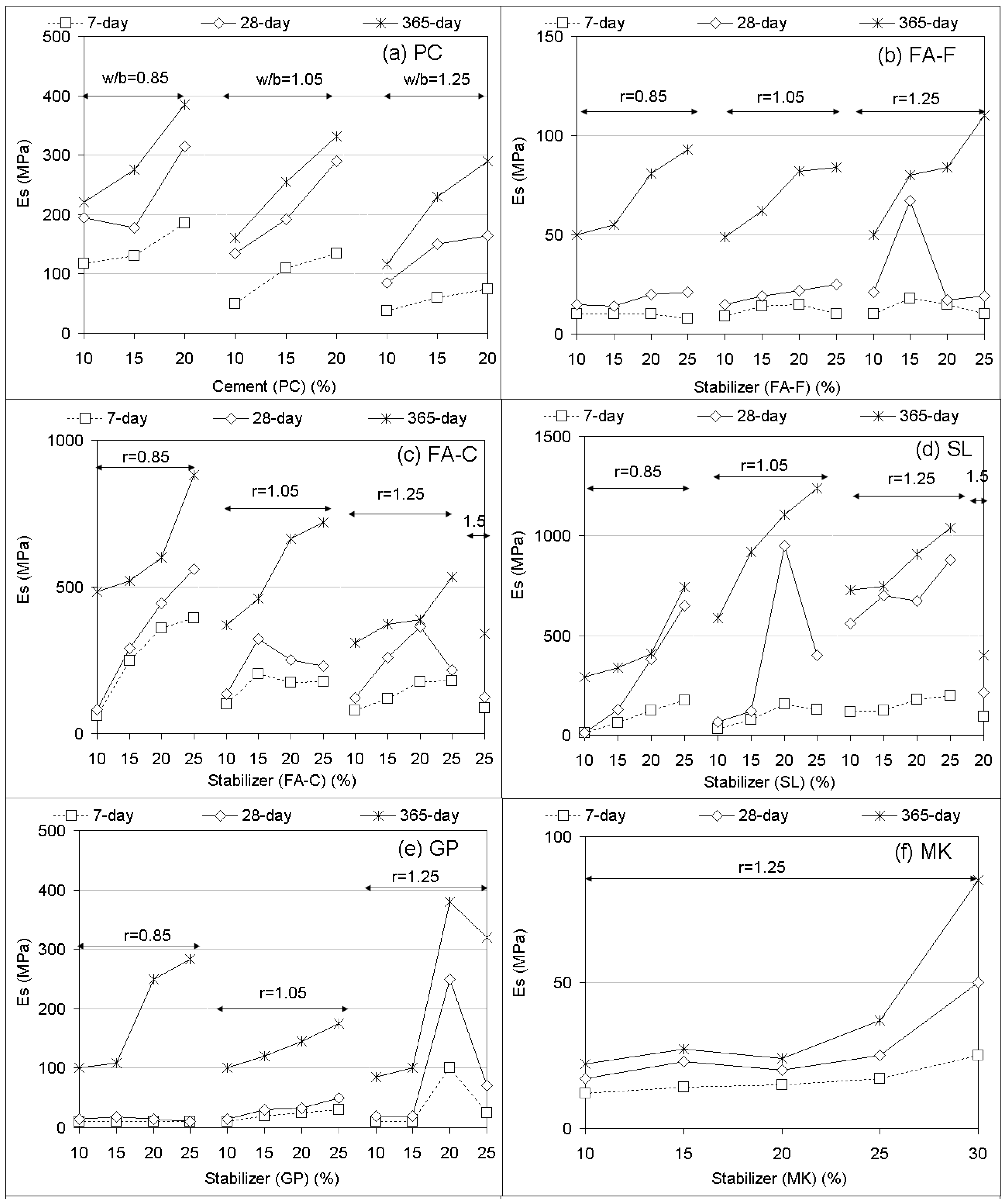
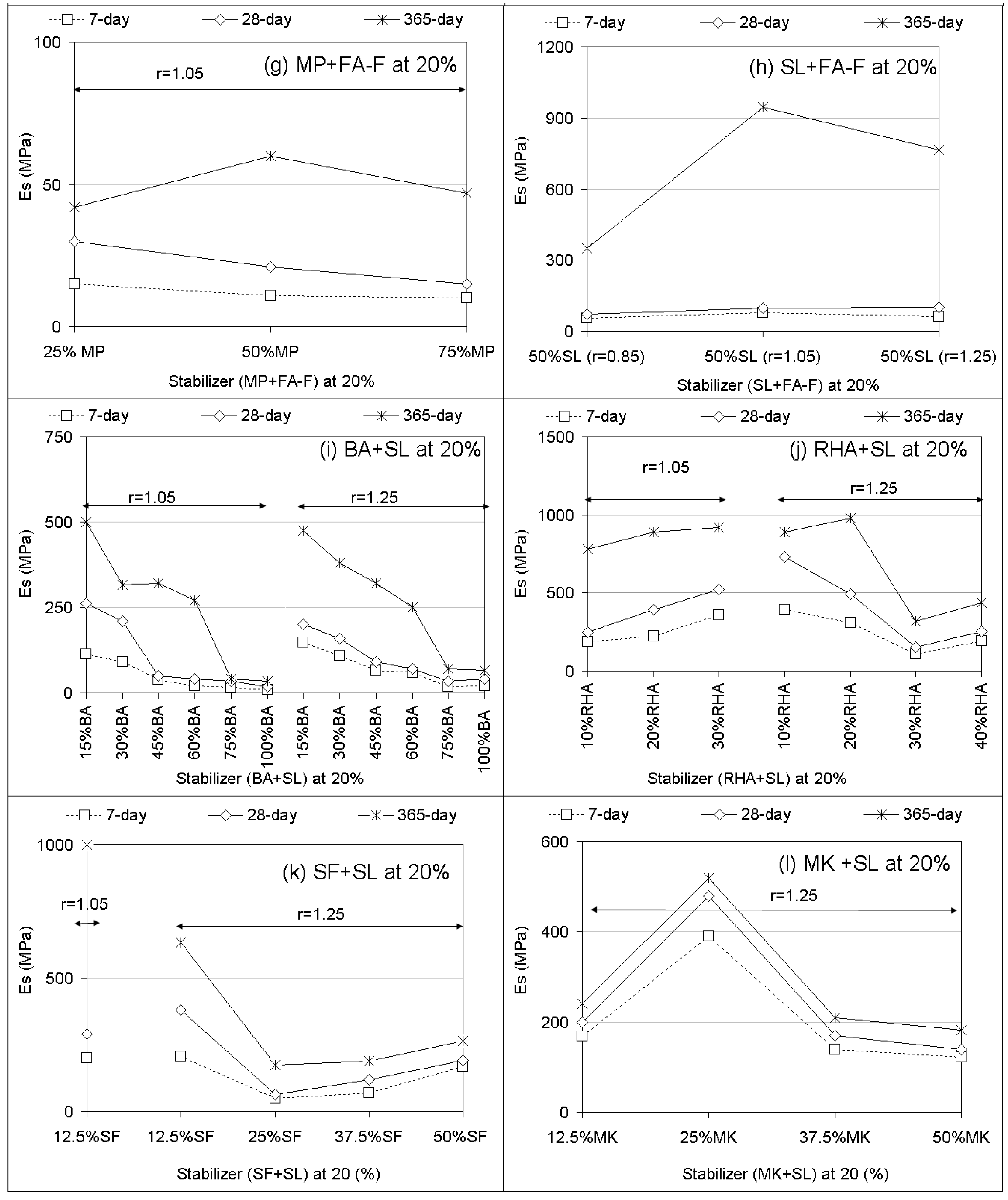
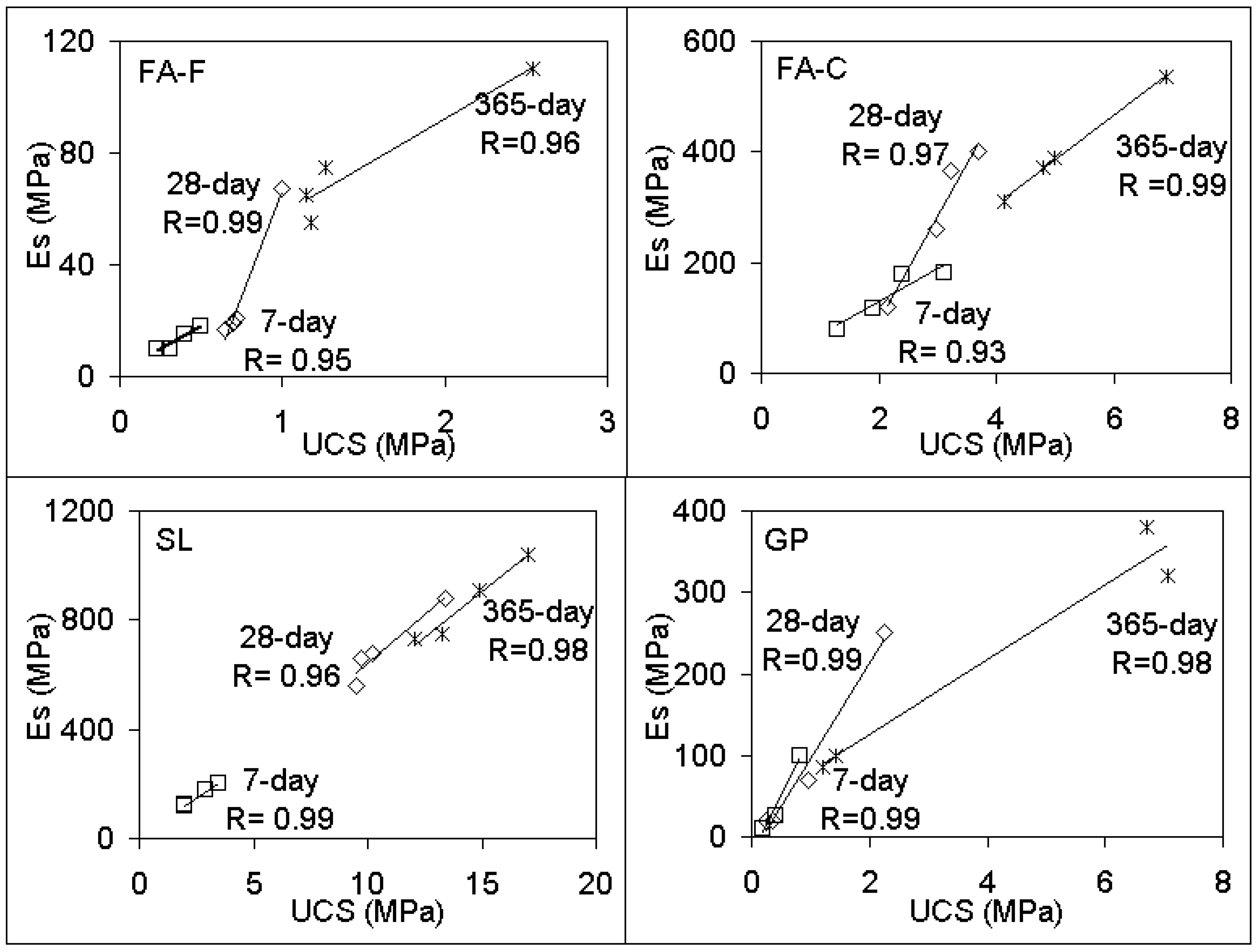
3.1. Unconfined Compressive Strength (UCS) Performances and Elastic Modulus
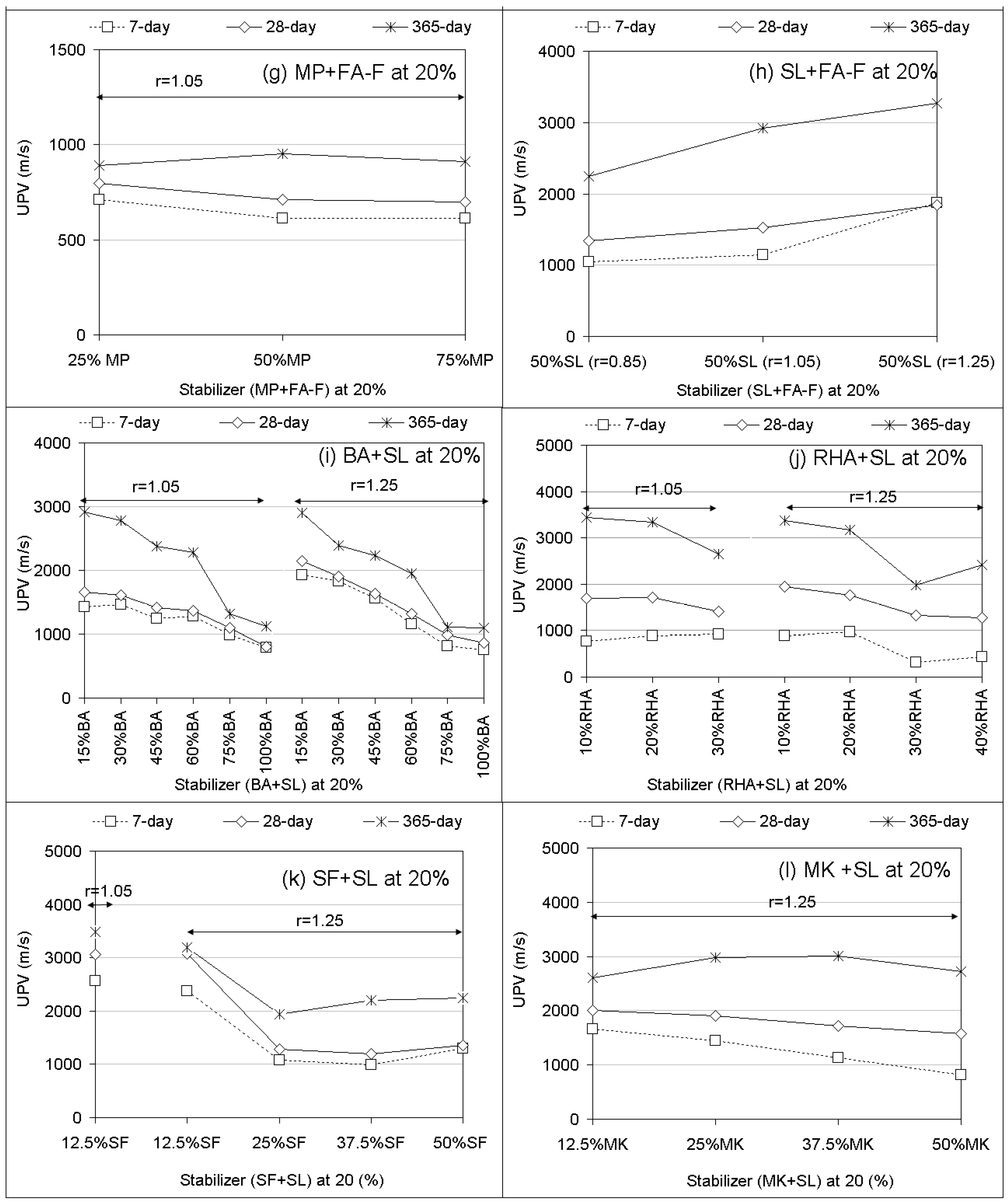
3.2. Ultrasonic Pulse Velocity (UPV) Performances
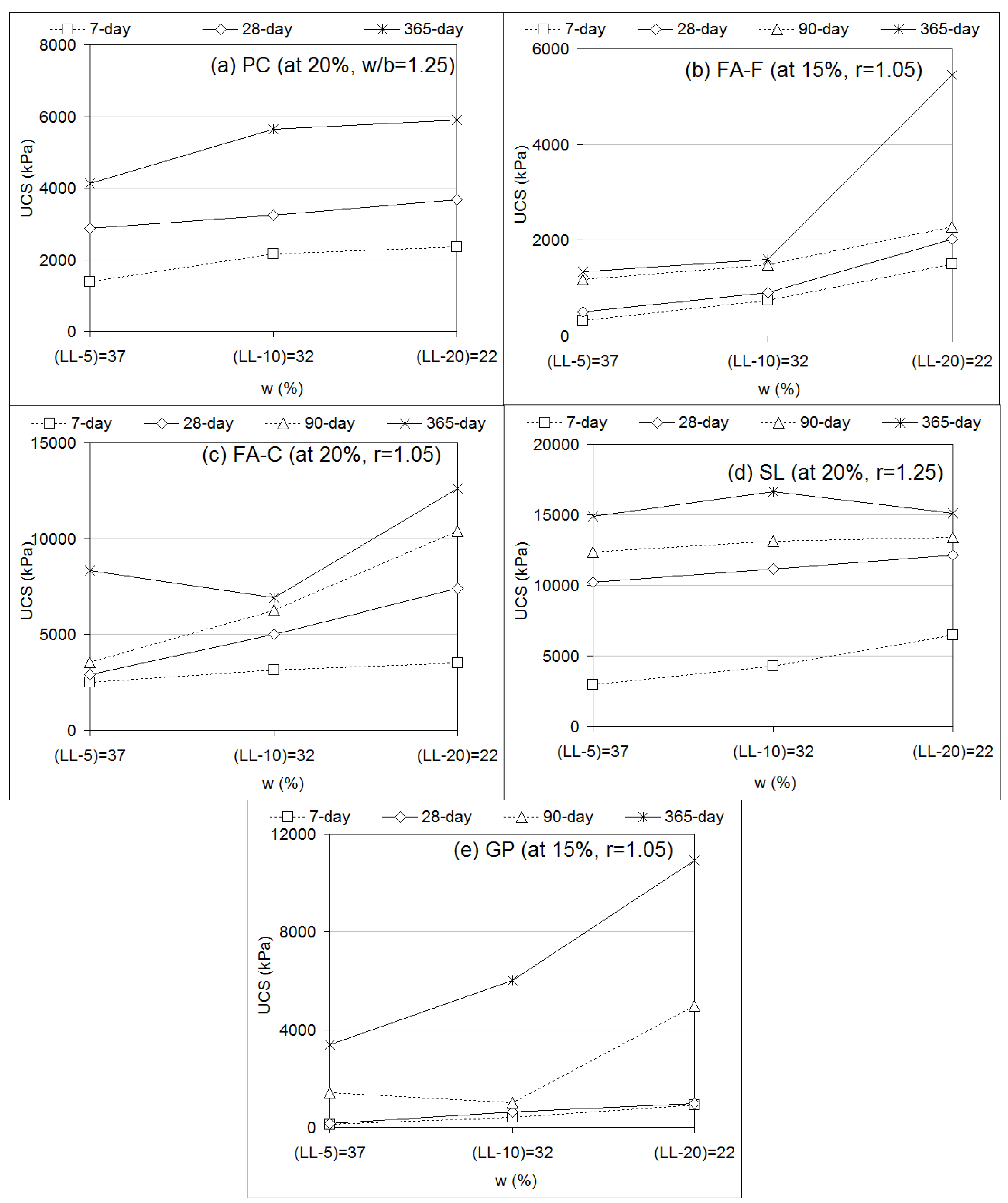
3.3. Effects of Molarity and Water Content
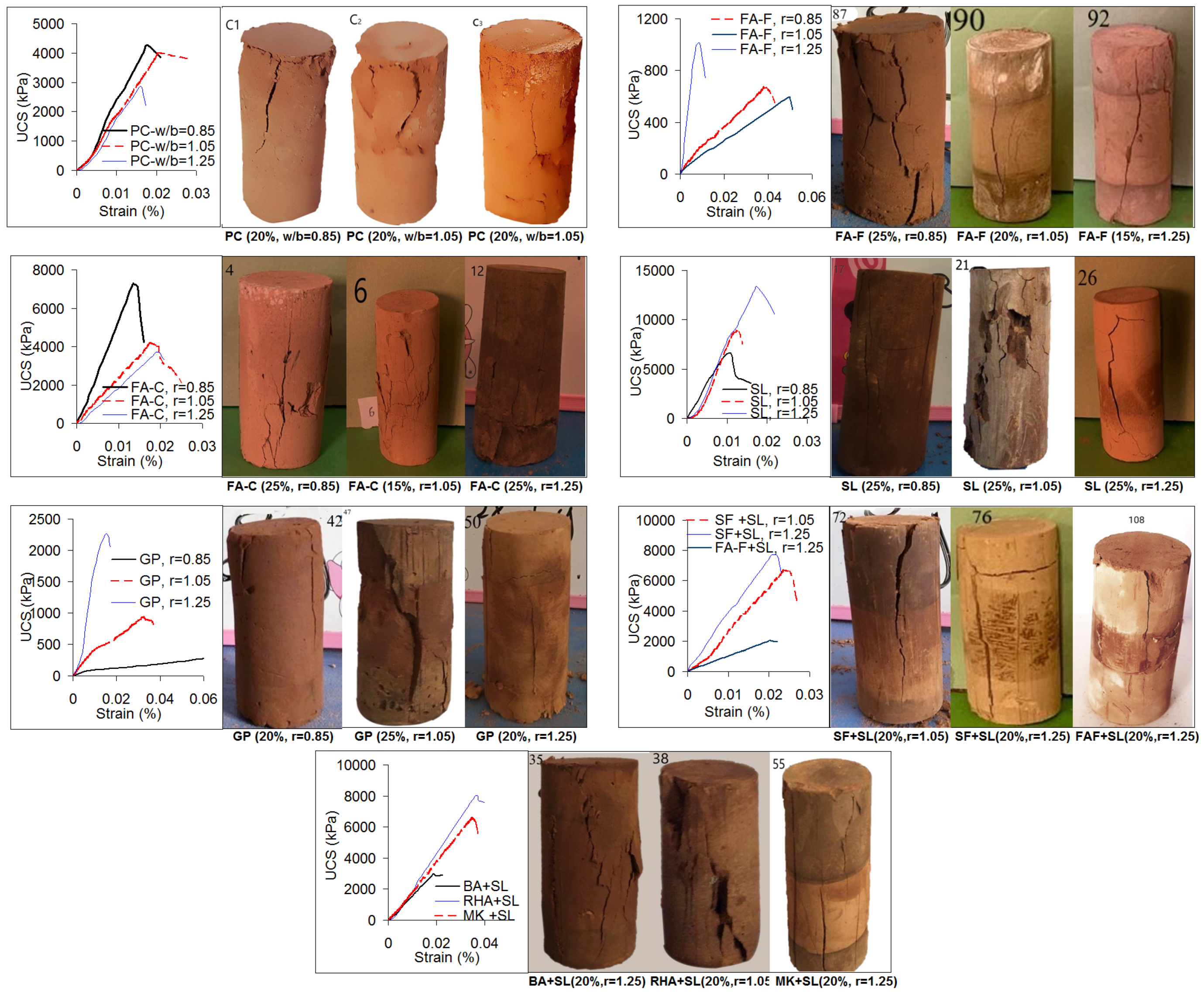
3.4. Stress–strain Curves and Failure Planes
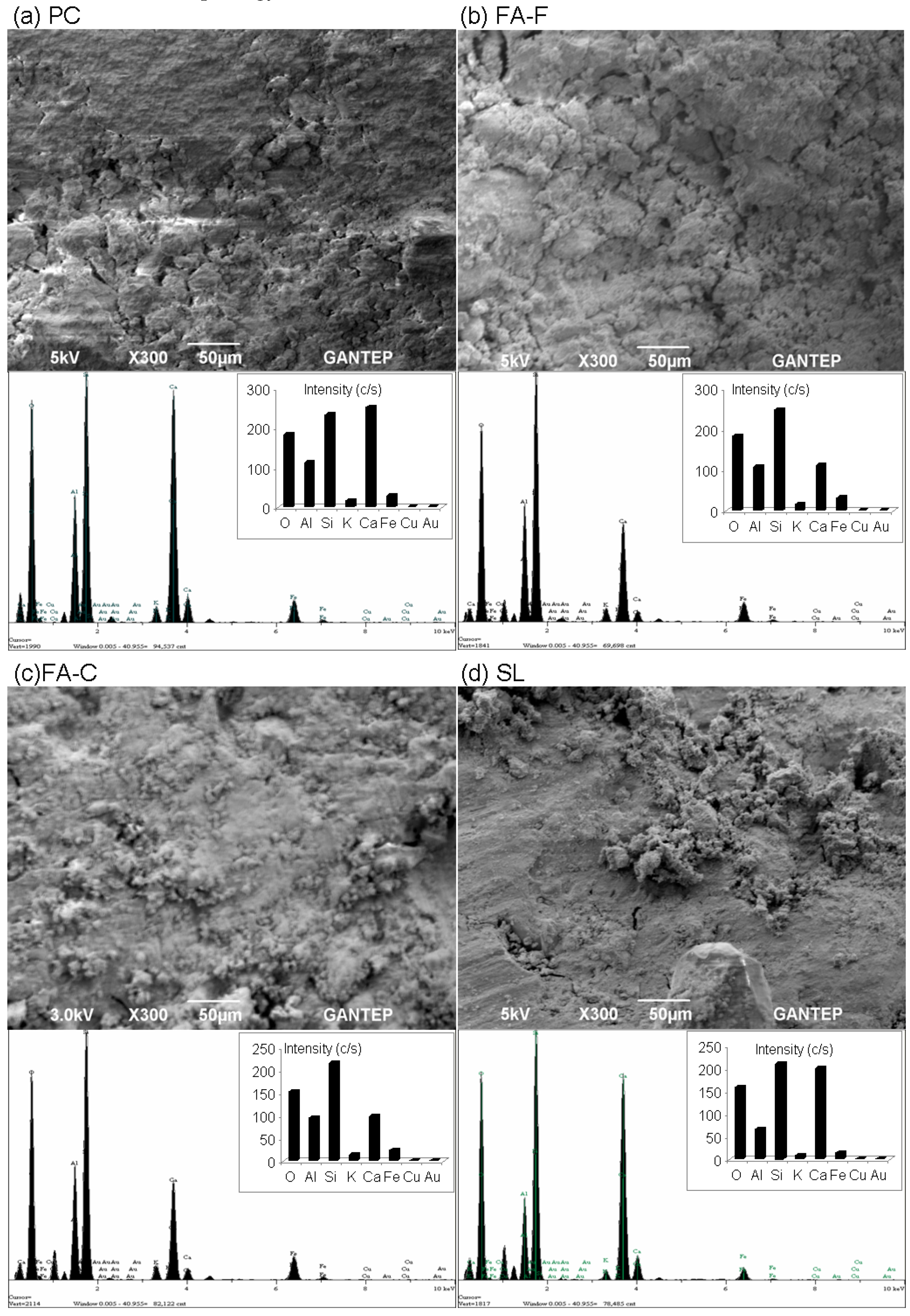
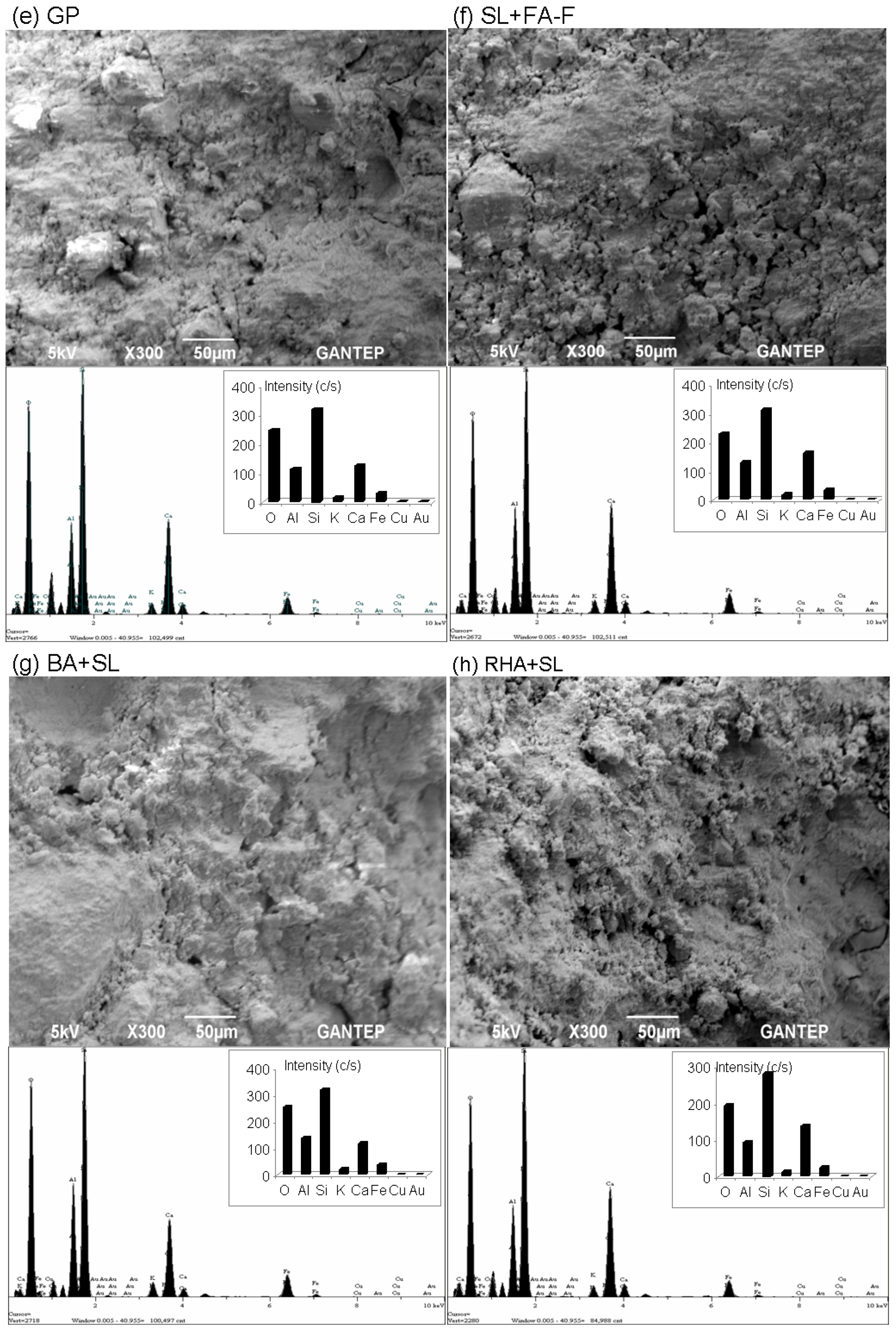
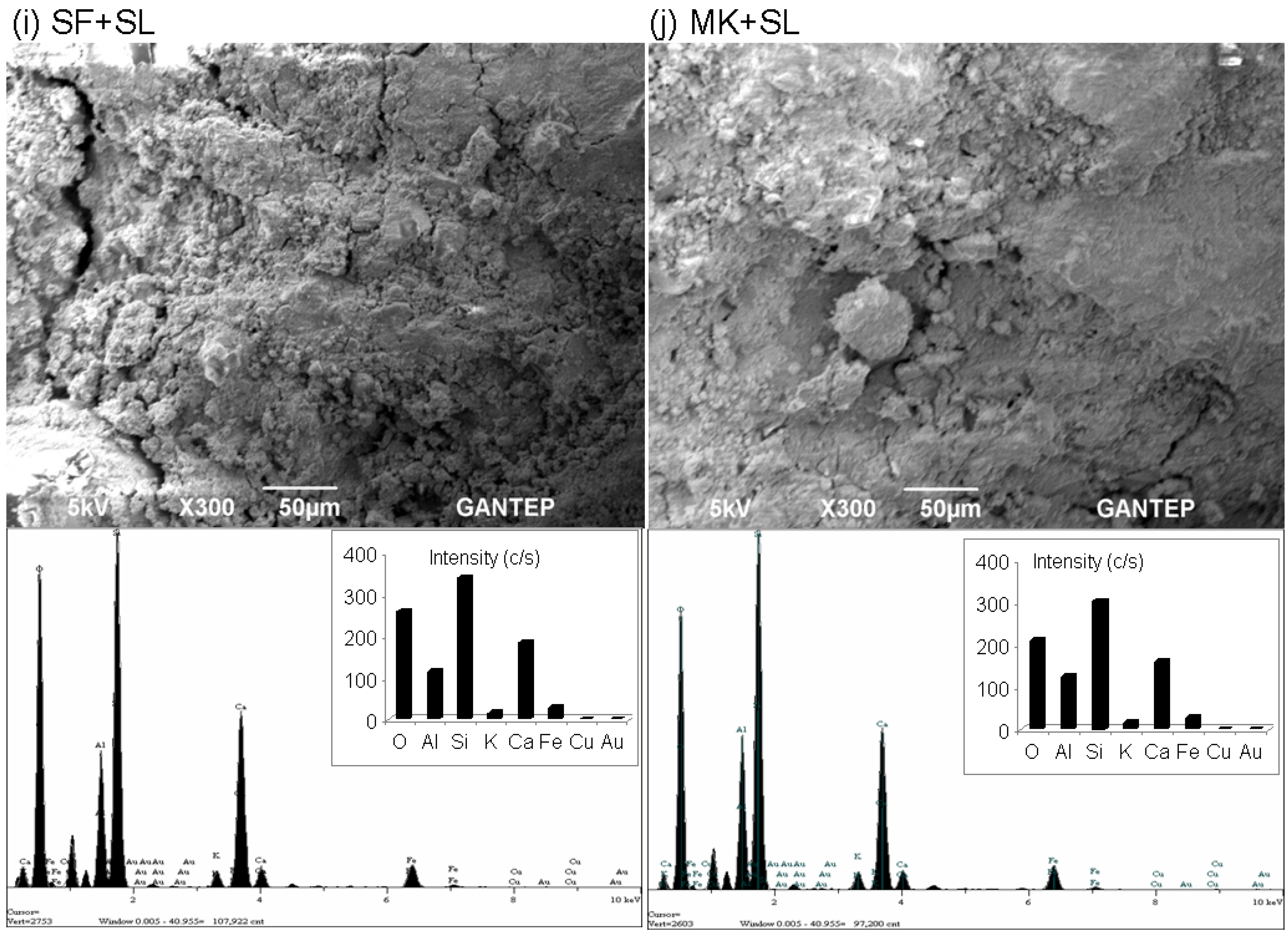
3.5. Microstructural Evaluation by Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Analyses
4. Conclusions
- (1)
- Compared to PC, UCS responses of geopolymer soilcrete specimens were low for FA-F, GP, MK, BA, and MP + FA-F specimens and were high for the specimens FA-C, SL and fewer proportions of SL combinations. Among the geopolymers, SL and SF + SL (12.5% SF) offer the most favorable UCS values, and FA-C follows. The variables affecting the UCS of geopolymer specimens are stabilizer type, proportion, alkaline ratio, and curing time. Es (elastic modulus) values estimated for geopolymer specimens offer strong correlations (R > 0.90) with UCS performances. Thus, they could be beneficial for the stiffness of soil-binder columns during design.
- (2)
- In most of the treatments with stabilizer only, increasing stabilizer proportions (10% to 25%) and alkaline ratios (r = 0.85 to r = 1.25) increases UCS performances with increasing curing time. In the combinations, increasing stabilizer proportions of BA, RHA, SF, and MK added to SL leads to a decreasing UCS.
- (3)
- Increasing curing time proportionally increases UCS for both PC and the geopolymer. However, the effect of long-term curing (90 and 365 days) on UCS of geopolymer specimens results in better performances than PC. In the majority of treatments, due to the geopolymer, UCS performances of long-term curing (365-day) markedly increase compared to those of short-term curing (7 and 28 days).
- (4)
- Most UCS performances of the geopolymer specimens can be sufficiently assessed within the performance criterion of the bearing capacity (0.2–5 MPa) recommended in previous studies. However, the effect of workability, dependent upon the affecting variables, should not be underestimated.
- (5)
- UPV performances mostly confirm the UCS results of geopolymer specimens.
- (6)
- UCS responses of geopolymer specimens increase with alkaline concentrations up to (i) 14 M for FA-F and SL, (ii) 10–12 M for FA-C, and (iii) 16 M for GP. Effects of molarity (8 to 16 M) on the geopolymer specimens (FA-F, FA-C, SL, GP) generally show strength development between 10 and 14 M. UCS increases with decreasing water content of soil (LL-5 = 37 to LL-20 = 22).
- (7)
- Stress–strain curves of geopolymer soilcrete specimens (28 days) show brittle behavior, which develop shear failure planes mostly in the axial splitting and near axial orientations. The behavior and failure modes are not found to be systematically dependent on the stabilizer type, stabilizer proportion, nor alkaline ratio of the geopolymer. Following completion of the UCS test, the geopolymer specimens remain sufficiently stable without fully disintegrating. This could be beneficial to assess the damage potential of soilcrete columns for post-failure responses.
- (8)
- From microstructure analyses (UCS specimens at 28 days), the intensity of the silica peak is identified in all geopolymer specimens. No alumina and calcium peaks are found. The peak of silica appears to be in good agreement with the strength development, specifically for the geopolymer specimens with combined stabilizers (SL + FA-F, BA + SL, RHA + SL, SF + SL, and MK + SL).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowles, J.E. Foundation Analysis and Design; McGraw-Hill: New York, NJ, USA, 1996. [Google Scholar]
- Coduto, D.P. Geotechnical Engineering: Principles and Practices; Prentice-Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Hasheminezhad, A.; Bahadori, H. Seismic response of shallow foundations over liquefiable soils improved by deep soil mixing columns. Comput. Geotech. 2019, 110, 251–273. [Google Scholar] [CrossRef]
- Kitazume, M.; Terashi, M. The Deep Mixing Method; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Porbaha, A. State of the art in deep mixing technology: part I. Basic concepts and overview. In Proceedings of the Institution of Civil Engineers-Ground Improvement; Thomas Telford Services Ltd.: London, UK, 1998; Volume 2, pp. 81–92. [Google Scholar]
- Frikha, W.; Zargayouna, H.; Boussetta, S.; Bouassida, M. Experimental study of Tunis soft soil improved by deep mixing column. Geotech. Geol. Eng. 2017, 35, 931–947. [Google Scholar] [CrossRef]
- Khedari, J.; Watsanasathaporn, P.; Hirunlabh, J. Development of fibre-based soil–cement block with low thermal conductivity. Cem. Concr. Compos. 2005, 27, 111–116. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Teixeira Pinto, A. Deep soft soil improvement by alkaline activation. In Proceedings of the Institution of Civil Engineers-Ground Improvement; Thomas Telford Services Ltd.: London, UK, 2011; Volume 164, pp. 73–82. [Google Scholar]
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Arulrajah, A.; Yaghoubi, M.; Disfani, M.M.; Horpibulsuk, S.; Bo, M.W.; Leong, M. Evaluation of fly ash-and slag-based geopolymers for the improvement of a soft marine clay by deep soil mixing. Soils Found. 2018, 58, 1358–1370. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2015. [Google Scholar]
- El Idrissi, A.C.; Roziere, E.; Loukili, A.; Darson, S. Design of geopolymer grouts: the effects of water content and mineral precursor. Eur. J. Environ. Civ. Eng. 2018, 22, 628–649. [Google Scholar] [CrossRef]
- Kuo, W.T.; Liu, M.Y.; Juang, C.U. Bonding behavior of repair material using fly-ash/ground granulated blast furnace slag-based geopolymer. Materials 2019, 12, 1697. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Wang, J.; Wang, C.; Huang, P.; Fang, C. Sulfate resistance of recycled aggregate concrete with GGBS and fly ash-based geopolymer. Materials 2019, 12, 1247. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Moura, D.; Ding, Y.; Jalali, S. Composition, strength and workability of alkali-activated metakaolin based mortars. Constr. Build. Mater. 2011, 25, 3732–3745. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Suksiripattanapong, C.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Calcium carbide residue: Alkaline activator for clay–fly ash geopolymer. Constr. Build. Mater. 2014, 69, 285–294. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127, 134–142. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-activated ground-granulated blast furnace slag for stabilization of marine soft clay. J. Mater. Civ. Eng. 2014, 27, 04014146. [Google Scholar] [CrossRef]
- Rios, S.; Cristelo, N.; Viana da Fonseca, A.; Ferreira, C. Structural performance of alkali-activated soil ash versus soil cement. J. Mater. Civ. Eng. 2015, 28, 04015125. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Investigation on soil–geopolymer with slag, fly ash and their blending. Arab. J. Sci. Eng. 2016, 41, 393–400. [Google Scholar] [CrossRef]
- Du, Y.J.; Yu, B.W.; Liu, K.; Jiang, N.J.; Liu, M.D. Physical, hydraulic, and mechanical properties of clayey soil stabilized by lightweight alkali-activated slag geopolymer. J. Mater. Civ. Eng. 2016, 29, 04016217. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Mechanical behavior and sulfate resistance of alkali activated stabilized clayey soil. Geotech. Geol. Eng. 2017, 35, 1907–1920. [Google Scholar] [CrossRef]
- Samson, G.; Cyr, M.; Gao, X.X. Formulation and characterization of blended alkali-activated materials based on flash-calcined metakaolin, fly ash and GGBS. Constr. Build. Mater. 2017, 144, 50–64. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Experimental investigation of using a recycled glass powder-based geopolymer to improve the mechanical behavior of clay soils. Constr. Build. Mater. 2018, 170, 302–313. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Using calcium carbide residue as an alkaline activator for glass powder-clay geopolymer. Constr. Build. Mater. 2018, 183, 417–428. [Google Scholar] [CrossRef]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; El Idrissi, A.C.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a new geopolymer grout: rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. Strength development of soil–fly ash geopolymer: assessment of soil, fly ash, alkali activators, and water. J. Mater. Civ. Eng. 2018, 30, 04018171. [Google Scholar] [CrossRef]
- Güllü, H.; Cevik, A.; Al-Ezzi, K.M.; Gülsan, M.E. On the rheology of using geopolymer for grouting: A comparative study with cement-based grout included fly ash and cold bonded fly ash. Constr. Build. Mater. 2019, 196, 594–610. [Google Scholar] [CrossRef]
- ASTM D2487-11. Standard Practice for Classification of Soils for Engineering Purposes; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Bhadriraju, V.; Puppala, A.J.; Madhyannapu, R.S.; Williammee, R. Laboratory procedure to obtain well-mixed soil binder samples of medium stiff to stiff expansive clayey soil for deep soil mixing simulation. Geotech. Test. J. 2007, 31, 225–238. [Google Scholar]
- Pakbaz, M.S.; Farzi, M. Comparison of the effect of mixing methods (dry vs. wet) on mechanical and hydraulic properties of treated soil with cement or lime. Appl. Clay Sci. 2015, 105, 156–169. [Google Scholar] [CrossRef]
- ASTM C150/C150M-17. Standard Specification for Portland cement; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C618-15. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Celik, F.; Canakci, H. Examination of the mechanical properties and failure pattern of soilcrete mixtures modified with rice husk ash. Eur. J. Environ. Civ. Eng. 2018. [Google Scholar] [CrossRef]
- Wen, N.; Zhao, Y.; Yu, Z.; Liu, M. A sludge and modified rice husk ash-based geopolymer: synthesis and characterization analysis. J. Clean. Prod. 2019, 226, 805–814. [Google Scholar] [CrossRef]
- ASTM C1240-15. Standard Specification for Silica Fume Used in Cementitious Mixtures; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ACI, 234R-96, 1996. Guide for the use of Silica Fume in Concrete; ACI: Farmington Hills, MI, USA, 1996; Volume 234, pp. 1–15. [Google Scholar]
- Cevik, A.; Alzeebaree, R.; Humur, G.; Nis, A.; Gulsan, M.E. Effect of nano-silica on the chemical durability and mechanical performance of fly ash based geopolymer concrete. Ceram. Int. 2018, 44, 12253–12264. [Google Scholar] [CrossRef]
- Mohd Ali, A.Z.; Sanjayan, J.; Guerrieri, M. Performance of geopolymer high strength concrete wall panels and cylinders when exposed to a hydrocarbon fire. Constr. Build. Mater. 2017, 137, 195–207. [Google Scholar] [CrossRef]
- Güllü, H.; Canakci, H.; Al Zangana, I.F. Use of cement based grout with glass powder for deep mixing. Constr. Build. Mater. 2017, 137, 12–20. [Google Scholar] [CrossRef]
- JGS 0821–2000. Practice for Marking and Curing Stabilized Soil Specimens without Compaction; Japanese Geotechnical Society Standard, Sengoku: Tokyo, Japan, 2000. [Google Scholar]
- ASTM D2166. Standard Test Method for Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM D5102-09. Standard Test Methods for Unconfined Compressive Strength of Compacted Soil-Lime Mixtures; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM C597-16. Standard Test Method for Pulse Velocity through Concrete; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Terashi, M.; Kitazume, M. QA/QC for deep-mixed ground: current practice and future research needs. In Proceedings of the Institution of Civil Engineers-Ground Improvement; ICE Publishing: London, UK, 2011; Volume 164, pp. 161–177. [Google Scholar]
- Abbey, S.J.; Ngambi, S.; Ngekpe, B.E. Understanding the performance of deep mixed column improved soils-a review. Int. J. Civ. Eng. Technol. (Ijciet) 2015, 6, 97–117. [Google Scholar]
- Anon, O.H. Classification of rocks and soils for engineering geological mapping, Part 1—rock and soil materials. In Report of the Commission of Engineering Geological Mapping; Bulletin of the International Association of Engineering Geology: New Castle Upon Tyne, UK, 1979; Volume 19, pp. 364–371. [Google Scholar]
- Patel, Y.J.; Shah, N. Enhancement of the properties of ground granulated blast furnace slag based self compacting geopolymer concrete by incorporating rice husk ash. Constr. Build. Mater. 2018, 171, 654–662. [Google Scholar] [CrossRef]
- Temuujin, J.V.; Van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Misra, A.; Biswas, D.; Upadhyaya, S. Physico-mechanical behavior of self-cementing class C fly ash-clay mixtures. Fuel 2005, 84, 1410–1422. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. High strength geopolymer binder based on waste-glass powder. Adv. Powder Technol. 2017, 28, 215–222. [Google Scholar] [CrossRef]
- Jain, N. Effect of nonpozzolanic and pozzolanic mineral admixtures on the hydration behavior of ordinary Portland cement. Constr. Build. Mater. 2012, 27, 39–44. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of blast furnace slag grades on fly ash based geopolymer waste forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Hwang, C.L.; Huynh, T.P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Paisitsrisawat, P.; Rattanasak, U. Strength and resistance to sulfate and sulfuric acid of ground fluidized bed combustion fly ash-silica fume alkali-activated composite. Adv. Powder Technol. 2014, 25, 1087–1093. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Ghorbel, E.; Wardeh, G. Optimization approach of granulated blast furnace slag and metakaolin based geopolymer mortars. Constr. Build. Mater. 2019, 198, 10–26. [Google Scholar] [CrossRef]
- Kawasaki, T.; Niina, A.; Saitoh, S.; Suzuki, Y.; Honjo, Y. Deep mixing method using cement hardening agent. In Proceedings of the 10th International Conference on Soil Mechanics and Foundation Engineering, Stockholm, Sweden, 15–19 June 1981; A.A.Balkema: Rotterdam, The Netherlands, 1981; Volume 3, pp. 721–724. [Google Scholar]
- Hassan, M. Engineering Characteristics of Cement Stabilized Soft Finnish Clay-a Laboratory Study Licentiate Thesis; Helsinki University of Technology: Helsinki, Finland, 2009; Available online: https://aaltodoc.aalto.fi/bitstream/handle/123456789/30181/lic_hassan_md.%20mamunul_2009.pdf?sequence=1&isAllowed=y (accessed on 22 June 2019).
- Kramer, S.L. Geotechnical Earthquake Engineering; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Mandal, T.; Tinjum, J.M.; Edil, T.B. Non-destructive testing of cementitiously stabilized materials using ultrasonic pulse velocity test. Transp. Geotech. 2016, 6, 97–107. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Hardjito, D. Studies of Fly Ash-based Geopolymer Concrete. Ph.D. Thesis, Curtin University, Perth, Australia, 2005. Available online: https://espace.curtin.edu.au/bitstream/handle/20.500.11937/634/18580_Hardjito2005.pdf?sequence=2&isAllowed=y (accessed on 24 June 2019).
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Lorenzo, G.A.; Bergado, D.T. Fundamental characteristics of cement-admixed clay in deep mixing. J. Mater. Civ. Eng. 2006, 18, 161–174. [Google Scholar] [CrossRef]
- Basu, A.; Mishra, D.A.; Roychowdhury, K. Rock failure modes under uniaxial compression, Brazilian, and point load tests. Bull. Eng. Geol. Environ. 2013, 72, 457–475. [Google Scholar] [CrossRef]
- Illampas, R.; Ioannou, I.; Charmpis, D.C. Adobe bricks under compression: experimental investigation and derivation of stress–strain equation. Constr. Build. Mater. 2014, 53, 83–90. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Xu, W.; Lo, T.Y.; Memon, S.A. Microstructure and reactivity of rich husk ash. Constr. Build. Mater. 2012, 29, 541–547. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Al Bakri, A.M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Heah, C.Y. Processing and characterization of calcined kaolin cement powder. Constr. Build. Mater. 2012, 30, 794–802. [Google Scholar] [CrossRef]
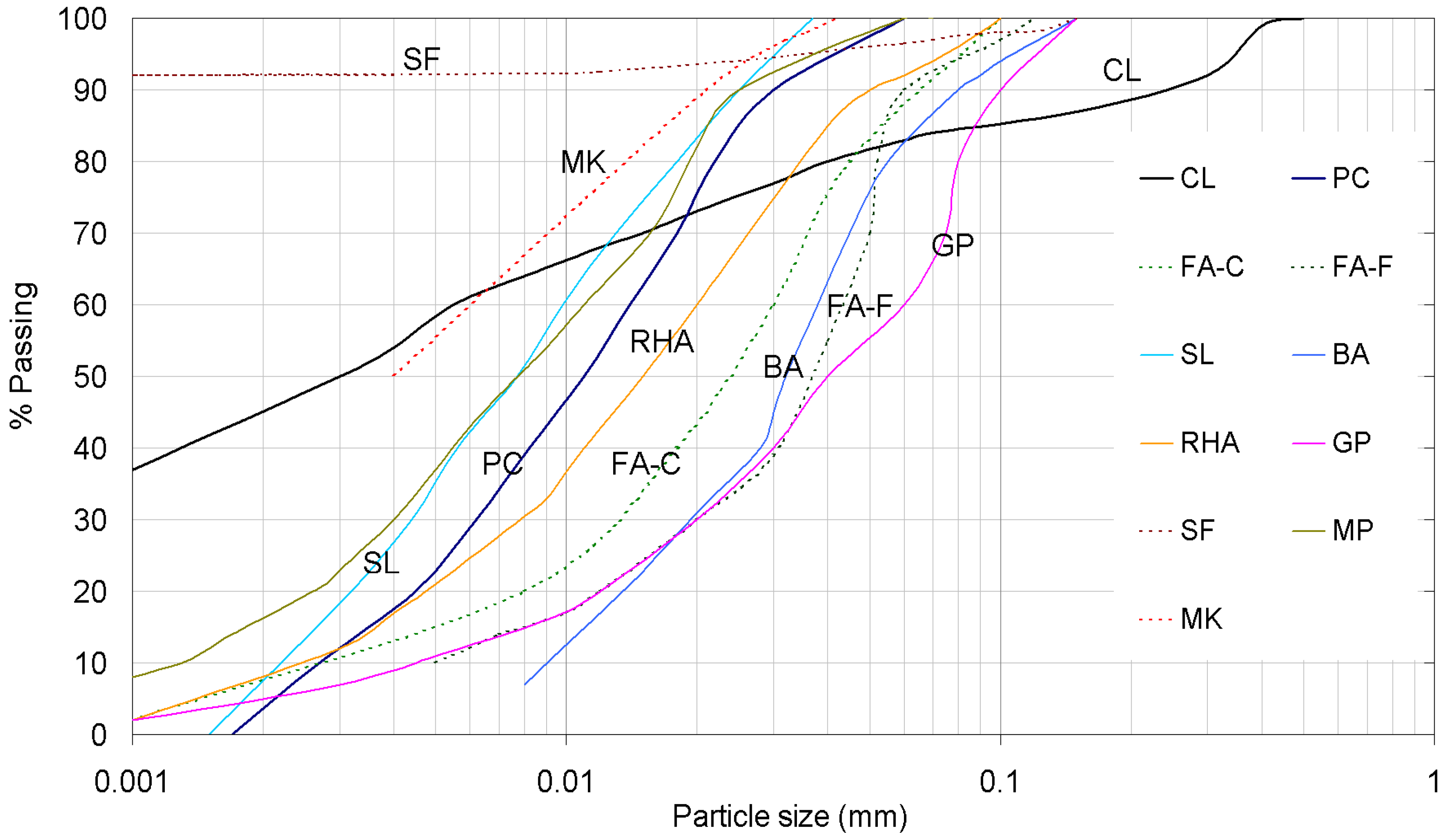



| (a) Chemical composition | |||||||||||
| Constituent (%) | CL | PC | FA-F | FA-C | SL | BA | RHA | GP | SF | MP | MK |
| CaO | 18.24 | 62.58 | 4.24 | 23.9 | 34.2 | 6.13 | 1.14 | 8.21 | 1.35 | 52.45 | 0.2 |
| Al2O3 | 6.36 | 5.31 | 24.4 | 7.97 | 10.6 | 34.3 | 0.54 | 1.00 | 0.39 | 0.39 | 45.7 |
| Fe2O3 | 10.7 | 4.04 | 7.1 | 5.34 | 1.28 | 15 | 0.16 | 0.52 | 1.21 | 0.78 | 0.31 |
| SO3 | 0.08 | 2.73 | 0.29 | 3.03 | 0.68 | 0.9 | 0.25 | 0.06 | 1.0 | 0.076 | 0.14 |
| MgO | 0.44 | 2.82 | 2.4 | 0.53 | 7.63 | 1.57 | 0.5 | 0.14 | 2.23 | 0.54 | 0.23 |
| SiO2 | 17.2 | 20.25 | 57.2 | 18.27 | 40.4 | 39.4 | 87.2 | 78 | 92.5 | 1.29 | 50.6 |
| K2O | 1.49 | 0.92 | 3.37 | 1.39 | - | 1.19 | 1.94 | 0.09 | 0.08 | 0.11 | 0.11 |
| (b) Physical and index properties | |||||||||||
| Property | CL | PC | FA-F | FA-C | SL | BA | RHA | GP | SF | MP | MK |
| Specific surface (m2/kg) | - | 326 | 379 | 290 | 575 | - | 1080 | 382 | 21,100 | 519 | 13,200 |
| Specific gravity | 2.7 | 3.15 | 2.25 | 2.7 | 2.88 | 2.16 | 2.03 | 2.54 | 2.2 | 2.71 | 2.6 |
| Cu (Coefficient of uniformity) | - | - | 8.4 | 10.7 | 4.33 | 4.75 | 7.1 | 13.9 | - | 9.3 | - |
| Cc (Coefficient of curvature) | - | - | 1.9 | 2.7 | 0.47 | 1.3 | 1.15 | 1.55 | - | 0.76 | - |
| Grout (Binder Only by PC) | Deep Mixing Soil | |||||
|---|---|---|---|---|---|---|
| w/b | PC (%) | PC (g) | W (g) | Soil (CL) (g) | w (%) | Water (g) |
| 0.85 | 10 | 100 | 85 | 1000 | (LL-5) = 37 | 370 |
| 0.85 | 15 | 150 | 127.5 | 1000 | (LL-5) = 37 | 370 |
| 0.85 | 20 | 200 | 170 | 1000 | (LL-5) = 37 | 370 |
| 1.05 | 10 | 100 | 105 | 1000 | (LL-5) = 37 | 370 |
| 1.05 | 15 | 150 | 157.5 | 1000 | (LL-5) = 37 | 370 |
| 1.05 | 20 | 200 | 210 | 1000 | (LL-5) = 37 | 370 |
| 1.25 | 10 | 100 | 125 | 1000 | (LL-5) = 37 | 370 |
| 1.25 | 15 | 150 | 187.5 | 1000 | (LL-5) = 37 | 370 |
| 1.25 | 20 | 200 | 250 | 1000 | (LL-5) = 37 | 370 |
| 1.25 | 20 | 200 | 250 | 1000 | (LL-10) = 32 | 320 |
| 1.25 | 20 | 200 | 250 | 1000 | (LL-20) = 22 | 320 |
| Geopolymer | Deep Mixing Soil | |||||||
|---|---|---|---|---|---|---|---|---|
| Stabilizer | Stabilizer (%) | r = Alkaline Activator/Stabilizer (%) | Stabilizer (g) | Alkaline Activator (g) | NaOH (M) | Soil (CL) (g) | w (%) | Water (g) |
| 10 | 0.85 | 100 | 85 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 15 | 0.85 | 150 | 127.5 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 20 | 0.85 | 200 | 170 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 25 | 0.85 | 250 | 212.5 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| FA-F | 10 | 1.05 | 100 | 105 | 12 M | 1000 | (LL-5) = 37 | 370 |
| FA-C | 15 | 1.05 | 150 | 157.5 | 12 M | 1000 | (LL-5) = 37 | 370 |
| SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| GP | 25 | 1.05 | 250 | 262.5 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 10 | 1.25 | 100 | 125 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 15 | 1.25 | 150 | 187.5 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 25 | 1.25 | 250 | 312.5 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 25 | 1.5 | 250 | 375 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 20 | 1.5 | 200 | 300 | 12 M | 1000 | (LL-5) = 37 | 370 | |
| 25%MP + 75%FA-F | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%MP + 50%FA-F | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 75%MP + 25%FA-F | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%SL + 50%FA-F | 20 | 0.85 | 200 | 170 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%SL + 50%FA-F | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%SL + 50%FA-F | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 15%BA + 85%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 30%BA + 70%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 45BA% + 55%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 60BA% + 40%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 75%BA + 25%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 15%BA + 85%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 30%BA + 70%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 45BA% + 55%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 60BA% + 40%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 75%BA + 25%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 100%BA | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 100%BA | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 10%RHA + 90%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 20%RHA + 80%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 30%RHA + 70%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 10%RHA + 90%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 20%RHA + 80%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 30%RHA + 70%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 40%RHA + 60%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%GP + 50%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 12.5%SF + 87.5%SL | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 12.5%SF + 87.5%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 25%SF + 75%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 37.5%SF + 62.5%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%SF + 50%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| MK | 10 | 1.25 | 100 | 125 | 12 M | 1000 | (LL-5) = 37 | 370 |
| MK | 15 | 1.25 | 150 | 187.5 | 12 M | 1000 | (LL-5) = 37 | 370 |
| MK | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| MK | 25 | 1.25 | 250 | 312.5 | 12 M | 1000 | (LL-5) = 37 | 370 |
| MK | 30 | 1.25 | 300 | 375 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 12.5%MK + 87.5%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 25%MK + 75%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 37.5%MK + 62.5%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| 50%MK + 50%SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-5) = 37 | 370 |
| GP | 15 | 1.05 | 150 | 157.5 | 14 M | 1000 | (LL-5) = 37 | 370 |
| FA-F | 15 | 1.05 | 15 | 157.5 | 14 M | 1000 | (LL-5) = 37 | 370 |
| FA-C | 20 | 1.05 | 200 | 210 | 14 M | 1000 | (LL-5) = 37 | 370 |
| SL | 20 | 1.25 | 200 | 250 | 14 M | 1000 | (LL-5) = 37 | 370 |
| GP | 15 | 1.05 | 150 | 157.5 | 10 M | 1000 | (LL-5) = 37 | 370 |
| FA-F | 15 | 1.05 | 15 | 157.5 | 10 M | 1000 | (LL-5) = 37 | 370 |
| FA-C | 20 | 1.05 | 200 | 210 | 10 M | 1000 | (LL-5) = 37 | 370 |
| SL | 20 | 1.25 | 200 | 250 | 10 M | 1000 | (LL-5) = 37 | 370 |
| GP | 15 | 1.05 | 150 | 157.5 | 8 M | 1000 | (LL-5) = 37 | 370 |
| FA-F | 15 | 1.05 | 15 | 157.5 | 8 M | 1000 | (LL-5) = 37 | 370 |
| FA-C | 20 | 1.05 | 200 | 210 | 8 M | 1000 | (LL-5) = 37 | 370 |
| SL | 20 | 1.25 | 200 | 250 | 8 M | 1000 | (LL-5) = 37 | 370 |
| GP | 15 | 1.05 | 150 | 157.5 | 16 M | 1000 | (LL-5) = 37 | 370 |
| FA-F | 15 | 1.05 | 15 | 157.5 | 16 M | 1000 | (LL-5) = 37 | 370 |
| FA-C | 20 | 1.05 | 200 | 210 | 16 M | 1000 | (LL-5) = 37 | 370 |
| SL | 20 | 1.25 | 200 | 250 | 16 M | 1000 | (LL-5) = 37 | 370 |
| GP | 15 | 1.05 | 150 | 157.5 | 12 M | 1000 | (LL-10) = 32 | 320 |
| GP | 15 | 1.05 | 150 | 157.5 | 12 M | 1000 | (LL-20) = 22 | 220 |
| FA-F | 15 | 1.05 | 150 | 157.5 | 12 M | 1000 | (LL-10) = 32 | 320 |
| FA-F | 15 | 1.05 | 150 | 157.5 | 12 M | 1000 | (LL-20) = 22 | 220 |
| FA-C | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-10) = 32 | 320 |
| FA-C | 20 | 1.05 | 200 | 210 | 12 M | 1000 | (LL-20) = 22 | 220 |
| SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-10) = 32 | 320 |
| SL | 20 | 1.25 | 200 | 250 | 12 M | 1000 | (LL-20) = 22 | 220 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canakci, H.; Güllü, H.; Alhashemy, A. Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing. Materials 2019, 12, 2542. https://doi.org/10.3390/ma12162542
Canakci H, Güllü H, Alhashemy A. Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing. Materials. 2019; 12(16):2542. https://doi.org/10.3390/ma12162542
Chicago/Turabian StyleCanakci, Hanifi, Hamza Güllü, and Ali Alhashemy. 2019. "Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing" Materials 12, no. 16: 2542. https://doi.org/10.3390/ma12162542
APA StyleCanakci, H., Güllü, H., & Alhashemy, A. (2019). Performances of Using Geopolymers Made with Various Stabilizers for Deep Mixing. Materials, 12(16), 2542. https://doi.org/10.3390/ma12162542





