On the Microstructure and Isothermal Oxidation at 800, 1200, and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at %) Alloy
Abstract
1. Introduction
2. Alloy Selection
2.1. Why Al-Rich, NbAl3-Based Alloy?
2.2. Why Cr, Hf, and Laves Phase?
2.3. Why Hf as a Reactive Element and not Y?
2.4. Why no Fe, Si, and Ti?
2.5. Why no Stable Solid Solution?
2.6. Why a Zone A Microstructure?
3. Experimental
4. Results
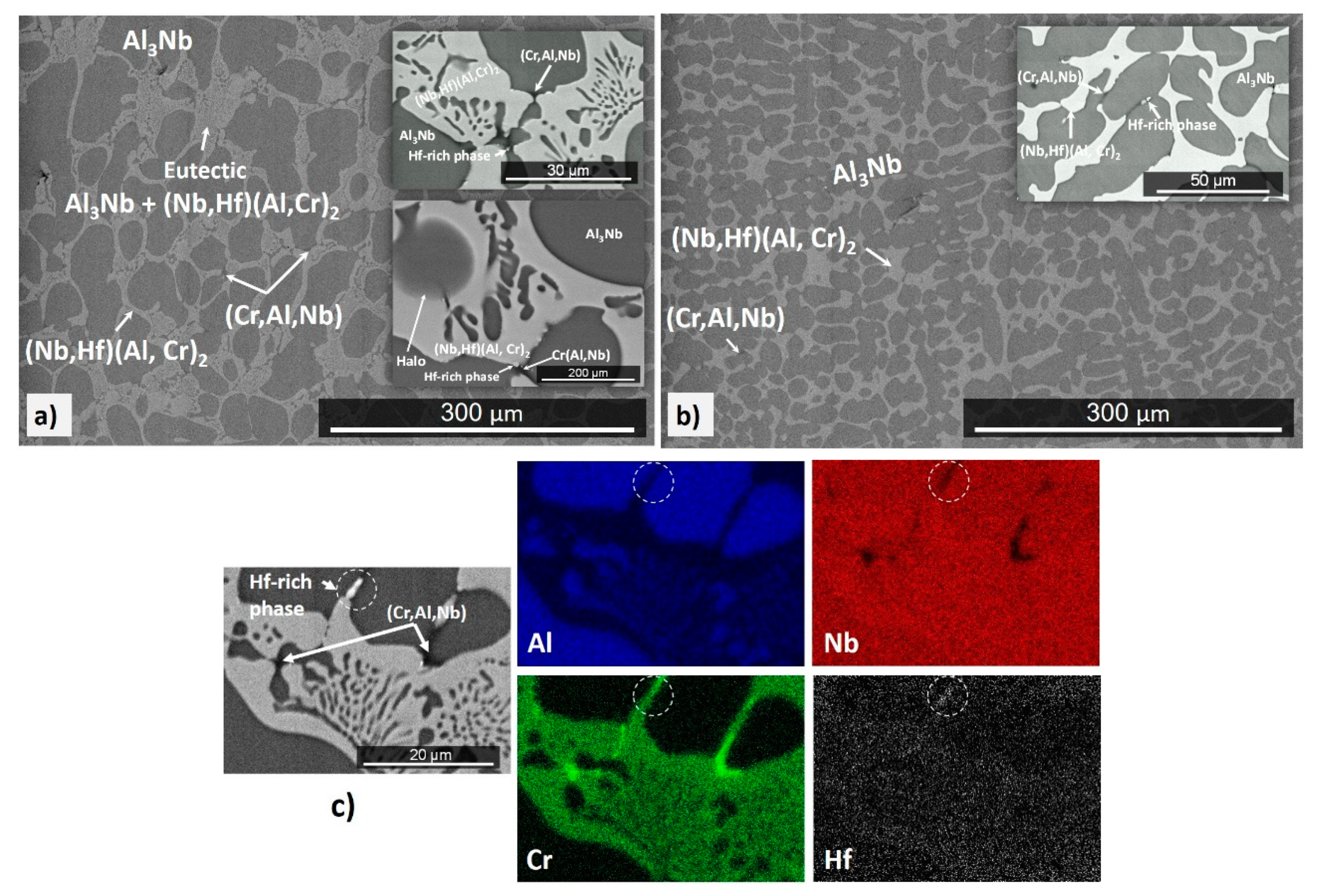
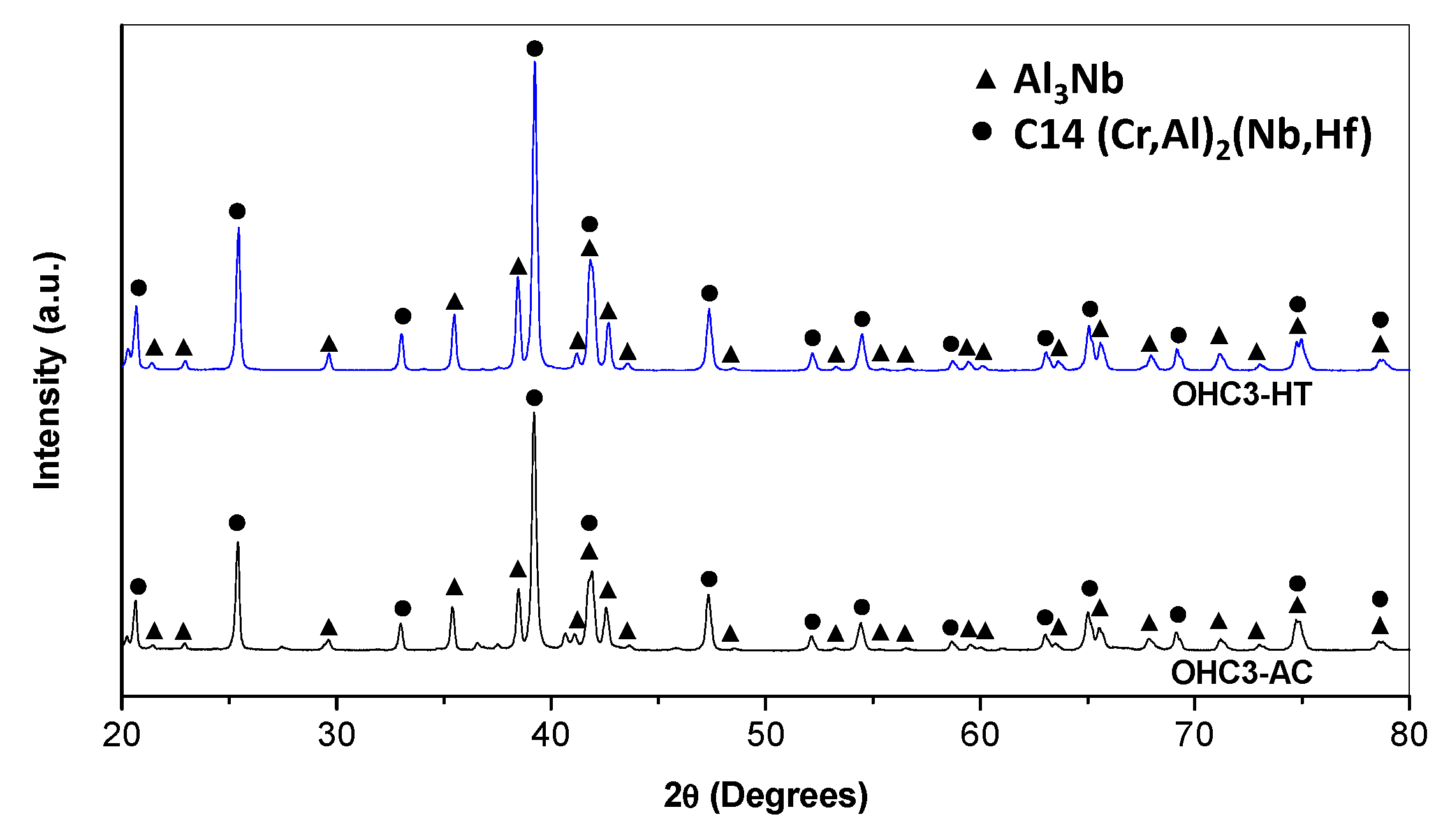
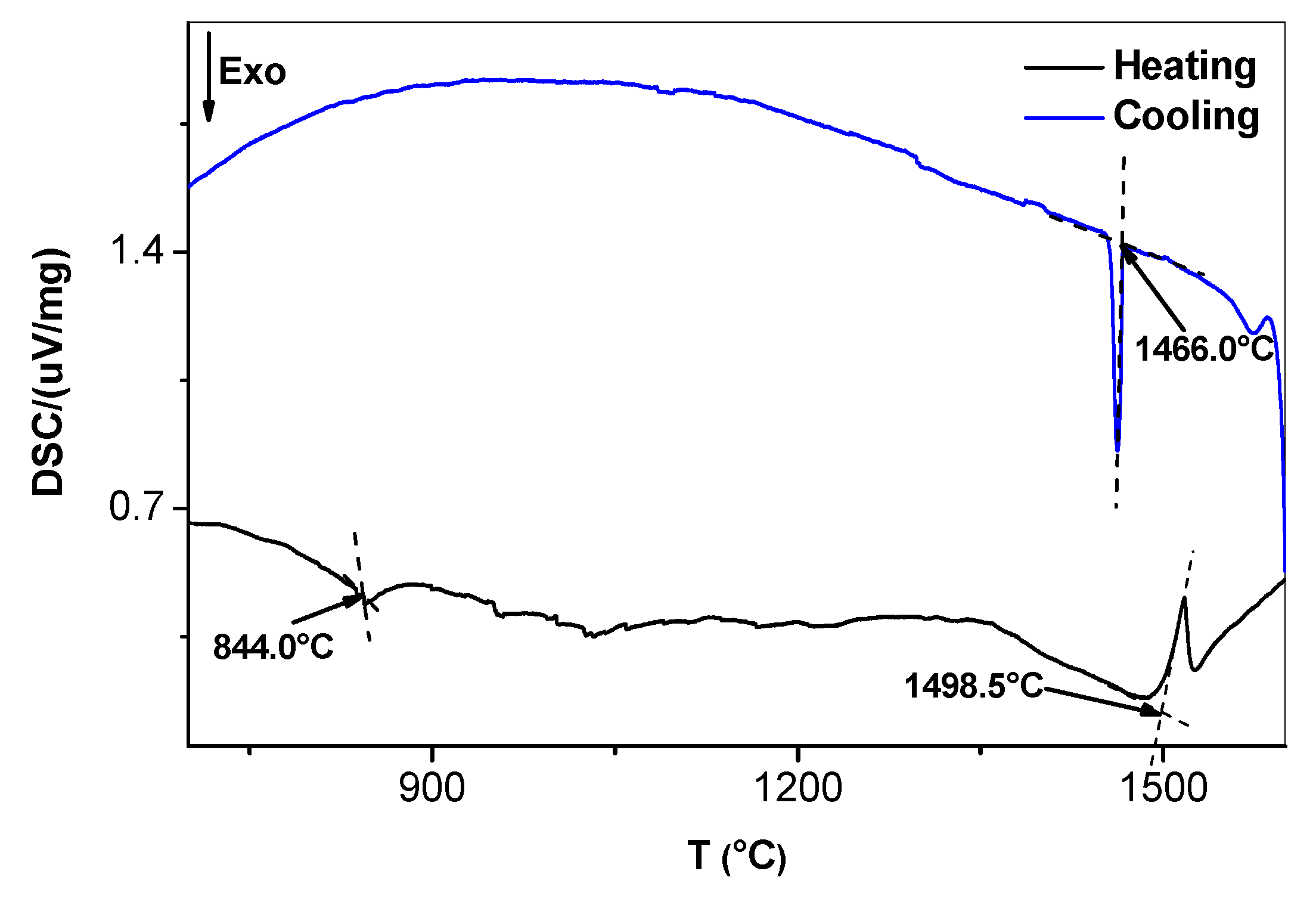
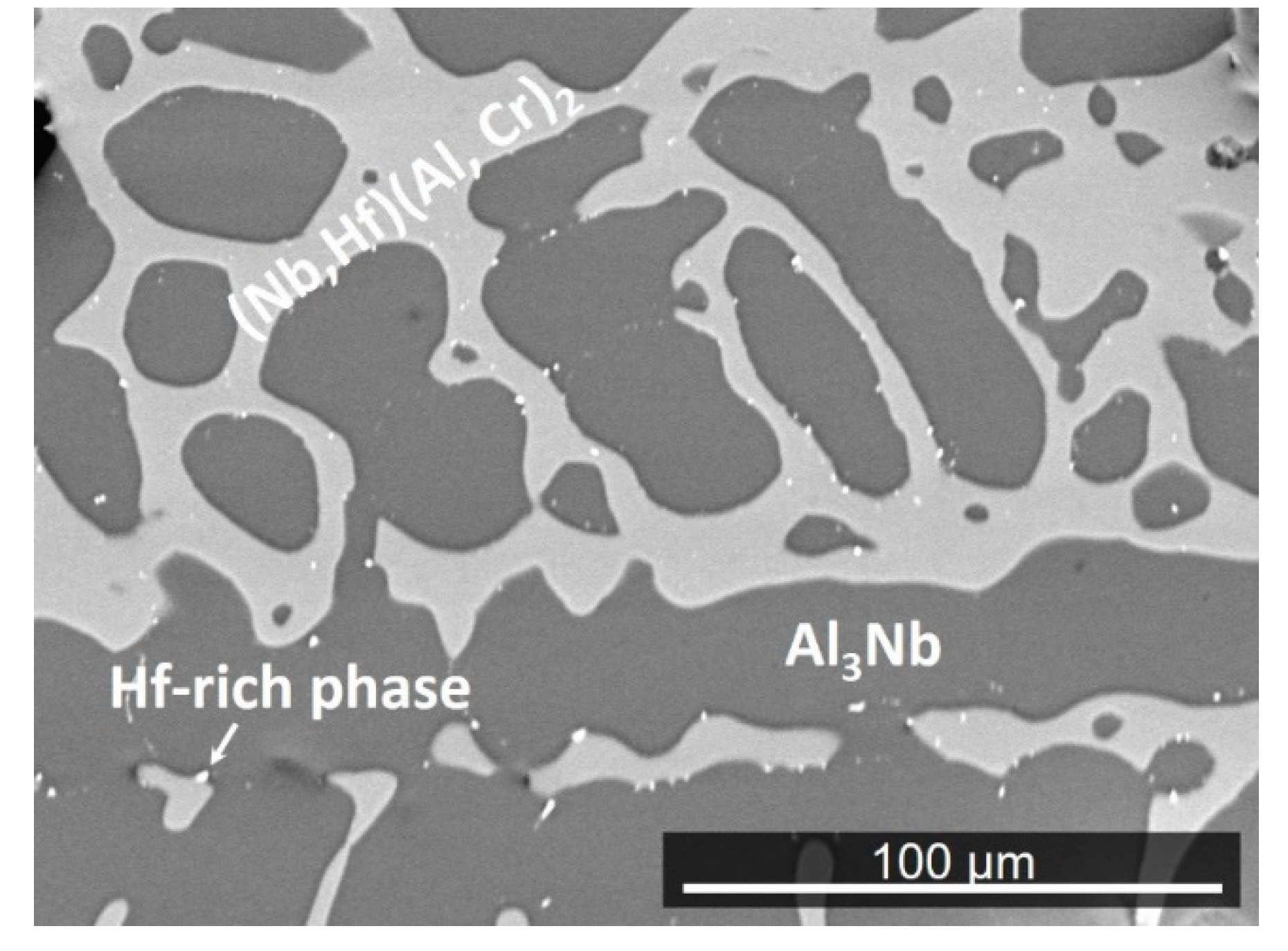
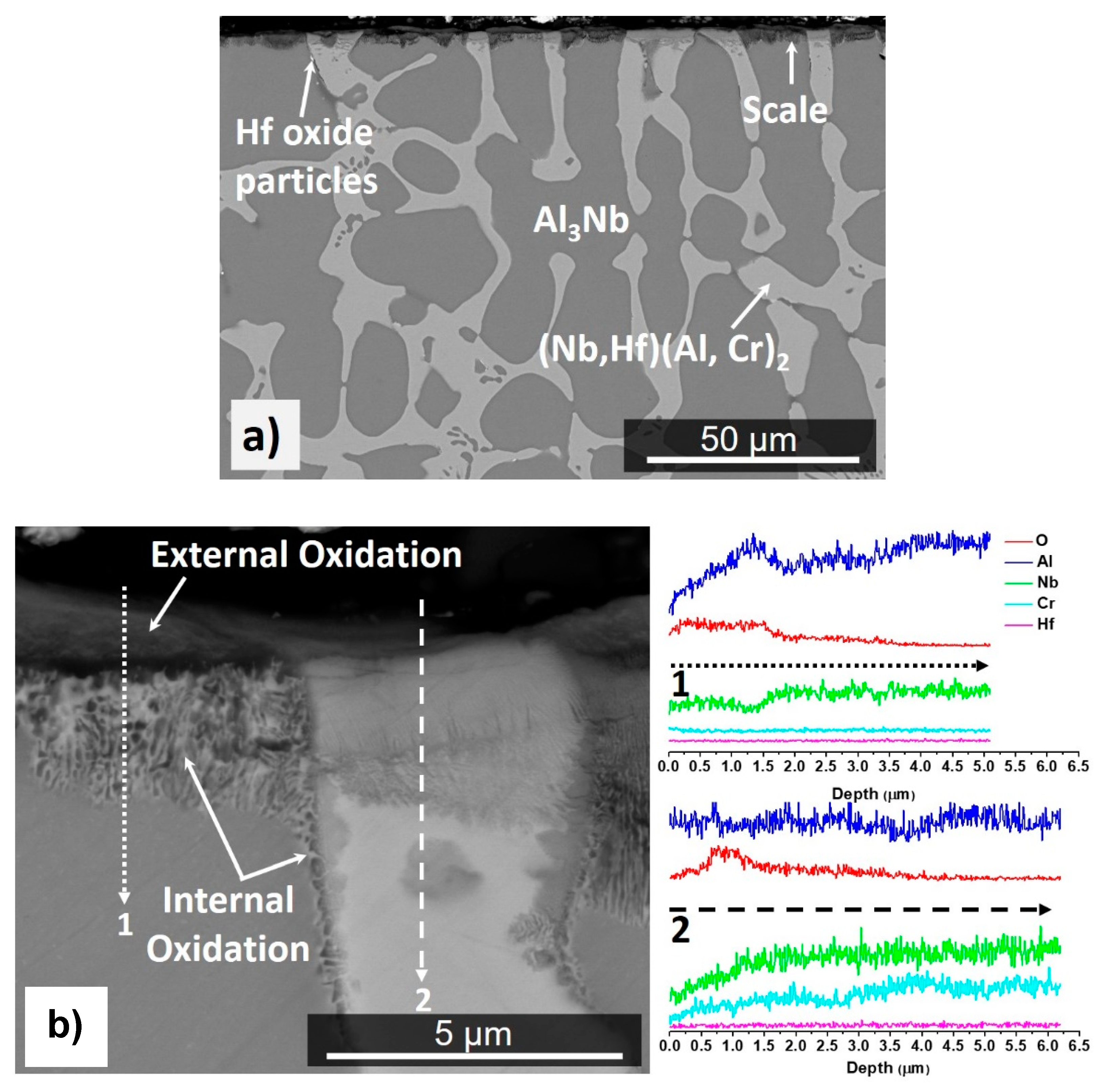
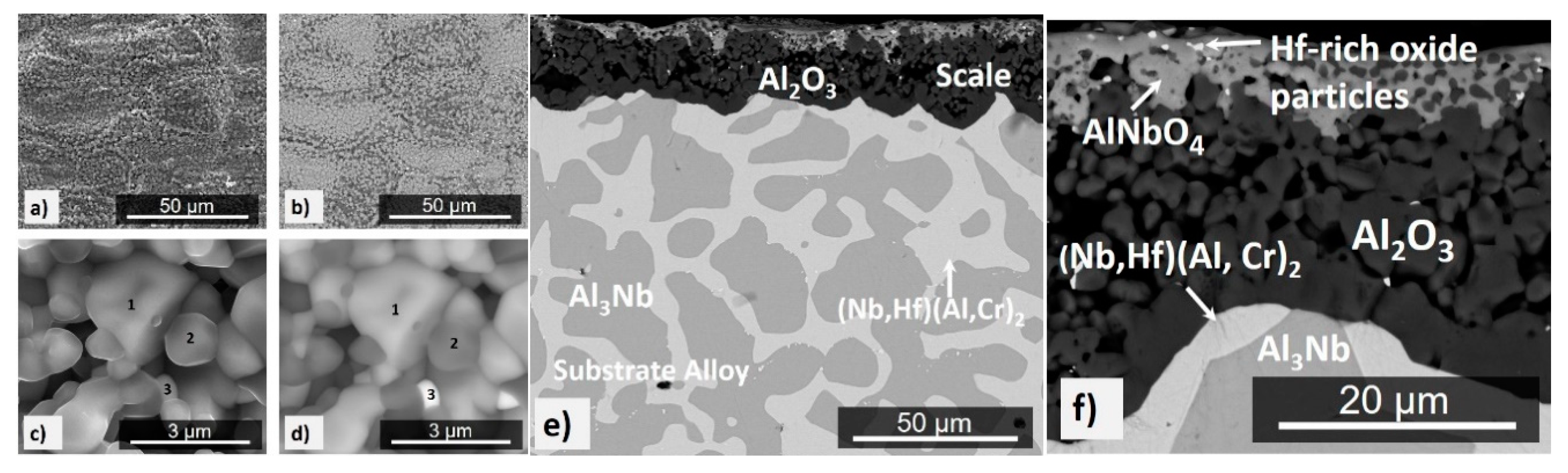
4.1. Cast and Heat-Treated Microstructures
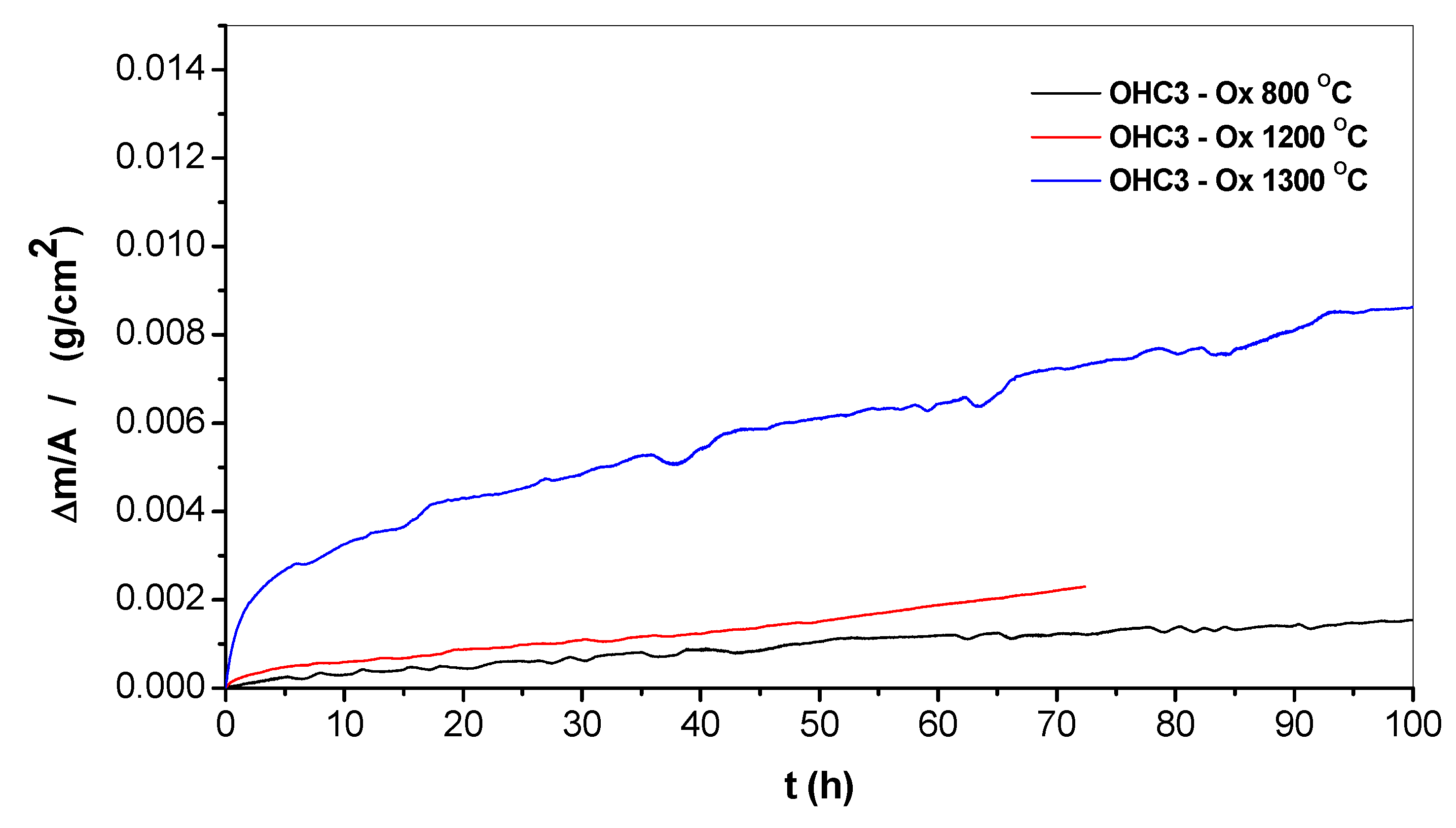
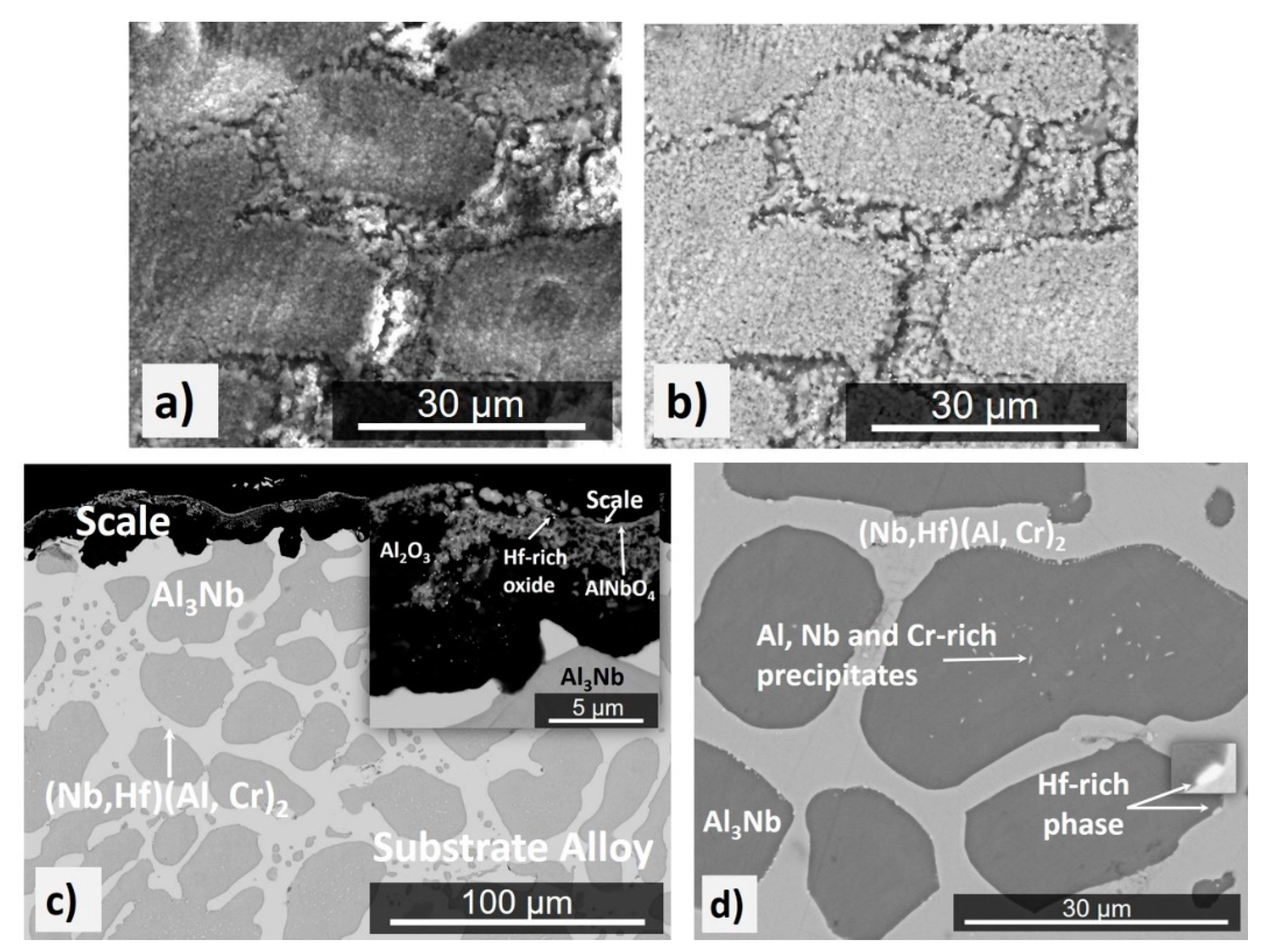
4.2. Isothermal Oxidation
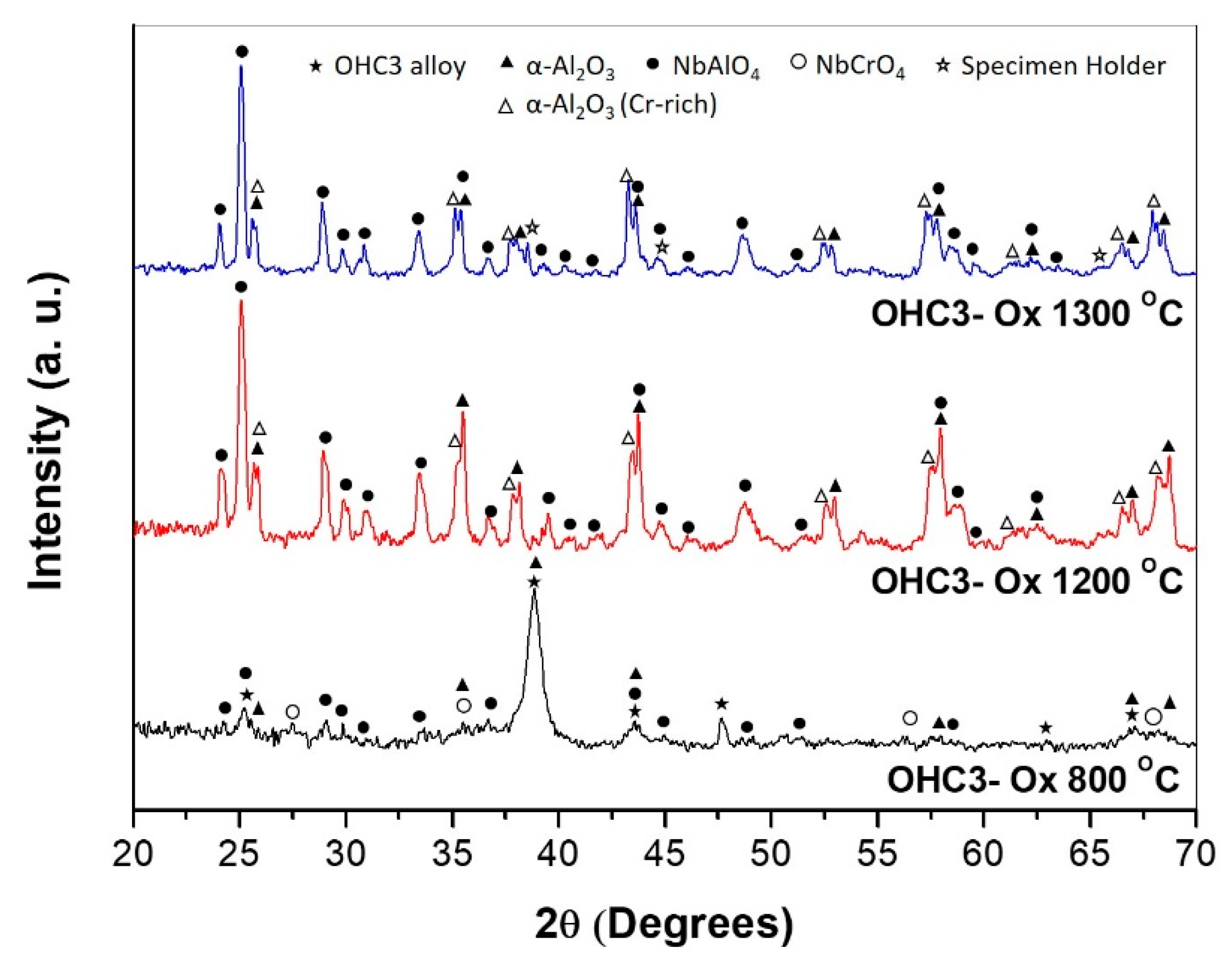
4.2.1. Oxidation at 800 °C
4.2.2. Oxidation at 1200 °C
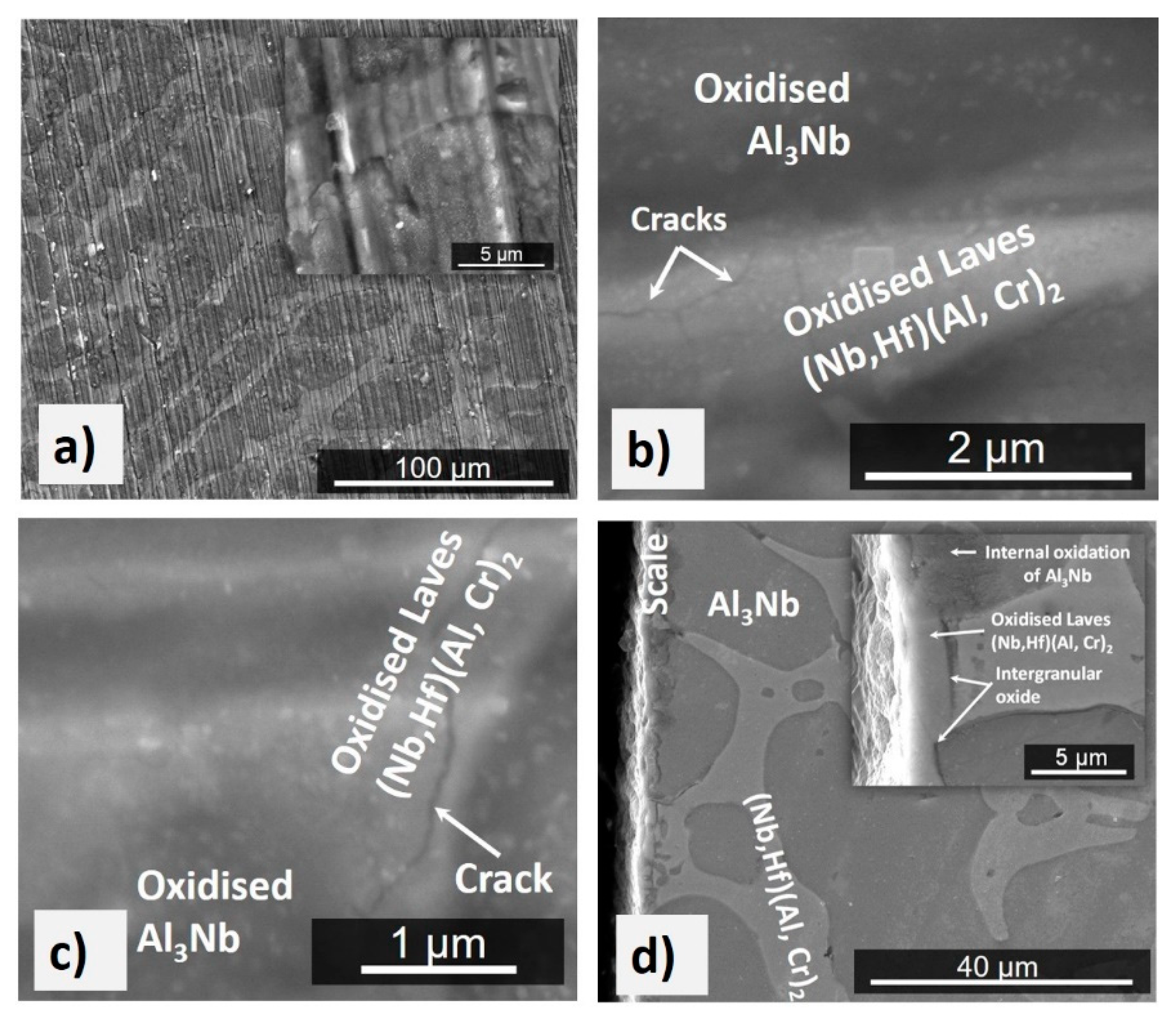
4.2.3. Oxidation at 1300 °C
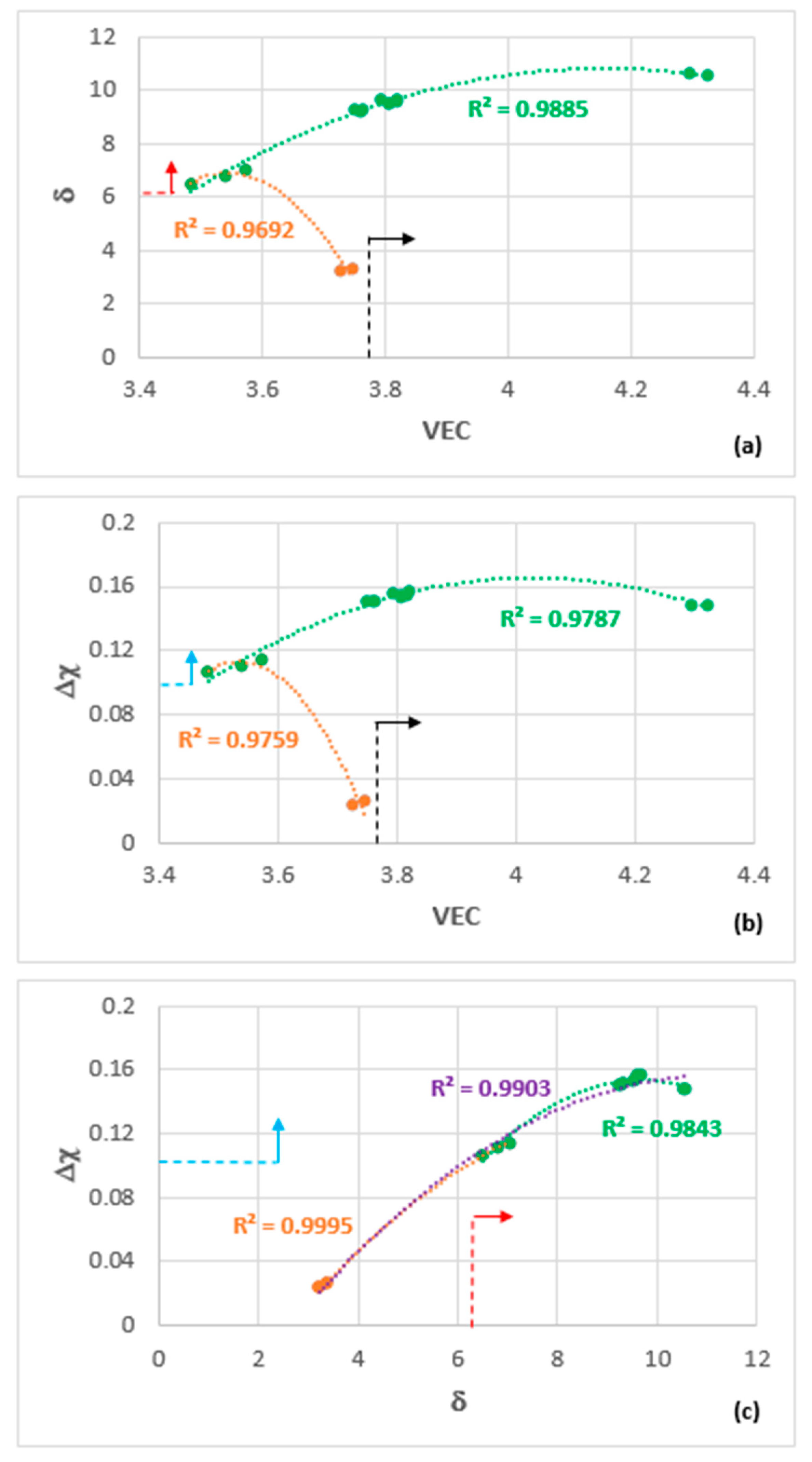
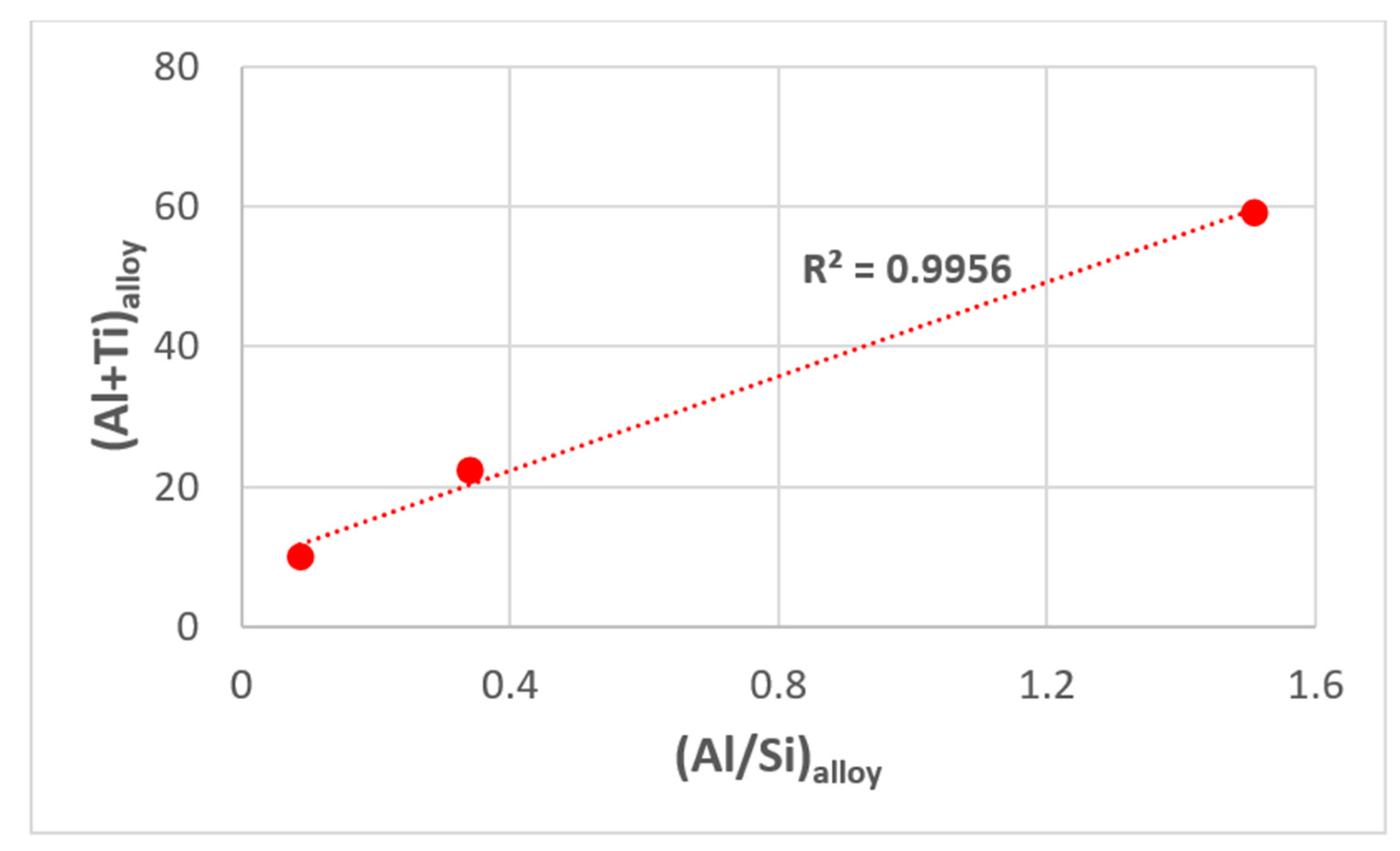
5. Discussion
5.1. Microstructures
5.2. Oxidation
5.2.1. Oxidation at 800 °C
5.2.2. Oxidation at 1200 and 1300 °C
6. Summary and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harada, H.; Murakami, H. Design of Ni-Base Superalloys. In Computational Materials Design; Saito, T., Ed.; Springer: Berlin, Germany, 1999; Volume 34, p. 40. [Google Scholar]
- Wlodek, S.T. The Stability of Superalloys in Long Term Stability of High Temperature Materials; Fuchs, G.E., Dannemann, K.A., Deragon, T.C., Eds.; TMS: Warrendale, PA, USA, 1999; p. 18. [Google Scholar]
- Balsone, S.J.; Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.C.; Chatterjee, A.; Heffernan, T.M. Materials beyond superalloys—Exploiting high-temperature composites. In Structural Intermetallics; Hemker, K.J., Dimiduk, D.M., Clemens., H., Darolia, R., Inui, M., Larsen, J.M., Sikka, V.K., Whittenberger, J.D., Eds.; TMS: Warrendale, PA, USA, 2001; pp. 99–108. [Google Scholar]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef]
- Noebe, R.N.; Bowman, R.R.; Cullers, C.L.; Raj, S.V. Flow and fracture behaviour of NiAl in relation to the brittle to ductile transition temperature. In High Temperature Ordered Intermetallic Alloys IV; Johnson, L.A., Pope, D.P., Stiegler, J.O., Eds.; Defense Technical Information Center: Fort Belvoir, VA, USA, 1991; Volume 213, p. 589. [Google Scholar]
- Dilip, M.S. MoSi2 and other silicides as high temperature structural materials. In Superalloys; Antolovich, S.D., Stusrud, R.W., Klarstrom, D.L., Eds.; TMS: Warrendale, PA, USA, 1992; pp. 409–422. [Google Scholar]
- Frasier, F.R.; Baker, D.R.; Kaufman, M.J. Solidification Microstructures and Mechanical Properties of NbAl3 Based Alloys. In High Temperature Nb Alloys; Stephens, J.J., Ahmad, I., Eds.; TMS: Warrendale, PA, USA, 1991; pp. 121–134. [Google Scholar]
- Shah, D.D.M.; Anton, D.L.; Musson, C.W. Feasibility study of intermetallic composites. Mat. Res. Soc. Symp. Proc. 1990, 194, 333–340. [Google Scholar] [CrossRef]
- Duquette, D.J. Environmental Resistance of Intermetallic Compounds and Composite Materials. In Critical Issues in the Development of high Temperature Structural Materials; Stoloff, N.S., Duquette, D.J., Giamci, A.F., Eds.; TMS: Warrendale, PA, USA, 1993; p. 431. [Google Scholar]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I—The bcc Nb solid solution. J. Alloy. Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Mendiratta, M.G.; Dimiduk, D.M. Microstructures and mechanical behaviour of Nb-Ti base beta + silicide alloys. Mat. Res. Soc. Symp. Proc. 1994, 322, 491–502. [Google Scholar] [CrossRef]
- Tewari, R.; Song, H.; Dey, G.K.; Chatterjee, A.; Vasudevan, V.K. Microstructural evolution in niobium-based alloys. Metall. Mater. Trans. A 2008, 39, 1506–1518. [Google Scholar] [CrossRef]
- Yuan, S.; Jia, L.; Ma, L.; Cui, R.; Su, L.; Zhang, H. The microstructure optimising of the Nb-14Si-22Ti-4Cr-2Al-2Hf alloy processed by directional solidification. Mater. Lett. 2012, 84, 124–127. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X (X = Al, Ge, Si, Sn) in Nb-silicide based alloys. Materials 2018, 11, 395. [Google Scholar] [CrossRef]
- Hunag, Q.; Guo, X.-P.; Kang, Y.-W.; Song, J.-X.; Qu, S.-Y.; Han, Y.-F. Microstructures and mechanical properties of directionally solidified multi-element Nb-Si alloy. Prog. Nat. Sci. Mater. Int. 2011, 21, 146–152. [Google Scholar] [CrossRef]
- Sekido, N.; Kimura, Y.; Miura, S.; Mishima, Y. Microstructure development of unidirectionally solidified (Nb)/Nb3Si eutectic alloys. Mater. Sci. Eng. A 2007, 444, 51–57. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Zacharis, E.; Utton, C.; Tsakiropoulos, P. A study of the effects of Hf and Sn on the microstructure, hardness and oxidation of Nb-18Si silicide based alloys without Ti addition. Materials 2018, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Subramanian, P.; Zhao, J.C.; Bewlay, B.; Darolia, R.; Schafrik, R. Turbine Blade for Extreme Temperature Conditions. U.S. Patent 7,189,459 B2, 13 March 2007. [Google Scholar]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.7Si2.4Ti2.4Al3Hf0.5 and Nb1.3Si2.4Ti2.4Al3.5Hf0.4 alloys. Materials 2019, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Smialek, J.L.; Barrett, C.A.; Schaeffer, J.C. Design for Oxidation Resistance. In ASM Handbook: Materials Selection and Design; ASM International: Materials Park, OH, USA, 1997; Volume 20, pp. 589–602. [Google Scholar]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Begley, R.T. Columbium alloy development at Westinghouse. In Evolution of Refractory Metals and Alloys; Dalder, E.N.C., Grobstein, T., Olsen, C.S., Eds.; TMS: Warrendale, PA, USA, 1994; pp. 29–48. [Google Scholar]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Ta and Cr additions in the microstructure of Nb-Ti-Si-Al in situ composites. Intermetallics 2006, 14, 639–659. [Google Scholar] [CrossRef]
- Fujiwara, T.; Yasuda, K.; Kodama, H. High temperature oxidation of Nb3Al based alloys, in High Temperature Ordered Intermetallic Alloys, V. Mat. Res. Soc. Symp. Proc. 1993, 288, 959–964. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys; Part II. J. Alloy. Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.45Si2.7Ti2.25Al3.25Hf0.35 and Nb1.35Si2.3Ti2.3Al3.7Hf0.35 alloys. Materials 2019, 12, 759. [Google Scholar] [CrossRef]
- Hernandez-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at %) alloy. Materials 2019, 12, 1806. [Google Scholar] [CrossRef]
- Hernandez-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation of silica and alumina scale forming Si-23Fe-15Cr-15Ti-1Nb and Si-25Nb-5Al-5Cr-5Ti (at %) silicide alloys. Materials 2019, 12, 1091. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X. Preparation and oxidation resistance of silicide/aluminide composite coatings on a Nb-Ti-Si based alloy. Surf. Coat. Technol. 2015, 274, 18–25. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- Meier, G.H. Fundamentals of the Oxidation of high Temperature Intermetallics in Oxidation of High-Temperature Intermetallics; Grobstein, T., Doychak, J., Eds.; TMS: Warrendale, PA, USA, 1989; pp. 1–15. [Google Scholar]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. Ab initio investigation of the Nb-Al system. Comp. Mater. Sci. 2015, 107, 116–121. [Google Scholar] [CrossRef]
- Misra, A.K. Identification of Chemically Compatible Reinforcement Materials for Nb Aluminides from Thermodynamic Considerations. In High Temperature Niobium Alloys; Stephens, J.J., Ahmad, I., Eds.; TMS: Warrendale, PA, USA, 1991; pp. 135–142. [Google Scholar]
- Meier, G.H.; Pettit, F.S. The oxidation behaviour of intermetallic compounds. Mater. Sci. Eng. A 1992, 153, 548–560. [Google Scholar] [CrossRef]
- Svedberg, R. Oxides associated with the improved air oxidation performance of some Niobium intermetallics and alloys. In Properties of High Temperature Alloys; Foroulis, Z.A., Pettit, F.S., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1976; p. 331. [Google Scholar]
- Perkins, R.A.; Chiang, K.T.; Meier, G.H. Formation of alumina on Nb-Al alloys. Scr. Metall. 1988, 22, 419–424. [Google Scholar] [CrossRef]
- Fleischer, R.L.; Zababa, R.J. Mechanical Properties of Diverse Binary High Temperature Intermetallic Compounds; Technical Report 89CRD201; GE Research and Development Centre: Niskayuna, NY, USA, November 1989. [Google Scholar]
- Von Keitz, A.; Sauthoff, G. Laves phases for high-temperatures—Part II: Stability and mechanical properties. Intermetallics 2002, 10, 497–510. [Google Scholar] [CrossRef]
- Brady, M.P.; Tortorelli, P.F.; Walker, L.R. Correlation of alloy microstructure with oxidation behaviour in chromia-forming intermetallic-reinforced Cr alloys. Mater. High Temp. 2000, 17, 235–243. [Google Scholar] [CrossRef]
- Brady, M.P.; Tortorelli, P.F. Alloy design of intermetallics for protective scale formation and for use as precursors for complex ceramic phase surfaces. Intermetallics 2004, 12, 779–789. [Google Scholar] [CrossRef]
- Hebsur, M.H.; Stephens, J.R.; Smailek, J.L.; Barrett, C.A.; Fox, D.S. Influence of Alloying Elements on the Oxidation Behaviour of NbAl3 in Oxidation of High Temperature Intermetallics; Grobstein, T., Doychak, J., Eds.; The Metallurgical Society of AIME and TMS: Pittsburgh, PA, USA, 1989; pp. 171–183. [Google Scholar]
- Doychak, J.; Hebsur, M.G. Protective Al2O3 Scale formation on NbAl3-Base Alloys. Oxid. Met. 1991, 36, 113–141. [Google Scholar] [CrossRef]
- Stein, F.; He, C.; Wossack, I. The liquidus surface of the Cr-Al-Nb system and re-investigation of the Cr-Nb and Al-Cr phase diagrams. J. Alloy. Compd. 2014, 598, 253–265. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, Z.; Lu, S.; Jianye, Z.; Guangming, L. Effect of Al additions on the oxidation behaviour of Laves phase NbCr2 alloys at 1373 K and 1474 K. Int. J. Refract. Metal. Hard Mater. 2009, 27, 659–663. [Google Scholar] [CrossRef]
- Perkins, R.A.; Chiang, K.T.; Meier, G.H.; Miller, R.A. Effect of Alloying, Rapid Solidification and Surface Kinetics on the High Temperature Environmental Resistance of Niobium AFOSR Report; LMSC-F352227; Lockheed Missiles & Space Company Inc.: Palo Alto, CA, USA, 1989. [Google Scholar]
- Jiang, C.-L.; Jiao, Z.; Zeng, W.; Liu, F.-S.; Tang, B.; Liu, Q.-J. Effects of different phases, compositional change and doping on ductility improvement of NbAl3 phases. J. Alloy. Compd. 2019, 788, 172–182. [Google Scholar] [CrossRef]
- Munitz, A.; Gokhale, A.B.; Abbaschian, R. The effect of supercooling on the microstructure of Al-Nb alloys. J. Mater. Sci. 2000, 35, 2263–2271. [Google Scholar] [CrossRef]
- Zhao, J.C.; Jackson, M.R.; Peluso, L.A. Evaluation of phase relations in the Nb-Cr-Al sytem at 1000 °C using diffusion-multiple approach. J. Phase Equilibria Diffus. 2004, 25, 152–159. [Google Scholar]
- Ebrahimi, F.; Ruiz-Aparicio, J.G.L. Diffusivity in the Nb-Ti-Al ternary solid solution. J. Alloy. Compd. 1996, 245, 1–9. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der Oxydation von Legierungen. Z. Elektrochem. 1959, 63, 772–782. [Google Scholar]
- Lauf, R.J.; Altstetter, C.J. Diffusion and trapping of oxygen in refractory metal alloys. Acta Metall. 1979, 27, 1157–1163. [Google Scholar] [CrossRef]
- Fujita, M.; Kaneno, Y.; Takasugi, T. Phase field and room temperature mechanical properties of C15 Laves phase in Nb-Hf-Cr and Nb-Ta-Cr alloy systems. J. Alloy. Compd. 2006, 424, 283–288. [Google Scholar] [CrossRef]
- Pint, B.T. Optimization of Reactive-Element additions to improve oxidation performance of Alumina-Forming alloys. J. Am. Ceram. Soc. 2003, 86, 686–695. [Google Scholar] [CrossRef]
- Tweddle, A.; Tsakiropoulos, P. Role of Y and Ge additions in Nb-silicide based alloys. University of Sheffield: Sheffield, UK, 2015; Unpublished work. [Google Scholar]
- Gama, S. Aluminium-Iron-Niobium, Ternary Alloys; Wiley-VCH: Weinheim, Germany, 1992; Volume 5, p. 281. [Google Scholar]
- Raghavan, V. The Al-Fe-Hf (Aluminium-Iron-Hafnium) system, Phase Diagrams Ternary Iron Alloys. Indian Inst. Met. 1992, 1, 115–119. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Ge addition on the microstructure of Nb-18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar] [CrossRef]
- Xu, Z.; Utton, C.; Tsakiropoulos, P. A study of the effect of 2 at % Sn on the microstructure and isothermal oxidation at 800 and 1200 °C of Nb-24Ti-18Si based alloys with Al and/or Cr additions. Materials 2018, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Lacy, L.L.; Robinson, M.B.; Rathy, T.J. Containerless undercooling and solidification in drop tubes. J. Cryst. Growth 1981, 51, 47–60. [Google Scholar] [CrossRef]
- Lacy, L.L.; Robinson, M.B.; Rathy, T. Containerless undercooling and solidification of bulk metastable Nb3Ge alloys. J. Appl. Phys. 1982, 53, 682–689. [Google Scholar] [CrossRef]
- Bendersky, L.; Biancaniello, F.S.; Boettinger, W.J.; Perepezko, J.H. Microstructural characterisation of rapidly solidified Nb-Si alloys. Mater. Sci. Eng. 1987, 89, 151–159. [Google Scholar] [CrossRef]
- Han, X.J.; Wei, B. Microstructural characteristics of Ni-Sb eutectic lloys under substantial undercooling and containerless solidification conditions. Metall. Mater. Trans. 2002, 33, 1221–1228. [Google Scholar] [CrossRef]
- Mullis, A.M.; Clopet, C.R.; Cochrane, R.F. Determination of the origin of anomalous eutectic structures from in situ observation of recalescence behavior. Mater. Sci. Forum 2014, 790–797, 349–354. [Google Scholar] [CrossRef]
- Li, M.; Nagashio, K.; Kuribayashi, K. Re-examination of the solidification behaviour of undercooled Ni-Sn eutectic melt. Acta Mater. 2002, 50, 3239–3250. [Google Scholar] [CrossRef]
- Li, M.; Kuribayashi, K. Nucleation controlled microstructures and anomalous eutectic formation in undercooled Co-Sn and Ni-Si eutectic melts. Metall. Mater. Trans. 2003, 34, 2999–3008. [Google Scholar] [CrossRef]
- Loser, W.; Hermann, R.; Leonhardt, M.; Stephan, D.; Bormann, R. Metastable phase formation in undercooled near-eutectic Nb-Al alloys. Mater. Sci. Eng. A 1997, 224, 53–60. [Google Scholar] [CrossRef]
- Kofstad, P. Formation of Compact Scales at High Temperatures. In High Temperature Oxidation of Metals (Corrosion Monographs Series); In Tech Open: Oslo, Norway, 1966. [Google Scholar]
- Steinhorst, M.; Grabke, H.J. Oxidation of Niobium Aluminide NbAl3. Mater. Sci. Eng. A 1989, 120, 55–59. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakao, Y. Undercooling in Ni3Sn-Ni3Sn2 eutectic system alloys, Kyushu Inst. Technol. Acad. Rep. 1981, 42, 43–52. [Google Scholar]
- Wang, Y.; Gao, J.; Kolbe, M.; Ren, Y.; Matson, D. Metastable solidification of hypereutectic Co2Si-CoSi composition: Microstructural studies and in-situ observations. Acta Mater. 2018, 142, 172–180. [Google Scholar] [CrossRef]
- Prymak, O.; Stein, F. The ternary Cr-Al-Nb phase diagram: Experimental investigations of isothermal sections at 1150, 1300 and 1450 °C. J. Alloy. Compd. 2012, 513, 378–386. [Google Scholar] [CrossRef]
- Mahdouk, K.; Gachon, J.C. A thermodynamic study of the Al-Cr-Nb ternary system. J. Alloy. Compd. 2001, 321, 232–236. [Google Scholar] [CrossRef]
- Thoma, D.J.; Perepezko, J.H. An experimental evaluation of the phase relationships and solubilities in the Nb-Cr system. Mater. Sci. Eng. A 1992, 424, 97–108. [Google Scholar] [CrossRef]
- Ivanchenko, V. Light Metal Systems. Part 1. Phys. Chem. 2004, 360–370. [Google Scholar]
- Grabke, H.J.; Steinhorst, M.; Brumm, M.; Wiemer, D. Oxidation and Intergranular Disintegration of the Aluminides NiAl and NbAl3 and Phases in the system Nb-Ni-Al. Oxid. Met. 1991, 35, 199–222. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Grabke, H.J. Mechanism of the intergranular Disintegration (Pest) of the Intermetallic Compound NbAl3. Scr. Metall. Mater. 1993, 28, 747–752. [Google Scholar] [CrossRef]
- Souza, S.A.; Ferrandini, P.L.; Souza, E.A. On the properties of the eutectic alloy Al3(Nb,Cr) + Cr(Al,Nb). J. Alloy. Compd. 2008, 464, 162–167. [Google Scholar] [CrossRef]
- Rios, C.T.; Milenkovic, S.; Ferrandini, P.L.; Caram, R. Directional solidification, microstructure and properties of the Al3Nb-Nb2Al eutectic. J. Cryst. Growth 2005, 275, 153–158. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I. Phase transformation s in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Wang, Y.; Smialek, J.L.; Suneson, M. Oxidation behavior of Hf-Modified Al Coatings on Inconel-718 at 1050 °C. J. Coat. Sci. Technol. 2014, 1, 25–45. [Google Scholar] [CrossRef]
- Pint, B.A.; DiStefano, J.R.; Wright, I.G. Oxidation resistance: One barrier to moving beyond Ni-base superalloys. Mater. Sci. Eng. A 2006, 415, 255–263. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of Aluminium Oxide Scales on High-Temperature Alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]
- Perkins, R.A.; Meier, G.H. The oxidation behaviour and protection of niobium. JOM 1990, 42, 17–21. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. The impact of Ti and temperature on the stability of Nb5Si3 phases: A first-principles study. Sci. Technol. Adv. Mater. 2017, 18, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Wang, J.; Du, Y.; Holec, D.; Nie, X.; Jin, Z. Predicting an alloying strategy for improving fracture toughness of C15 NbCr2 Laves phase: A first-principles study. Comp. Mater. Sci. 2016, 123, 59–64. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Pint, B.A. Oxidation behaviour of Cr-Cr2Nb alloys. In Fundamental Aspects of High Temperature Corrosion; Shores, D.A., Hou, P.Y., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1996; pp. 74–85. [Google Scholar]
- Zheng, H.; Lu, Z.; Lu, S.; Su, Z.Q.; Quan, F. Study on scaling of mechanically alloyed and hot pressed NbCr2 Laves phase at 1200 °C. Int. J. Refract. Met. Hard Mater. 2008, 26, 1–4. [Google Scholar] [CrossRef]
- Nesbitt, J.A.; Lowell, C.E. Prediction of the high temperature oxidative life of intermetallics. Mat. Res. Soc. Symp. Proc. 1993, 288, 107–118. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Beyond Nickel Based Superalloys. In Encyclopaedia of Aerospace Engineering; Blockley, R., Shyy, W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Thandorn, T.; Tsakiropoulos, P. Study of the role of B addition on the microstructure of the Nb-24Ti-18Si-8B alloy. Intermetallics 2010, 18, 1033–1038. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. On the Nb-Ge binary system. Metall. Mater. Trans. A 2015, 46, 5526–5536. [Google Scholar] [CrossRef]
- Utton, C.A.; Papadimitriou, I.; Kinoshita, H.; Tsakiropoulos, P. Experimental and thermodynamic assessment of the Ge-Nb-Si ternary phase diagram. J. Alloy. Compd. 2017, 717, 303–316. [Google Scholar] [CrossRef]
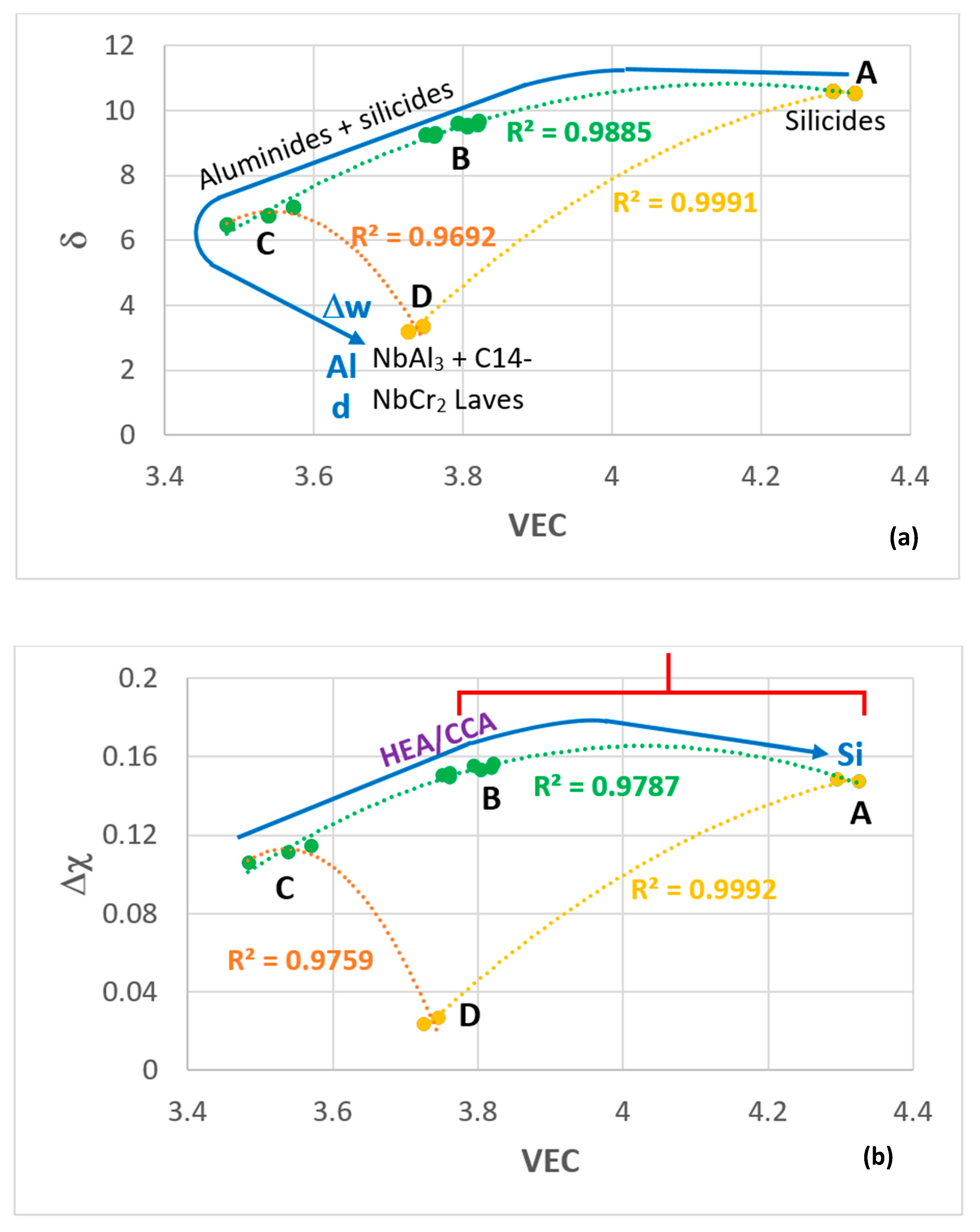
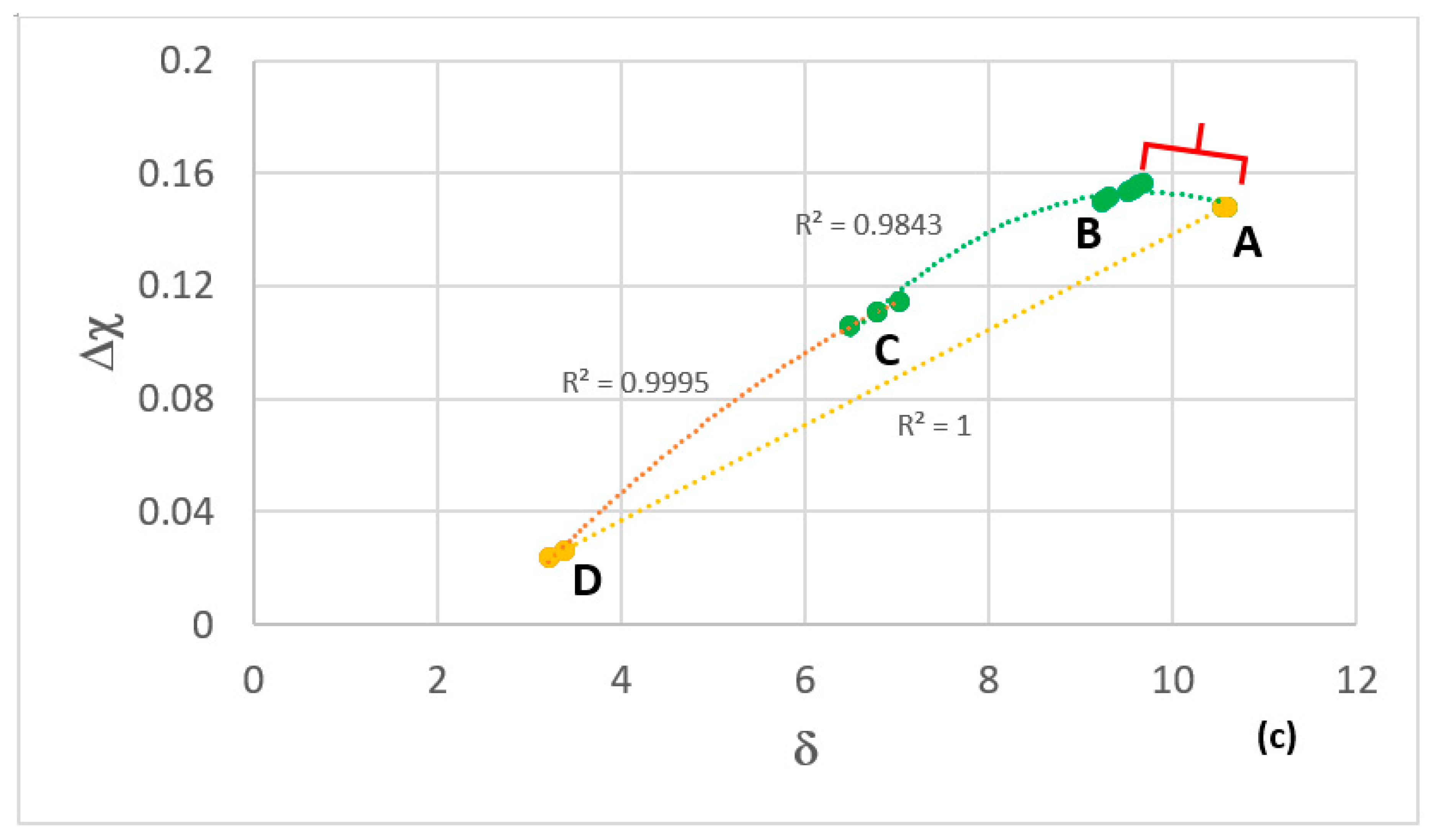
| Temperature | n | Parabolic Rate Constant Kp (g2·cm−4·s−1) | Mass Change (mg/cm2) |
|---|---|---|---|
| 800 °C | 0.68 | 6.72 × 10−12 | 1.54 |
| 1200 °C | 0.56 | 1.78 × 10−11 (0–43 h) 5.7 × 10−11 (43–80 h) | 4.47 |
| 1300 °C | 0.41 | 1.88 × 10−10 | 8.5 |
| MAP Area | Alloy [ref] | Alloying Elements (at %) | Phases (AC and HT) | Mass Change (mg/cm2) pest Behaviour | kp (g2cm−4s−1) | Scale & Thickness (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cr | Hf | Nb | Si | Ti | 800 °C | 1200 °C | 1200 °C | 1200 °C | |||
| A | OHC5 [30] | 5 | 5 | 25 | 60 | 5 | TM6Si5 C40-TMSi2 | 0.22 no pest | 0.85 | 1.4 10−12 | αAl2O3 5–10 | |
| B | MG5 [29] | 32.5 | 3.5 | 14.5 | 27 | 22.5 | DO22-TiAl3 Ti5Si4, TiAl D88-TM5Si3 TiSi | 0.5 no pest | 1.6 | 8 10−12 | Niobates αAl2O3 < 10 | |
| MG6 [29] | 37 | 3.5 | 13.5 | 23 | 23 | DO22-TiAl3 Ti5Si4, TiAl D88-TM5Si3 TiSi TM9Si7Al4 | 0.37 no pest | 2.3 | 3 10−13 | Niobates αAl2O3 ≤ 10 | ||
| MG7 [21] | 35 | 4 | 13 | 24 | 24 | DO22-TiAl3 Ti5Si4, TiAl D88-TM5Si3 TiSi | 0.68 no pest | 2.6 | 1 10−11 | Niobates αAl2O3 5 | ||
| C | Zone A in MG7 | 54 | 1.7 | 11 | 12.8 | 20.5 | DO22-TiAl3 D88-TM5Si3 | |||||
| D | OHC3 | 68 | 6 | 0.5 | 25.5 | DO22-NbAl3 C14-NbCr2 | 1.54 no pest | 4.47 | 5.7 10−11 −1.8 10−11 | NbAlO4 αAl2O3 7–32 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Negrete, O.; Tsakiropoulos, P. On the Microstructure and Isothermal Oxidation at 800, 1200, and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at %) Alloy. Materials 2019, 12, 2531. https://doi.org/10.3390/ma12162531
Hernández-Negrete O, Tsakiropoulos P. On the Microstructure and Isothermal Oxidation at 800, 1200, and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at %) Alloy. Materials. 2019; 12(16):2531. https://doi.org/10.3390/ma12162531
Chicago/Turabian StyleHernández-Negrete, Ofelia, and Panos Tsakiropoulos. 2019. "On the Microstructure and Isothermal Oxidation at 800, 1200, and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at %) Alloy" Materials 12, no. 16: 2531. https://doi.org/10.3390/ma12162531
APA StyleHernández-Negrete, O., & Tsakiropoulos, P. (2019). On the Microstructure and Isothermal Oxidation at 800, 1200, and 1300 °C of the Al-25.5Nb-6Cr-0.5Hf (at %) Alloy. Materials, 12(16), 2531. https://doi.org/10.3390/ma12162531






